Abstract
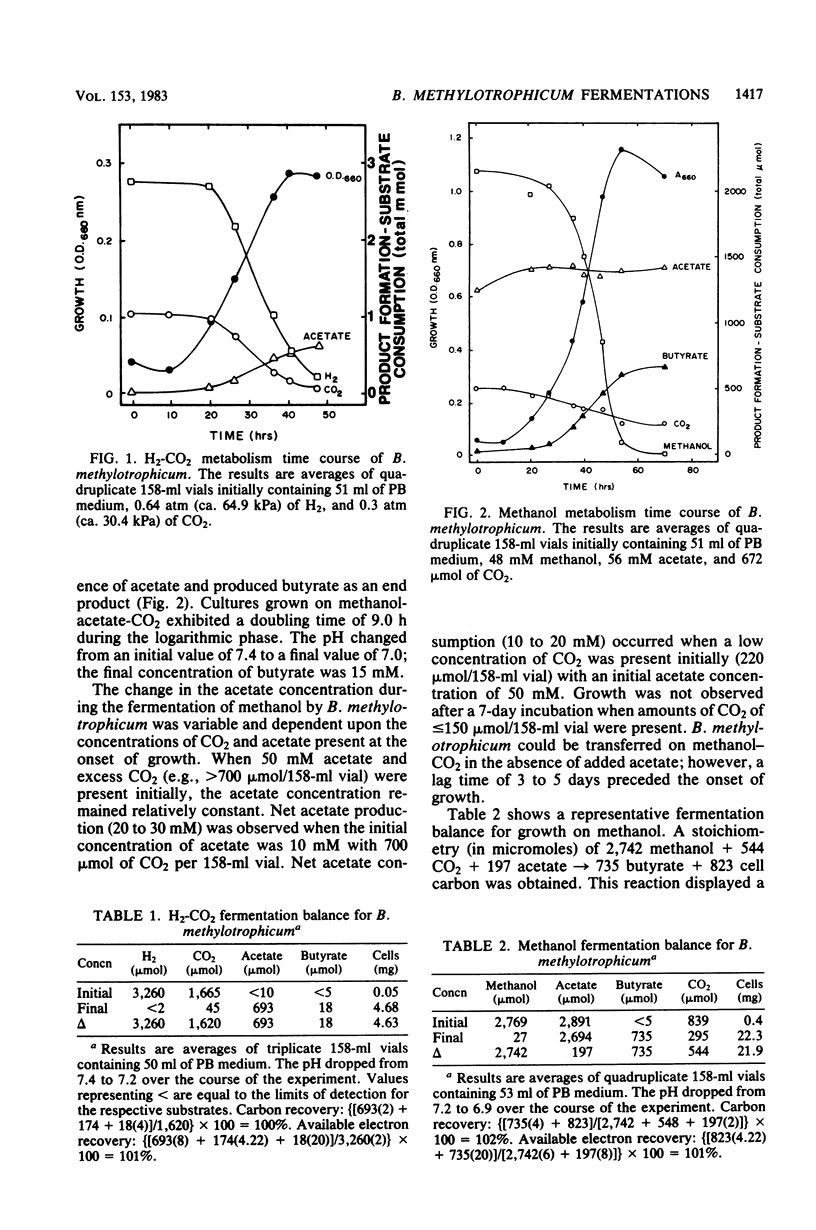
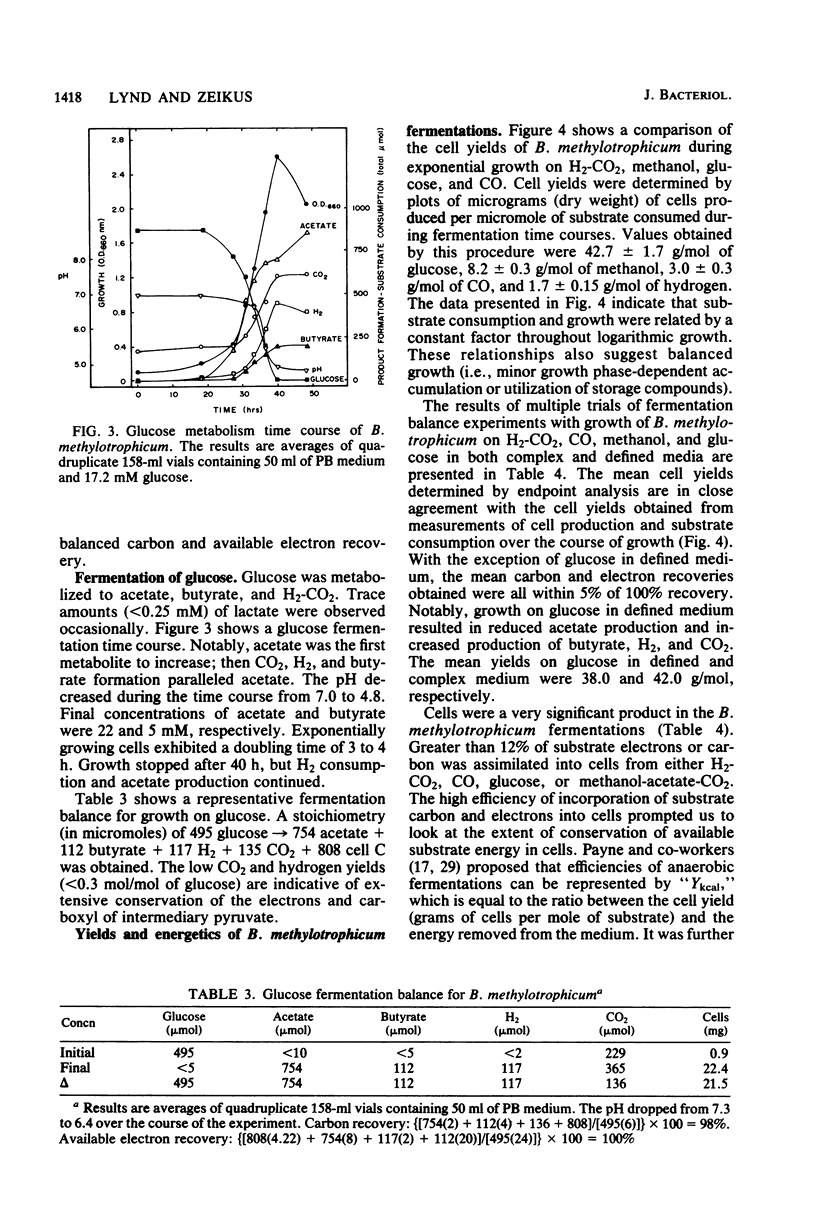
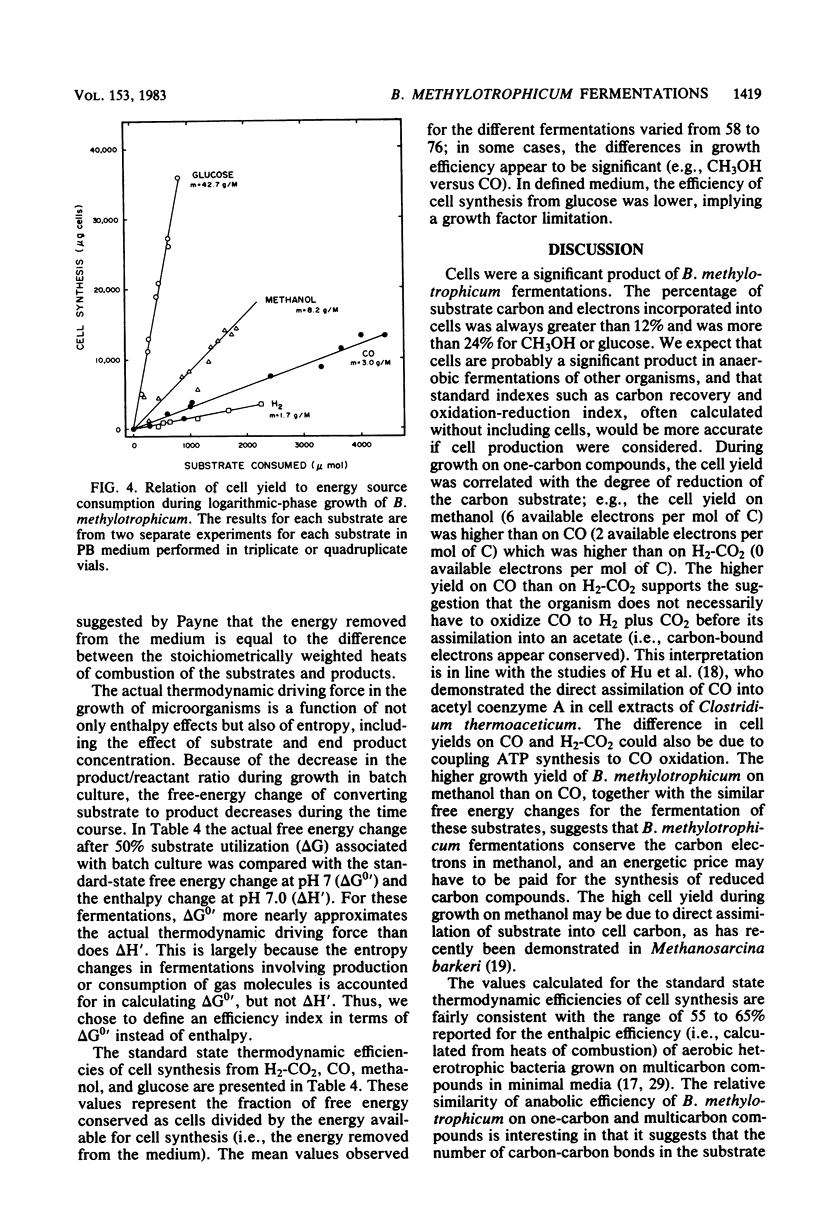
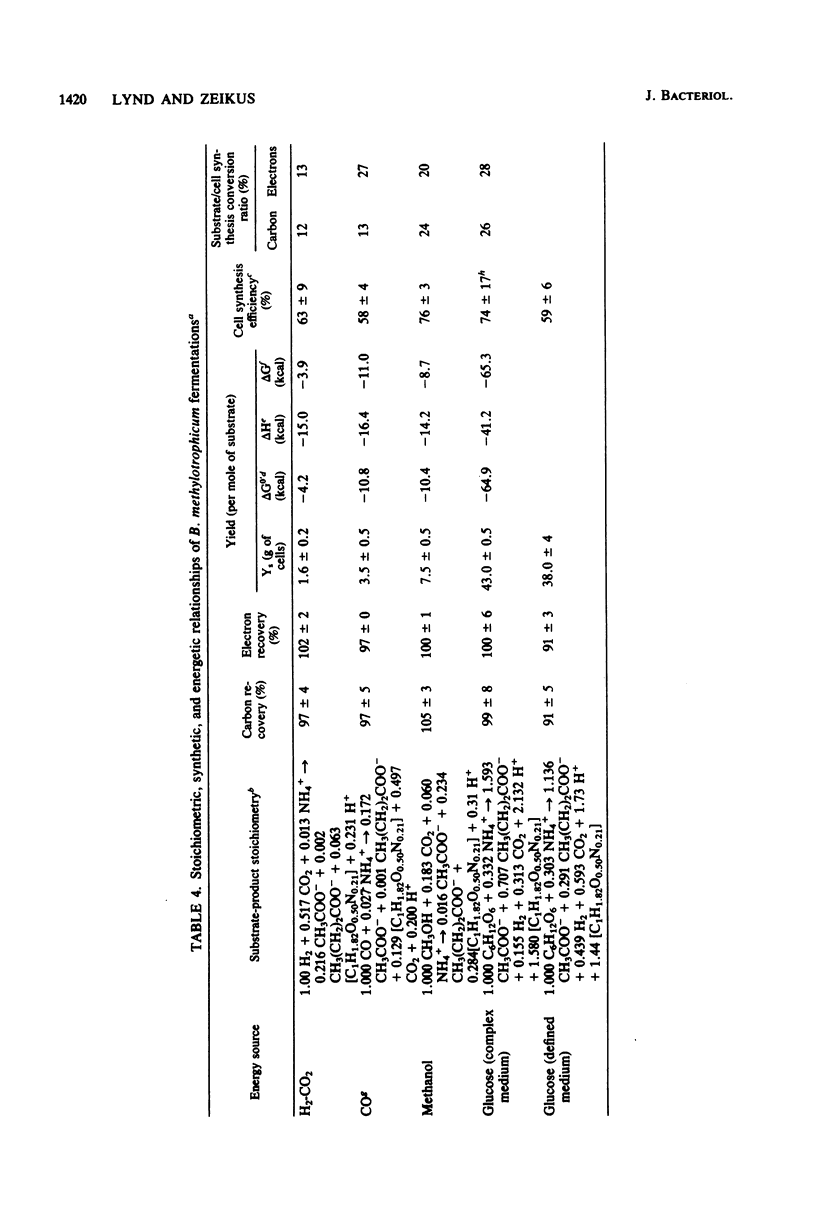
The fermentative metabolism of Butyribacterium methylotrophicum grown on either H2-CO2, methanol, glucose, or CO is described. The following reaction stoichiometries were obtained: 1.00 H2 + 0.52 CO2 leads to 0.22 acetate + 0.06 cell C; 1 methanol + 0.18 CO2 + 0.01 acetate leads to 0.24 butyrate + 0.29 cell C; and 1.00 glucose leads to 0.31 CO2 + 1.59 acetate + 0.21 butyrate + 0.13 H2 + 1.58 cell C. Cell yields of 1.7 g (dry weight) per mol of H2, 8.2 g (dry weight) per mol of methanol, 42.7 g (dry weight) per mol of glucose, and 3.0 g (dry weight) per mol of CO were obtained from linear plots of cell synthesis and substrate consumption. Doubling times of 9.0, 9.0, and 3 to 4 h were observed during batch growth on H2-CO2, methanol, and glucose, respectively. Indicative of a growth factor limitation, glucose fermentation in defined medium displayed a lower cell synthesis efficiency than when yeast extract (0.05%) was present. B. methylotrophicum fermentation displayed atypically high substrate/cell carbon synthesis conversion ratios for an anaerobe, as greater than 24% of the carbon was assimilated into cells during growth on methanol or glucose. The data indicate that B. methylotrophicum conserves carbon-bound electrons during growth on single-carbon or multicarbon substrates.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen J. R., Gottschalk G., Schlegel H. G. Clostridium formicoaceticum nov. spec. isolation, description and distinction from C. aceticum and C. thermoaceticum. Arch Mikrobiol. 1970;72(2):154–174. doi: 10.1007/BF00409521. [DOI] [PubMed] [Google Scholar]
- Andreesen J. R., Schaupp A., Neurauter C., Brown A., Ljungdahl L. G. Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: effect of metals on growth yield, enzymes, and the synthesis of acetate from CO 2 . J Bacteriol. 1973 May;114(2):743–751. doi: 10.1128/jb.114.2.743-751.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A., Kamen M. D., Bornstein B. T. The Synthesis of Butyric and Caproic Acids from Ethanol and Acetic Acid by Clostridium Kluyveri. Proc Natl Acad Sci U S A. 1945 Dec;31(12):373–381. doi: 10.1073/pnas.31.12.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A., Kamen M. D. Carbon Dioxide Utilization in the Synthesis of Acetic Acid by Clostridium Thermoaceticum. Proc Natl Acad Sci U S A. 1945 Aug;31(8):219–225. doi: 10.1073/pnas.31.8.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A., Kamen M. D., Haas V. Carbon Dioxide Utilization in the Synthesis of Acetic and Butyric Acids by Butyribacterium Rettgeri. Proc Natl Acad Sci U S A. 1945 Nov;31(11):355–360. doi: 10.1073/pnas.31.11.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A., Ruben S., Beck J. V. Radioactive Carbon as an Indicator of Carbon Dioxide Reduction: IV. The Synthesis of Acetic Acid from Carbon Dioxide by Clostridium Acidi-Urici. Proc Natl Acad Sci U S A. 1940 Aug 15;26(8):477–482. doi: 10.1073/pnas.26.8.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M., Mayer F., Gottschalk G. Clostridium aceticum (Wieringa), a microorganism producing acetic acid from molecular hydrogen and carbon dioxide. Arch Microbiol. 1981 Jan;128(3):288–293. doi: 10.1007/BF00422532. [DOI] [PubMed] [Google Scholar]
- Drake H. L., Hu S. I., Wood H. G. Purification of five components from Clostridium thermoaceticum which catalyze synthesis of acetate from pyruvate and methyltetrahydrofolate. Properties of phosphotransacetylase. J Biol Chem. 1981 Nov 10;256(21):11137–11144. [PubMed] [Google Scholar]
- Genthner B. R., Bryant M. P. Growth of Eubacterium limosum with Carbon Monoxide as the Energy Source. Appl Environ Microbiol. 1982 Jan;43(1):70–74. doi: 10.1128/aem.43.1.70-74.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Davis C. L., Bryant M. P. Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2-CO2-utilizing species. Appl Environ Microbiol. 1981 Jul;42(1):12–19. doi: 10.1128/aem.42.1.12-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghambeer R. K., Wood H. G., Schulman M., Ljungdahl L. Total synthesis of acetate from CO2. 3. Inhibition by alkylhalides of the synthesis from CO2, methyltetrahydrofolate, and methyl-B12 by Clostridium thermoaceticum. Arch Biochem Biophys. 1971 Apr;143(2):471–484. doi: 10.1016/0003-9861(71)90232-3. [DOI] [PubMed] [Google Scholar]
- Hu S. I., Drake H. L., Wood H. G. Synthesis of acetyl coenzyme A from carbon monoxide, methyltetrahydrofolate, and coenzyme A by enzymes from Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):440–448. doi: 10.1128/jb.149.2.440-448.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy W. R., Zeikus J. G. One-carbon metabolism in methanogens: evidence for synthesis of a two-carbon cellular intermediate and unification of catabolism and anabolism in Methanosarcina barkeri. J Bacteriol. 1982 Aug;151(2):932–941. doi: 10.1128/jb.151.2.932-941.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzycki J. A., Wolkin R. H., Zeikus J. G. Comparison of unitrophic and mixotrophic substrate metabolism by acetate-adapted strain of Methanosarcina barkeri. J Bacteriol. 1982 Jan;149(1):247–254. doi: 10.1128/jb.149.1.247-254.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl L. G. Total synthesis of acetate from CO2 by heterotrophic bacteria. Annu Rev Microbiol. 1969;23:515–538. doi: 10.1146/annurev.mi.23.100169.002503. [DOI] [PubMed] [Google Scholar]
- Lynd L., Kerby R., Zeikus J. G. Carbon monoxide metabolism of the methylotrophic acidogen Butyribacterium methylotrophicum. J Bacteriol. 1982 Jan;149(1):255–263. doi: 10.1128/jb.149.1.255-263.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien W. E., Ljungdahl L. G. Fermentation of fructose and synthesis of acetate from carbon dioxide by Clostridium formicoaceticum. J Bacteriol. 1972 Feb;109(2):626–632. doi: 10.1128/jb.109.2.626-632.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D. J., Wood H. G., Ghambeer R. K., Ljungdahl L. G. Total synthesis of acetate from carbon dioxide. Retention of deuterium during carboxylation of trideuteriomethyltetrahydrofolate or trideuteriomethylcobalamin. Biochemistry. 1972 Aug 1;11(16):3074–3080. doi: 10.1021/bi00766a021. [DOI] [PubMed] [Google Scholar]
- Parker D. J., Wu T. F., Wood H. G. Total synthesis of acetate from CO 2 : methyltetrahydrofolate, an intermediate, and a procedure for separation of the folates. J Bacteriol. 1971 Nov;108(2):770–776. doi: 10.1128/jb.108.2.770-776.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J., Williams M. L. Carbon assimilation from simple and complex media by prtotrophic heterotrophic bacteria. Biotechnol Bioeng. 1976 Nov;18(11):1653–1655. doi: 10.1002/bit.260181117. [DOI] [PubMed] [Google Scholar]
- Schulman M., Ghambeer R. K., Ljungdahl L. G., Wood H. G. Total synthesis of acetate from CO2. VII. Evidence with Clostridium thermoaceticum that the carboxyl of acetate is derived from the carboxyl of pyruvate by transcarboxylation and not by fixation of CO2. J Biol Chem. 1973 Sep 25;248(18):6255–6261. [PubMed] [Google Scholar]
- Schulman M., Parker D., Ljungdahl L. G., Wood H. G. Total synthesis of acetate from CO 2 . V. Determination by mass analysis of the different types of acetate formed from 13 CO 2 by heterotrophic bacteria. J Bacteriol. 1972 Feb;109(2):633–644. doi: 10.1128/jb.109.2.633-644.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner R. S., Wolfe R. S., Ljungdahl L. G. Tetrahydrofolate enzyme levels in Acetobacterium woodii and their implication in the synthesis of acetate from CO2. J Bacteriol. 1978 May;134(2):668–670. doi: 10.1128/jb.134.2.668-670.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Acetate metabolism in Methanosarcina barkeri. Arch Microbiol. 1978 Nov 13;119(2):175–182. doi: 10.1007/BF00964270. [DOI] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. One carbon metabolism in methanogenic bacteria. Cellular characterization and growth of Methanosarcina barkeri. Arch Microbiol. 1978 Oct 4;119(1):49–57. doi: 10.1007/BF00407927. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G. Chemical and fuel production by anaerobic bacteria. Annu Rev Microbiol. 1980;34:423–464. doi: 10.1146/annurev.mi.34.100180.002231. [DOI] [PubMed] [Google Scholar]


