Abstract
Opsins are a class of retinal-binding, seven transmembrane helix proteins that function as light-responsive ion pumps or sensory receptors. Previously, genes encoding opsins had been identified in animals and the Archaea but not in fungi or other eukaryotic microorganisms. Here, we report the identification and mutational analysis of an opsin gene, nop-1, from the eukaryotic filamentous fungus Neurospora crassa. The nop-1 amino acid sequence predicts a protein that shares up to 81.8% amino acid identity with archaeal opsins in the 22 retinal binding pocket residues, including the conserved lysine residue that forms a Schiff base linkage with retinal. Evolutionary analysis revealed relatedness not only between NOP-1 and archaeal opsins but also between NOP-1 and several fungal opsin-related proteins that lack the Schiff base lysine residue. The results provide evidence for a eukaryotic opsin family homologous to the archaeal opsins, providing a plausible link between archaeal and visual opsins. Extensive analysis of Δnop-1 strains did not reveal obvious defects in light-regulated processes under normal laboratory conditions. However, results from Northern analysis support light and conidiation-based regulation of nop-1 gene expression, and NOP-1 protein heterologously expressed in Pichia pastoris is labeled by using all-trans [3H]retinal, suggesting that NOP-1 functions as a rhodopsin in N. crassa photobiology.
Retinal is a chromophore that binds to integral membrane proteins (opsins) to form light-absorbing pigments known as rhodopsins. Visual rhodopsins contain the chromophore 11-cis retinal, which has intrinsic qualities optimal for vision (1). In contrast, archaeal rhodopsins contain all-trans retinal and function in light-activated ion pumping and phototaxis (reviewed in ref. 2). Archaeal and visual rhodopsins show little sequence identity but possess a similar secondary structure (3). This structure includes seven transmembrane α-helical domains and a conserved lysine residue in the seventh helix (helix G) that forms a Schiff base linkage with retinal (4). On light absorption, the retinal isomerizes and the Schiff base is deprotonated. These events are followed by conformational changes in the opsin protein and subsequent transduction of the light signal.
Four different archaeal rhodopsins have been identified in Halobacterium salinarum (reviewed in refs. 2 and 5). These are bacteriorhodopsin, (BR), halorhodopsin (HR), Sensory rhodopsin I (SRI), and sensory rhodopsin II (SRII). BR and HR are ion pumps that are activated by orange light under semianaerobic conditions; they function by expelling hydrogen ions from the cell or taking in extracellular chloride ions, respectively. The pumping action of BR and HR hyperpolarizes the membrane, driving ATP synthesis during anaerobic growth. SRI controls phototaxis of the cell to orange light during anaerobic conditions to energize pumping of BR and HR. SRII also controls cell motility but is produced only in oxygen, where it induces an avoidance response to blue-green light.
Biochemical evidence implicating the existence of rhodopsins in eukaryotic microorganisms has been presented (6, 7). However, genes encoding eukaryotic opsins have not been reported outside of animals, although many fungi, algae, and protists possess light-regulated processes. For example, in the filamentous fungus Neurospora crassa, blue light is a key regulator of growth and differentiation (reviewed in ref. 8). N. crassa hyphae grow by apical extension, branching to form a network known as a mycelium. Blue light induces carotenoid biosynthesis and hyperpolarizes the cell membrane in mycelial cells. When N. crassa is deprived of nutrients or water, the mycelium differentiates aerial hyphae; these structures give rise to vegetative spores called conidia. Blue light accelerates conidiation and increases the conidial yield, and conidial formation is governed by a circadian clock reset by blue light. Under nitrogen starvation, the mycelium produces female sexual structures termed protoperithecia, which mature into perithecia with beak structures through which sexual spores (ascospores) are expelled after fertilization. Blue light increases the production of protoperithecia and also activates the phototropism exhibited by perithecial beaks.
In this study, we identify a gene for a potential light receptor in N. crassa. The deduced amino acid sequence of nop-1 (New eukaryotic opsin 1) reveals seven putative transmembrane helix domains and conserved residues important for retinal binding and H+ pumping in the archaeal opsin family. We report the results of evolutionary analyses of the gene sequence, the chromosomal map position of nop-1, expression pattern for nop-1 during the N. crassa life cycle, and phenotypic analysis of a Δnop-1 mutant. We demonstrate that heterologously expressed NOP-1 protein binds all-trans [3H]retinal.
MATERIALS AND METHODS
N. crassa Growth Conditions, Library Screening, Chromosomal Mapping, and Northern Analysis.
Wild-type N. crassa strains were 74-OR23–1A (74A; obtained from R. L. Weiss, University of California, Los Angeles) and 74-OR8–1a (74a; obtained from the Fungal Genetics Stock Center, Kansas City, KS). Growth and genetic manipulations of N. crassa were as described (9).
The nop-1 gene was identified as a 961-bp cDNA from a λ ZapII library (Stratagene) as part of the Neurospora Genome Project (University of New Mexico). Sequence analysis indicated that the cDNA was truncated at the amino terminus, missing roughly 280 bp. The cDNA was labeled (10) and used to screen the N. crassa BARGEM7 λ genomic library (11). Three hybridizing plaques were purified and converted to double-stranded plasmids (11). Southern analysis (10) indicated that the three plasmids overlapped in the genetic material contained. One plasmid (1-1; Fig. 1A) contained a 5-kilobase insert centered on the nop-1 ORF. Subclones of the 1-1 plasmid were sequenced as described (12). Contigs were assembled by using sequencher Version 3.0 (Gene Codes, Ann Arbor, MI). Identification of ORFs and DNA sequence translation and comparisons were performed by using macdnasis Version 3.2 (Hitachi).
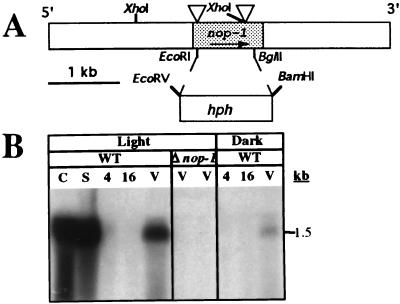
Figure 1.
nop-1 gene structure and expression. (A) Gene structure. The bar represents the 5′ → 3′ genomic region of nop-1. The shaded area corresponds to nop-1 ORF. Triangles indicate the two intron positions. The hph gene replacement between the EcoRI and BglII sites of nop-1 is shown by dotted lines. (B) Northern analysis. Samples contained 25 μg of total RNA isolated from light or dark-grown cultures as indicated. Δnop-1 strains are 39-1 (left) and 83-5 (right). WT, wild type; C, conidia; S, SCM plates; 4, 4-hr germlings; 16, 16-hr germlings; V, VM plates.
The nop-1 gene was mapped by following several single-base polymorphisms in a 541-bp region overlapping the nop-1 ORF among progeny in the multicent-2 mapping set (13). This region was amplified from parental and progeny strains by using nop-1-specific primers and Qiagen (Chatsworth, CA) PCR Master Mix. Sequencing was performed as described (12) by using the amplification primers. Sequences from each of the 38 multicent-2 progeny were scored with respect to the Oak Ridge and Mauriceville parental strains by using sequencher Version 3.0 for comparisons (data not shown).
Total RNA was isolated from N. crassa strains during various stages of growth and development. Conidia were propagated on solid Vogel’s minimal medium (VM; ref. 9) for 3 days in the dark at 30°C followed by 4 days in light at room temperature. Germling cultures were shaken at 200 rpm for 4 or 16 hours in liquid VM at 25°C in constant light or darkness after inoculating with 5 × 106 conidial cells per ml. For plate cultures, 1 μl of a conidial suspension was inoculated in the center of a VM or synthetic crossing medium (SCM; ref. 9) cellophane-overlaid plate and incubated at room temperature for 3 days in constant light or darkness (VM) or 6 days in constant light (SCM). Germlings were collected by centrifuging, whereas plate cultures were scraped with a spatula. Total RNA was extracted from cell pads as described (14). Northern blot analysis was performed essentially as described (15), except with Nytran Plus membranes (Schleicher & Schuell). Hybridization was performed as described (16) with an 810-bp fragment of the nop-1 ORF lacking the first 147 bp used as the probe.
Database Searches and Phylogenetic Analyses.
Searches of the nr, dbEST, and unfinished microbial genome sequence databases were conducted by using appropriate programs from the blast Version 2.0 suite or psi-blast (17), with the default settings used. When psi-blast was used, iterative searches were conducted until the program converged on a constant set of subject sequences.
The inferred amino acid sequence of NOP-1 was aligned with homologous sequences from archaea, fungi, and visual pigments. Because transmembrane regions of opsins are better conserved in both sequence and length than are the extramembrane strands, alignment was conducted by dividing the sequences into putative transmembrane-helix segments and extramembrane regions established by comparison with the structure of BR (18). The transmembrane helices and extramembrane strands were aligned as individual blocks by using clustalw (19). Preliminary trees constructed from alignments that included visual opsins always grouped the visual opsins together but placed them inconsistently and with relatively low levels of support in the phylogenetic trees inferred (see http://biology.unm.edu/∼ngp/home.html; data not shown). Because the relationship between visual pigments and archaeal opsins is uncertain (20), we excluded the visual opsins in later analyses.
The final alignment (see http://biology.unm.edu/∼ngp/home.html) was used in tree-building analyses by using several different methods, including maximum parsimony (MP) with test version d64 of paup* 4.0 (D. L. Swofford), maximum likelihood (ML) with the Accepted Point Mutation (PAM) model of evolution (21) with empirical amino acid frequencies as implemented in both puzzle 4.0 (K. Strimmer and A. von Haeseler, Zoologisches Institut, Universitat Munchen, Munchen, Germany) and paml (Z. Yang, University of California, Berkeley, CA), and distance analysis by neighbor joining (NJ) of γ distances (α = 2; ref. 22) estimated by using mega Version 1.01 (S. Kumar, K. Tamura, and M. Nei, Pennsylvania State University). Support for specific groupings was evaluated by using 500 bootstrap replicates and quartet-puzzling values (12). Support values of 70% and greater were considered indicators of reliable groupings (see ref. 23).
Construction and Analysis of Δnop-1 Strains.
A nop-1 gene-replacement construct (pJAB6) was made by replacing most of the nop-1 ORF in genomic clone 1-1 with an EcoRV–BamHI fragment from pCSN44 containing the Escherichia coli hph gene, which confers hygromycin B resistance (ref. 24; Fig. 1A). Strain 74A conidia were electroporated with pJAB6, and transformants were selected for hygromycin resistance as described (10, 25). The presence of the Δnop-1 mutation was verified by using Southern analysis (data not shown). Sexual crosses between heterokaryotic transformants and wild-type strain 74a were used to isolate Δnop-1 homokaryons (strains 39-1, 40-10, 80-1, 83-5, and 86-2). Sensitivity of strains to the H+-ATPase inhibitor oligomycin was tested by using VM plates containing 100 ng/ml oligomycin. Incubation was for 4 days at room temperature in constant light or total darkness.
[3H]Retinal Binding to NOP-1.
Construction of Pichia pastoris strains transformed with a control plasmid without insert (strain V1) or a nop-1 expression plasmid (strain L4), and isolation of crude membrane fractions will be described in detail elsewhere (J.A.B., E. N. Spudich, K. Scott, K.A.B., and J. L. Spudich, unpublished data). Samples (100 μl) from V1 (4.22 mg/ml) or L4 (4.64 mg/ml) were incubated with all-trans [3H]retinal (112 pmol; 224,000 cpm) overnight in the dark at room temperature. The Schiff base linkage was reduced by using sodium cyanoborohydride essentially as described (26), and samples were then subjected to SDS/PAGE electrophoresis (27). Gels were prepared for fluorography by using EN3HANCE according to the manufacturer’s recommendations (Dupont/NEN).
RESULTS
nop-1 Gene Organization, Chromosomal Mapping, Expression, and Sequence Analysis.
The nop-1 cDNA clone, designated NM4H11, was identified as an amino-terminal-truncated expressed sequence tag (EST) from a N. crassa mycelial library (28). The cDNA was used as a probe to identify a 4.97-kilobase genomic clone (Fig. 1A). Comparison of the genomic and cDNA sequences revealed one intron in the amino acid coding region. The existence of a second intron upstream of the cDNA sequence was suggested by the presence of consensus splice sequences in the genomic clone and confirmed after analysis of a second EST from an evening-specific cDNA library containing the complete amino-terminal region of the nop-1 ORF (Clone b4a12ne.f1; University of Oklahoma’s Advanced Center for Genome Technology). The distribution of nop-1 alleles observed during inheritance of single-base polymorphisms most closely matched scores for ars-1 (36/38 matches) and nic-3 (35/38 matches) on the left arm of LGVII (ref. 29; data not shown), implying this location for nop-1.
Northern analysis was used to measure steady-state nop-1 RNA levels under various environmental conditions, including constant light and darkness (Fig. 1B). The nop-1 transcript is 1.5 kilobases and is abundant in conidia and sexually differentiated SCM plate cultures; both of these tissues were exposed to light. nop-1 is also expressed during vegetative growth on high nitrogen VM solid medium, with higher levels in light than dark. In contrast, nop-1 message is not detected during submerged growth in liquid VM (a condition that suppresses conidiation and sexual differentiation) in either constant light or darkness. Thus, the nop-1 transcript level is highest under conditions where conidia are produced and there has been exposure to light (conidia, SCM cultures, and illuminated VM plates).
The deduced amino acid sequence of nop-1 predicts seven transmembrane helical domains and extensive similarity to the archaeal rhodopsin family (Fig. 2; data not shown). Most notably, the amino acids present in each of the 22 putative retinal binding pocket positions of NOP-1 are conserved with at least one (and usually multiple) of the H. salinarum rhodopsins. Consideration of these residues alone shows identities of 68.1–81.8% between NOP-1 and the archaeal rhodopsins. In particular, NOP-1 contains the conserved lysine residue in helix G (Lys-263) that forms the Schiff base linkage with retinal in all archaeal rhodopsins.
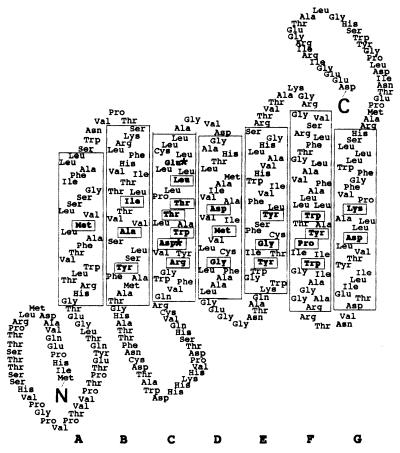
Figure 2.
Predicted topology of the NOP-1 protein. The seven transmembrane α-helices are designated A through G, and helix boundaries are based on those of BR (18). Arg-128 in helix C has been included in the pocket in accordance with the crystal structure of BR (30). Retinal-binding pocket residues conserved among the archaeal transport and sensory rhodopsins (31, 32) are boxed. ∗ mark positions homologous to Asp-85 and Asp-96 in BR. There are two in-frame methionines upstream of helix A; it is not known whether one or both are used for translation.
NOP-1 also contains Asp and Glu residues at positions corresponding to Asp-85 and Asp-96 in BR that are crucial for archaeal rhodopsin function (reviewed in refs. 2 and 5). Light absorption by BR causes the transfer of a proton from the Schiff base in helix G to Asp-85 in helix C; this leads to release of a proton to the medium and induces an open conformation of BR that allows proton uptake from the cytoplasm. Asp-96 is required for reprotonation of the Schiff base in BR; however, this residue is not conserved in the other three archaeal rhodopsins. Asp-85 is essential for the selectivity of ion transport; mutation of this residue to threonine converts BR into a chloride pump. SRI and SRII contain aspartate residues corresponding to Asp-85 in BR (Asp-76 and Asp-73, respectively). In the absence of its transducer, SRI is converted into a proton pump and phototaxis is disrupted; SRI-mediated proton transport is mechanistically the same as that for BR, with Asp-76 acting as a proton acceptor. In the case of SRII, substitution of Asn for Asp-73 results in constitutive signaling during phototaxis. Taken together, the sequence information suggests that NOP-1 can bind retinal and may also pump protons or other ions across a cell membrane.
NOP-1 also exhibits sequence similarity to six predicted seven-helix transmembrane proteins from various fungal species. These proteins include YDR033w, Yro2p/YBR054w, and Hsp30p/YCR021c from the budding yeast Saccharomyces cerevisiae (the relationship between Yro2p and archaeal opsins has been noted previously; ref. 33); a hypothetical protein identified by genomic sequencing in the fission yeast Schizosaccharomyces pombe; an Hsp30 homologue from the basidiomycete Coriolus versicolor, and an incomplete sequence derived from a set of overlapping ESTs from the filamentous ascomycete Emericella nidulans. S. cerevisiae Hsp30p is an integral plasma membrane protein that down-regulates the plasma membrane H+-ATPase in response to heat shock and exposure to weak acids (34). Functions for the remaining fungal proteins are unknown. With the exception of NOP-1, all of the related fungal proteins, including the predicted E. nidulans protein (E. N. Spudich and J. L. Spudich, personal communication), lack the conserved lysine in helix G responsible for the Schiff base linkage with retinal; therefore, by definition, these are opsin-related proteins, rather than opsins per se. In addition, the fungal opsin-related proteins lack the two acidic residues important for ion pumping in BR; however, the significance of this is unclear in proteins without a Schiff base lysine.
Phylogenetic Analysis.
Because the loops connecting the seven transmembrane helices of opsins exhibit substantial length variation as a result of insertions and deletions, the assignment of homology outside of the transmembrane helices is problematic when distantly related sequences are compared. We therefore explored the phylogeny of the opsins and opsin-related proteins using both the complete alignment and a shorter alignment containing only the transmembrane helices. Phylogenetic analyses with both alignments and various analytical methods produced results consistent with the general features shown in the tree presented (Fig. 3). Specifically, we found three major groups, one corresponding to the archaeal sequences, a second containing the sequences from S. cerevisiae, S. pombe, and C. versicolor (the Yro/Hsp30 group), and a third corresponding to NOP-1 and the sequence from E. nidulans (the NOP-1 group). In all cases, the branch leading to the NOP-1 group was placed between the archaeal and Yro/Hsp30 fungal groups.
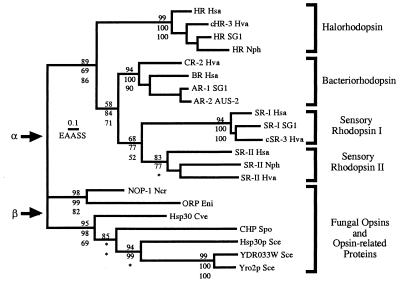
Figure 3.
Phylogenetic relationship between NOP-1 and related proteins from fungi and archaea. Phylogeny of the opsins was estimated by ML quartet puzzling, NJ of γ distances with α = 2, and MP. Analyses were conducted by using the complete alignment. Estimates of branch lengths were obtained by ML, assuming the PAM model of evolution with empirical amino acid frequencies, and the scale bar indicates 0.1 estimated amino acid substitutions per site (EAASS) by using this model. Support for the monophyly of subgroups of archaeal rhodopsins and all groupings within the fungal proteins is shown, with the quartet puzzling values above branches and the NJ and MP bootstrap proportions below branches. Cases in which alternative groupings were supported by NJ or parsimony are indicated by ∗ (within the fungal proteins, NJ and parsimony bootstrap consensus trees reverse the branching order of C. versicolor Hsp30 and the S. pombe conserved hypothetical protein). Two possible tree roots, α and β, are indicated by arrows. Cve, Coriolus versicolor; Eni, Emericella nidulans (the sexual form of Aspergillus nidulans); Hsa, Halobacterium salinarum; Hva, Haloarcula vallismortis; Ncr, Neurospora crassa; Nph, Natronomonas pharaonis; Sce, Saccharomyces cerevisiae; Spo, Schizosaccharomyces pombe. International Collaboration (IC) accession numbers: HR Hsa, P16102; cHR-3 Hva, P94853; HR SG1, P25964; HR Nph, P15647; CR-2 Hva, Q53496; BR Hsa, P02945; SR-1 SG1, P19585; AR-2 AUS-2, P29563; SR-I Hsa, P25964; SR-I SG1, P33743; cSR-3 Hva, Q48334; SR-II Hsa, P71411; SR-II Nph, P42196; SR-II Hva, P42197; NOP-1 Ncr, AF135863; ORP Eni, AA787158, AA785169, AA786492; Hsp30 Cve, AB003518; CHP Spo, AL031824; Hsp30p Sce, S31838; YDR033W Sce, S61586; Yro2p Sce, P38079.
The tree-building methods used result in unrooted trees unless assumptions are invoked regarding either relative rates of divergence or the existence of appropriate outgroup sequences. Placement of the root between the eukaryotes and archaea (root α in Fig. 3) is suggested by organismal phylogeny in the absence of horizontal gene transfer, and also by midpoint rooting (MP) and the estimation of ML trees assuming a molecular clock. However, placement of the root within the fungal groups (root β), is consistent with the presence of residues involved in retinal binding and ion pumping in NOP-1 and is supported by midpoint rooting of NJ and ML trees. The evolution of opsins and related proteins has not been strictly clock-like, based on a likelihood-ratio test (35), suggesting that the methods used to infer the root position should be viewed as heuristics that can be evaluated in the context of additional information, such as organismal phylogeny. Recognizable opsin homologues are absent from the complete genome sequences of several different archaeotes, a circumstance that argues for either independent opsin gene losses in these organisms or a horizontal opsin gene transfer between ancestors of the halophilic archaea and fungi. If multiple losses of opsin genes have occurred, placement of the root between the eukaryotes and archaea (root α) is more conservative, because this root does not presuppose the presence of two separate opsin subgroups in nonhalophilic archaeotes.
The high degree of sequence similarity between NOP-1 and the archaeal opsins in the retinal-binding and ion-pumping residues raises the possibility that NOP-1 is the result of a horizontal gene transfer from an archaeote. This is of particular interest in light of recent proposals regarding the prokaryotic origin of eukaryotic genes and the role of horizontal gene transfer in the evolution of fungal genomes (36, 37). However, the presence of an apparent NOP-1 orthologue in E. nidulans is inconsistent with a recent gene transfer. Furthermore, the presence of an opsin-related protein in the basidiomycete C. versicolor (38) suggests that Yro/Hsp30 gene clade was established at least 400 million years ago (see ref. 39). Based on these observations, the high degree of divergence between archaeal rhodopsins and NOP-1, and the apparent monophyletic nature of the archaeal group, we conclude that NOP-1 is unlikely to reflect a horizontal gene transfer from a halophilic archaeote.
Phenotypic Analysis of Δnop-1 Strains.
The phylogenetic analyses suggested that NOP-1 might function as a light-responsive sensory receptor or ion pump in N. crassa. As a first step in determining nop-1 functions, a Δnop-1 mutation was made by targeted integration of a construct in which the nop-1 gene was replaced by the hph gene (ref. 25; Fig. 1A). Southern analysis was used to identify several heterokaryotic transformants; Δnop-1 homokaryons were then isolated by using a sexual cross (data not shown). The results from Northern analysis indicate that the nop-1 transcript is absent in the Δnop-1 homokaryons (Fig. 1B), consistent with gene loss in these strains.
Loss of nop-1 does not cause any detectable phenotype during the life cycle of N. crassa. Conidia from Δnop-1strains have wild-type viability and exhibit normal morphology and mass accumulation during germination in light or dark-grown liquid cultures (data not shown). There is no obvious difference in growth rate, morphology, or pigmentation between wild-type and Δnop-1 strains grown on solid medium in light or dark, and dry weights of Δnop-1 and wild type are similar in cultures grown on solid medium in constant light. Analysis of the circadian rhythm indicated that period length, light-induced phase shifting, and photosuppression are normal in Δnop-1 mutants (data not shown). Δnop-1 mutants exhibit normal growth rate and morphology on solid medium with glycerol, succinate, or acetate as carbon sources and glutamate, arginine, histidine, or leucine as nitrogen sources in light or dark (data not shown). Finally, no defects were noted during the sexual cycle when Δnop-1 strains were used as male or female parents.
Because NOP-1 shows similarity to S. cerevisiae Hsp30p, which modulates the activity of the plasma membrane H+-ATPase during heat shock (34), we tested Δnop-1 strains for sensitivity to environmental stresses. Δnop-1 liquid culture germlings exhibit normal viability after incubation at 52°C, Δnop-1 strains possess wild-type growth rates at high temperature (42°C), and Δnop-1 mutants have normal growth rate and morphology under hypertonic conditions in light and dark (data not shown). Δnop-1 strains display wild-type sensitivity to the superoxide-generating agent paraquat (in light; data not shown). However, Δnop-1 germlings exhibit slightly (20%) greater resistance to hydrogen peroxide than wild-type (over a 4-hour period in light; data not shown).
Because it possesses the residues in BR that are required for transporting H+, we hypothesized that NOP-1 may function as a proton pump in N. crassa. Hence, it is possible that phenotypes resulting from a pumping defect are not exhibited by Δnop-1 mutants because another H+ pump can cover for NOP-1 function(s). Therefore, we tested the sensitivity of the Δnop-1 mutant to inhibitors for the three H+-ATPases of N. crassa. Wild-type and Δnop-1 strains possess identical colony morphologies and reduced growth rates in light when grown in the presence of the plasma membrane H+-ATPase inhibitor sodium orthovanadate (ref. 40; data not shown). When grown on VM plates containing the vacuolar ATPase inhibitor concanamycin (at 400 nM; ref. 41), the Δnop-1 mutant exhibits more extensive aerial hyphal development and denser conidiation than wild type after 2 days of growth in constant light; however, by day 3, the Δnop-1 and wild-type strains are indistinguishable (data not shown). There were no differences in morphology between wild type and the Δnop-1 mutant when incubated in the dark with the mitochondrial ATPase inhibitor oligomycin (42) at 100 ng/ml (Fig. 4A). However, morphological differences between Δnop-1 and wild type were observed during growth in constant light in the presence of oligomycin (Fig. 4A). In light, wild type possesses long aerial hyphae and conidia are produced in concentric rings, whereas aerial hyphae are shorter and conidiation occurs in dense patches randomly distributed over the center of the colony in the Δnop-1 mutant. Thus, exposure to oligomycin produces a synthetic effect in Δnop-1 strains that is light-dependent. This effect may result from compensation by the mitochondrial H+-ATPase for a light-regulated conidiation function of NOP-1 in the Δnop-1 mutants. Alternatively, it may be due to an indirect effect of oligomycin, either from reduced ATP levels or some other physiological change.
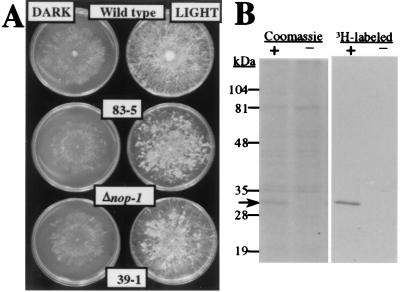
Figure 4.
Oligomycin-dependent phenotype of Δnop-1 N. crassa strains and retinal binding of NOP-1 heterologously expressed in P. pastoris. (A) Sensitivity to oligomycin. Wild-type and Δnop-1 N. crassa strains were cultured on 100 ng/ml oligomycin-containing medium in constant light or darkness for 4 days at room temperature and then photographed. (B) Binding of all-trans retinal to NOP-1. Crude membrane fractions from P. pastoris strains containing nop-1 overexpression (+) or control (−) plasmid were incubated with all-trans [3H]retinal, the linkage reduced by sodium cyanoborohydride, and samples analyzed by using SDS/PAGE followed by fluorography. The position of the NOP-1 protein is indicated by the arrow.
Binding of NOP-1 to All-Trans Retinal.
To further verify that NOP-1 is a rhodopsin, we took advantage of a procedure for labeling archaeal rhodopsins by using [3H]retinal (26). For these studies, we used crude membrane fractions from P. pastoris strains containing a control or nop-1 overexpression plasmid (strains V1 or L4, respectively; J.A.B., E. N. Spudich, K. Scott, K.A.B., and J. L. Spudich, unpublished data). NOP-1 protein from the L4 strain has a hexahistidine epitope at the carboxyl terminus. The membrane fractions were incubated with all-trans [3H]-retinal and the resultant Schiff base linkage reduced by using sodium cyanoborohydride. After gel electrophoresis and fluorography, the results demonstrated the presence of a single labeled species in L4 strain samples containing NOP-1 protein (Fig. 4B). This labeled protein corresponds to a Coomassie blue-stainable and anti-histidine epitope antibody-reactive protein present in the L4 strain but not in the control strain V1 (Fig. 4B; data not shown).
DISCUSSION
Although filamentous fungi exhibit a diverse array of light responses, the corresponding photoreceptors are poorly characterized. Therefore, discovery of the nop-1 gene has important implications for fungal photobiology. The high degree of sequence conservation between NOP-1 and archaeal opsins suggests a role for NOP-1 in transduction of light energy for ion pumping and/or sensory perception. Considering this sequence conservation, it is surprising that Δnop-1 strains have no apparent defects in light signaling under standard laboratory conditions. This result may reflect the existence of other functionally redundant opsins or opsin-related proteins (see below) that can compensate for loss of nop-1 in N. crassa, or a light signaling function in nature that is not apparent under the artificial conditions of the research laboratory. In any scenario, it is clear that determination of the actual function of nop-1 will require more extensive investigation.
Biochemical studies with NOP-1 protein heterologously expressed in P. pastoris demonstrate that NOP-1 protein binds all-trans retinal by using a Schiff base linkage. This result is further supported by UV-visible absorption spectroscopy with the same P. pastoris membranes (J.A.B., E. N. Spudich, K. Scott, K.A.B., and J. L. Spudich, unpublished data). In these preparations, NOP-1 forms a green-absorbing pigment in the presence of all-trans retinal (λmax 534 nm), with a spectral shape and bandwidth characteristic of archaeal rhodopsins. Furthermore, laser flash kinetic spectroscopy shows that the retinal-reconstituted pigment undergoes a photochemical reaction cycle with a near UV-absorbing intermediate typical of transient Schiff base deprotonation of the chromophore, as occurs in the photocycles of bacteriorhodopsin and SRI and SRII.
The retinal-binding properties of NOP-1 and the light and conidiation-based regulation of nop-1 gene expression support the hypothesis that NOP-1 plays a role in N. crassa photobiology. Mutations in two genes, wc-1 and wc-2, eliminate all known blue light responses in N. crassa (reviewed in ref. 8). The wc-1 and wc-2 genes have been cloned, and the predicted protein sequences specify transcription factors containing zinc finger and PER-ARNT/AHR-SIM domains (8). Because wc-1 and wc-2 regulate expression of the conidiation genes (43) con-5 and con-10 (44), it is plausible that nop-1 transcript levels are also controlled by a regulatory circuit involving the wc loci. Furthermore, NOP-1 may participate in a signal-transduction pathway that includes the WC-1 and WC-2 proteins in N. crassa.
The discovery of NOP-1 has important implications for the evolution of opsins and opsin-related proteins. The close relationship between NOP-1 and archaeal opsins indicates the existence of eukaryotic opsins of ancient origin. The deduced relationships among opsins and opsin-related proteins further suggest that the common ancestors of these proteins may have been involved in ion transport, with alternative functions being derived independently in both archaeal and fungal lineages. Among the fungal proteins, the position of the Yro/Hsp30 subgroup in phylogenetic trees suggests the existence of a subgroup that arose after the eukaryote–archaea split, whose members do not possess the residues important for retinal binding and associated light-regulated ion pumping activities (particularly if root α in Fig. 3 is accepted).
At present, it is not known whether fungal opsin-related proteins can bind a chromophore and function as light-responsive proteins in vivo. Studies of both vertebrate rhodopsin and bacteriorhodopsin demonstrate that variants with a mutation in the Schiff base lysine residue can still bind retinal and/or retinal analogues containing an n-alkylamine group (45–47). Mutant bacteriorhodopsin reconstituted with retinal can form pigments with absorption spectra indicative of noncovalent interactions between retinal and the binding pocket (46). Mutant versions of rhodopsin and bacteriorhodopsin reconstituted with n-alkylamine derivatives exhibit absorption maxima similar to wild type and can activate transducin (for rhodopsin; ref. 45) or function as an ion pump (BR; refs. 46 and 47). Hence, it is possible that opsin-related proteins can bind retinal or retinal analogues and bypass the need for a Schiff base linkage during light sensing. Therefore, it is of interest that we have recently discovered an EST for a N. crassa opsin-related protein with a well conserved retinal-binding pocket but lacking the Schiff base lysine residue during blast searches of the University of Oklahoma Advanced Center for Genome Technology database. Chromophore binding by this protein and possession of light-dependent functions in common with NOP-1 could explain the lack of phenotypes observed for Δnop-1 strains in our studies.
There has been a long-standing controversy regarding the relationship between archaeal opsins and the visual opsins present in animals. It is not clear whether the two groups represent homologous proteins that have diverged beyond recognizable sequence homology or are analogous proteins with separate evolutionary origins but similar structures (20). Although the discovery of NOP-1 does not resolve this issue directly, the existence of a fungal protein with clear evolutionary affinities to archaeal opsins places the notion of homology between archaeal and visual opsins within the realm of possibility. Although sequence differences suggest that the visual and archaeal proteins belong to different opsin groups, it is possible that the members of the two groups share ancestry in the opsin/opsin-related protein family, perhaps even among retinal-binding members. In fact, the ancient origin of fungal opsin homologues that we have inferred makes it likely that additional opsin homologues, including members of the NOP-1 and Yro/Hsp30 subfamilies, will be identified in other fungi. It is tempting to speculate that opsin homologues will be found in eukaryotic groups related to the fungi (12, 48) and that these opsins may be directly related to the visual opsins of animals.
Finally, our results raise interesting questions regarding the differences between S. cerevisiae and light-responsive filamentous ascomycetes such as N. crassa and E. nidulans. Because the entire genomic sequence is known for S. cerevisiae, it can be concluded that this organism lacks members of the NOP-1 opsin subgroup; this finding may reflect the apparent absence of photobiology in S. cerevisiae. The absence of opsin homologues in the genomes of some completely sequenced archaeotes may represent differences in photobiology among archaeotes, a hypothesis consistent with the assertion that the ancestral activity of opsins was light-activated ion pumping. The discovery of nop-1 and fungal opsin-related proteins illustrates the value of using genomic research with diverse organisms to address important questions in biology.
Acknowledgments
We thank John Spudich for assistance with Fig. 2; Elena and John Spudich for the generous gift of [3H]retinal; Elena Spudich for retinal-binding protocols; Jennifer Loros for advice on clock experiments; John Spudich, Elena Spudich, and Tom Vida for many helpful discussions; and Doug Ivey, Rebecca Kimball, and Qi Yang for comments on the manuscript. We acknowledge the Albuquerque High Performance Computing Center for computational support. This work was supported by Grant HRD-9550649 from the National Science Foundation.
Footnotes
Abbreviations BR, bacteriorhodopsin; HR, halorhodopsin; SRI, sensory rhodopsin I; SRII, sensory rhodopsin II; ML, maximum likelihood; MP, maximum parsimony; NJ, neighbor joining; EST, expressed sequence tag; VM, Vogel’s medium; SCM, synthetic crossing medium.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF135863).
References
- 1.Rando R R. Chem Biol. 1996;3:255–262. doi: 10.1016/s1074-5521(96)90105-2. [DOI] [PubMed] [Google Scholar]
- 2.Spudich J L. Mol Microbiol. 1998;28:1051–1058. doi: 10.1046/j.1365-2958.1998.00859.x. [DOI] [PubMed] [Google Scholar]
- 3.Fryxell K J, Meyerowitz E M. J Mol Evol. 1991;33:367–378. doi: 10.1007/BF02102867. [DOI] [PubMed] [Google Scholar]
- 4.Skulachev V P. Q Rev Biophys. 1993;26:177–199. doi: 10.1017/s0033583500004066. [DOI] [PubMed] [Google Scholar]
- 5.Khorana H G. Proc Natl Acad Sci USA. 1993;90:1166–1171. doi: 10.1073/pnas.90.4.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spudich J L, Zacks D N, Bogomolni R A. Isr J Chem. 1995;35:495–513. [Google Scholar]
- 7.Saranak J, Foster K W. Nature (London) 1997;387:465–466. doi: 10.1038/387465a0. [DOI] [PubMed] [Google Scholar]
- 8.Macino G, Arpaia G, Linden H, Ballario P. In: Society for General Microbiology Symposium 56. Caddick M X, Baumberg S, Hodgson D A, Phillips-Jones M K, editors. Cambridge, U.K.: Cambridge Univ. Press; 1998. pp. 213–224. [Google Scholar]
- 9.Davis R H, deSerres F J. Methods Enzymol. 1970;71:79–143. [Google Scholar]
- 10.Ivey F D, Hodge P N, Turner G E, Borkovich K A. Mol Biol Cell. 1996;7:1283–1297. doi: 10.1091/mbc.7.8.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pall M L, Brunelli J P. Fungal Genet Newslett. 1994;41:63–65. [Google Scholar]
- 12.Braun E L, Kang S, Nelson M A, Natvig D O. J Mol Evol. 1998;47:531–543. doi: 10.1007/pl00006409. [DOI] [PubMed] [Google Scholar]
- 13.Metzenberg R L, Stevens J N, Selker E U, Morzycka-Wroblewska E. Neurospora Newslett. 1984;31:35–40. [Google Scholar]
- 14.Sachs M S, Yanofsky C. Dev Biol. 1991;148:117–128. doi: 10.1016/0012-1606(91)90322-t. [DOI] [PubMed] [Google Scholar]
- 15.Tsui H-C T, Pease A J, Koehler T M, Winkler M E. In: Methods in Molecular Genetics. Adolph K W, editor. San Diego: Academic; 1994. pp. 197–200. [Google Scholar]
- 16.Church G M, Gilbert W. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Nucleic Acids Res. 1997;25:3389–3492. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grigorieff N, Ceska T A, Downing K H, Baldwin J M, Henderson R. J Mol Biol. 1996;259:393–421. doi: 10.1006/jmbi.1996.0328. [DOI] [PubMed] [Google Scholar]
- 19.Thompson J D, Higgins D G, Gibson T J. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson R, Schertler G F X. Phil Trans R Soc London Ser B. 1990;326:379–389. doi: 10.1098/rstb.1990.0019. [DOI] [PubMed] [Google Scholar]
- 21.Dayhoff M O, Schwartz R M, Orcutt B C. In: Atlas of Protein Sequence and Structure. Dayhoff M O, editor. Silver Spring, MD: Natl. Biomed. Res. Found.; 1978. pp. 345–352. [Google Scholar]
- 22.Ota T, Nei M. J Mol Evol. 1994;38:642–643. [Google Scholar]
- 23.Hillis D M, Bull J J. Syst Biol. 1993;42:182–192. [Google Scholar]
- 24.Staben C, Jensen B, Singer M, Pollock J, Schechtman M, Kinsey J, Selker E. Fungal Genet Newslett. 1989;36:79–81. [Google Scholar]
- 25.Ivey F D, Yang Q, Borkovich K A. Fungal Genet Biol. 1999;26:48–61. doi: 10.1006/fgbi.1998.1101. [DOI] [PubMed] [Google Scholar]
- 26.Spudich E N, Bogomolni R A, Spudich J L. Biochem Biophys Res Commun. 1983;112:332–338. doi: 10.1016/0006-291x(83)91835-1. [DOI] [PubMed] [Google Scholar]
- 27.Turner G E, Borkovich K A. J Biol Chem. 1993;268:14805–14811. [PubMed] [Google Scholar]
- 28.Nelson M A, Kang S, Braun E L, Crawford M E, Dolan P L, Leonard P M, Mitchell J, Armijo A M, Bean L, Blueyes E, et al. Fungal Genet Biol. 1997;21:348–363. doi: 10.1006/fgbi.1997.0986. [DOI] [PubMed] [Google Scholar]
- 29.Nelson M A, Natvig D O. Fungal Genet Newslett. 1998;45:44–54. [Google Scholar]
- 30.Luecke H, Richter H-T, Lanyi J K. Science. 1998;280:1934–1937. doi: 10.1126/science.280.5371.1934. [DOI] [PubMed] [Google Scholar]
- 31.Henderson R, Baldwin J M, Ceska T A, Semlin F, Beckmann E, Downing K H. J Mol Biol. 1990;213:899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- 32.Hoff W D, Jung K H, Spudich J L. Annu Rev Biophys Biomol Struct. 1997;26:223–258. doi: 10.1146/annurev.biophys.26.1.223. [DOI] [PubMed] [Google Scholar]
- 33.Graul R C, Sadee W. Pharm Res. 1997;14:1533–1541. doi: 10.1023/a:1012166015402. [DOI] [PubMed] [Google Scholar]
- 34.Braley R, Piper P W. FEBS Lett. 1997;418:123–126. doi: 10.1016/s0014-5793(97)01359-8. [DOI] [PubMed] [Google Scholar]
- 35.Felsenstein J. Annu Rev Genet. 1988;22:521–565. doi: 10.1146/annurev.ge.22.120188.002513. [DOI] [PubMed] [Google Scholar]
- 36.Doolittle W F. Trends Genet. 1998;14:307–311. doi: 10.1016/s0168-9525(98)01494-2. [DOI] [PubMed] [Google Scholar]
- 37.Prade R A, Griffith J, Kochut K, Arnold J, Timberlake W E. Proc Natl Acad Sci USA. 1997;94:14564–14569. doi: 10.1073/pnas.94.26.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iimura Y, Tatsumi K. FEBS Lett. 1997;412:370–374. doi: 10.1016/s0014-5793(97)00807-7. [DOI] [PubMed] [Google Scholar]
- 39.Berbee M L, Taylor J W. Can J Bot. 1993;71:1114–1127. [Google Scholar]
- 40.Bowman B J, Slayman C W. J Biol Chem. 1979;254:2928–2934. [PubMed] [Google Scholar]
- 41.Bowman E J, O’Neill F J, Bowman B J. J Biol Chem. 1997;272:14776–14786. doi: 10.1074/jbc.272.23.14776. [DOI] [PubMed] [Google Scholar]
- 42.Brody S, Dieckmann C, Mikolajczyk S. Mol Gen Genet. 1985;200:155–161. doi: 10.1007/BF00383329. [DOI] [PubMed] [Google Scholar]
- 43.Berlin V, Yanofsky C. Mol Cell Biol. 1985;5:849–855. doi: 10.1128/mcb.5.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lauter F-R, Russo V E A. Nucleic Acid Res. 1991;19:6883–6886. doi: 10.1093/nar/19.24.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhukovsky E A, Robinson P R, Oprian D D. Science. 1991;251:558–560. doi: 10.1126/science.1990431. [DOI] [PubMed] [Google Scholar]
- 46.Schweiger U, Tittor J, Oesterhelt D. Biochemistry. 1994;33:535–541. doi: 10.1021/bi00168a019. [DOI] [PubMed] [Google Scholar]
- 47.Friedman N, Druckmann S, Lanyi J, Needleman R, Lewis A, Ottolenghi M, Sheves M. Biochemistry. 1994;33:1971–1976. doi: 10.1021/bi00174a001. [DOI] [PubMed] [Google Scholar]
- 48.Baldauf S L, Doolittle W F. Proc Natl Acad Sci USA. 1997;94:12007–12012. doi: 10.1073/pnas.94.22.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]