Abstract
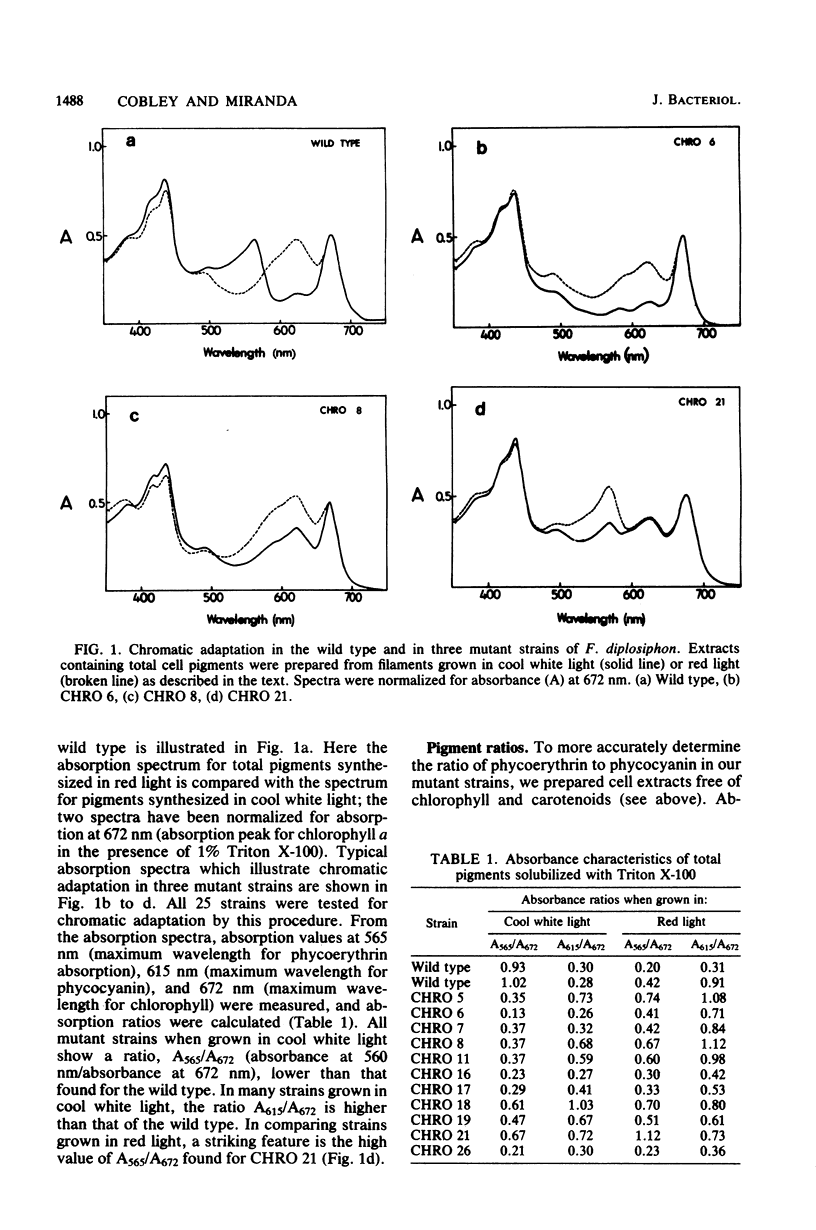
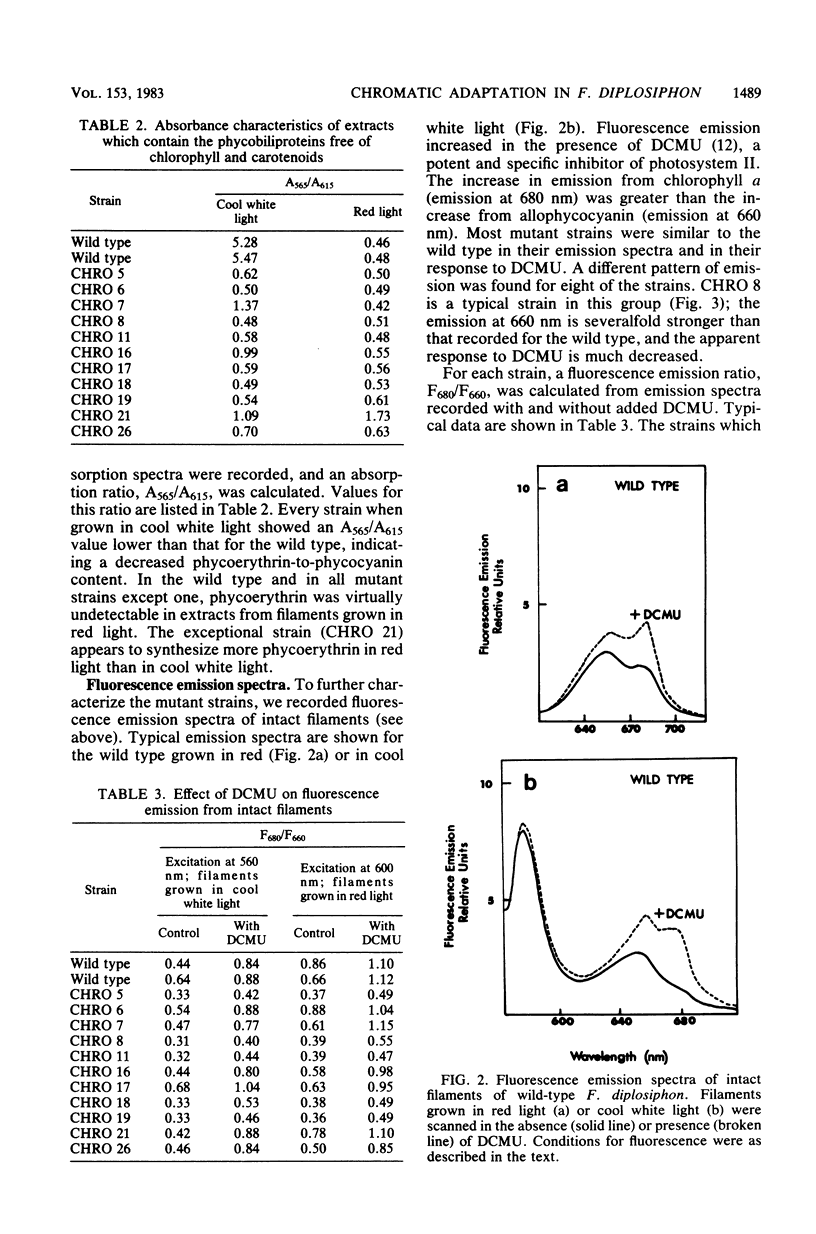
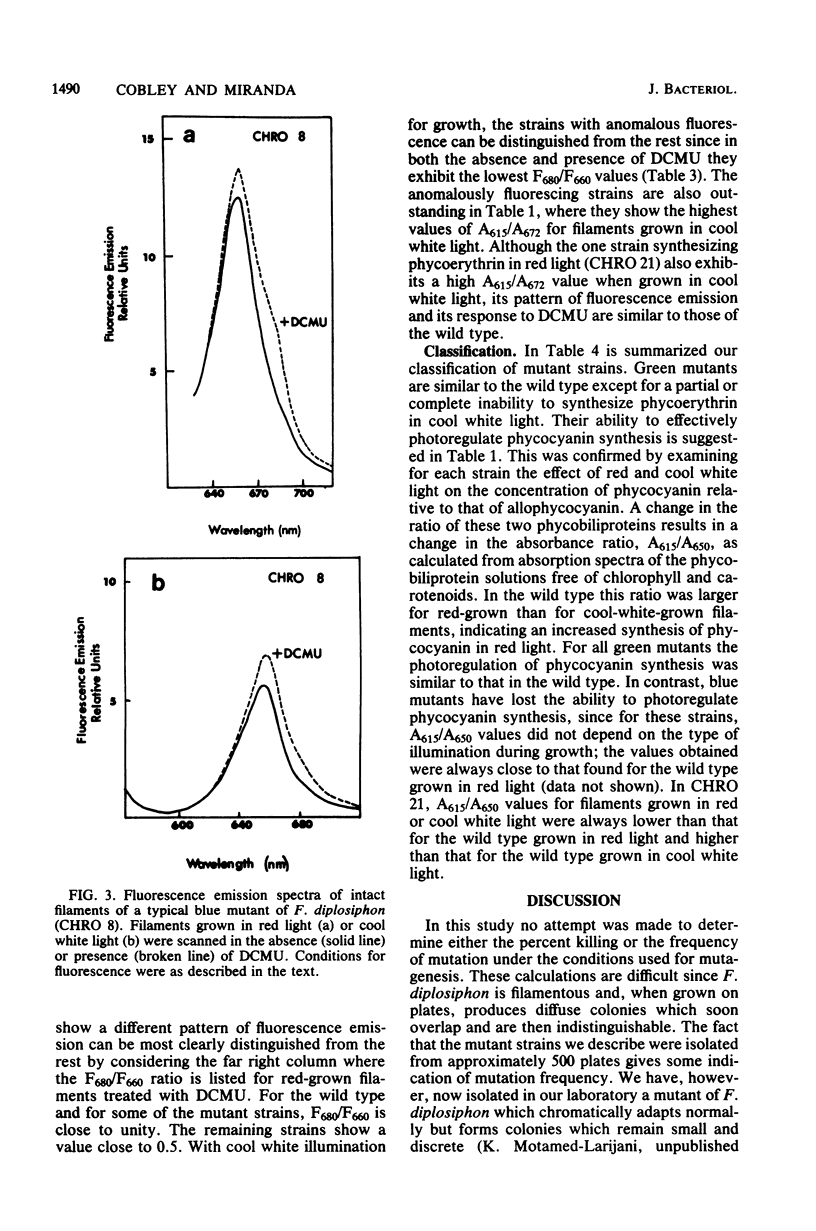
The chromatically adapting cyanobacterium, Fremyella diplosiphon, when grown in cool white fluorescent light, contains phycoerythrin as its predominant phycobiliprotein. When grown on agar plates with cool white illumination, mutant colonies deficient or devoid of phycoerythrin can be visibly distinguished from the wild type. A total of 25 anomalously pigmented strains were isolated and examined for their ability to chromatically adapt. Based on absorption spectra of cell extracts and on fluorescence emission spectra of intact filaments, we assigned each mutant to one of three classes. In green mutants (16 strains), the photoinduction of phycoerythrin synthesis by green light was lost or impaired, whereas the photorepression of phycocyanin synthesis by green light still functioned as in the wild type. In blue mutants (eight strains), both the ability to photoinduce phycoerythrin synthesis and the ability to photorepress phycocyanin synthesis were lost or impaired. Filaments of blue mutants exhibited a high fluorescence emission at 660 nm. A black mutant (one strain) exhibited partial induction of phycoerythrin and partial repression of phycocyanin in both red and cool white light. From the data, we suggest that in information transduction for chromatic adaptation, early events are common to both phycoerythrin and phycocyanin regulation and that blue mutants possess lesions in these early events. The lesions in green mutants occur in a subsequent branch of the information transduction pathway which is specific for phycoerythrin photoinduction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A., Bogorad L. Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol. 1973 Aug;58(2):419–435. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A., Bogorad L. Properties of subunits and aggregates of blue-green algal biliproteins. Biochemistry. 1971 Sep 14;10(19):3625–3634. doi: 10.1021/bi00795a022. [DOI] [PubMed] [Google Scholar]
- Bryant D. A. The photoregulated expression of multiple phycocyanin species. A general mechanism for the control of phycocyanin synthesis in chromatically adapting cyanobacteria. Eur J Biochem. 1981 Oct;119(2):425–429. doi: 10.1111/j.1432-1033.1981.tb05625.x. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. J Gen Microbiol. 1968 Jan;50(1):1–14. doi: 10.1099/00221287-50-1-1. [DOI] [PubMed] [Google Scholar]
- Gantt E., Conti S. F. Ultrastructure of blue-green algae. J Bacteriol. 1969 Mar;97(3):1486–1493. doi: 10.1128/jb.97.3.1486-1493.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A., Grabowski J., Zimmerman B. K. Phycobilisomes from blue-green and red algae: isolation criteria and dissociation characteristics. Plant Physiol. 1979 Apr;63(4):615–620. doi: 10.1104/pp.63.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendel S., Ohad I., Bogorad L. Control of Phycoerythrin Synthesis during Chromatic Adaptation. Plant Physiol. 1979 Nov;64(5):786–790. doi: 10.1104/pp.64.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haury J. F., Bogorad L. Action Spectra for Phycobiliprotein Synthesis in a Chromatically Adapting Cyanophyte, Fremyella diplosiphon. Plant Physiol. 1977 Dec;60(6):835–839. doi: 10.1104/pp.60.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. S., Ausubel F. M., Bogorad L. Cyanobacterial ribonucleic acid polymerases recognize lambda promoters. J Bacteriol. 1979 Oct;140(1):246–250. doi: 10.1128/jb.140.1.246-250.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J., Graham J. R., Wang R. T. Light Harvesting in Anacystis nidulans Studied in Pigment Mutants. Plant Physiol. 1980 Dec;66(6):1144–1149. doi: 10.1104/pp.66.6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J., Graham J. R., Wang R. T. Spontaneous pigment mutants of Anacystis nidulans selected by growth under far-red light. Arch Microbiol. 1980 Feb;124(2-3):143–148. doi: 10.1007/BF00427719. [DOI] [PubMed] [Google Scholar]
- Ohad I., Clayton R. K., Bogorad L. Photoreversible absorbance changes in solutions of allophycocyanin purified from Fremyella diplosiphon: Temperature dependence and quantum efficiency. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5655–5659. doi: 10.1073/pnas.76.11.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandeau de Marsac N. Occurrence and nature of chromatic adaptation in cyanobacteria. J Bacteriol. 1977 Apr;130(1):82–91. doi: 10.1128/jb.130.1.82-91.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Gingrich J. C., Glazer A. N. Cyanobacterial phycobilisomes. Particles from Synechocystis 6701 and two pigment mutants. J Cell Biol. 1980 Jun;85(3):558–566. doi: 10.1083/jcb.85.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]


