Abstract
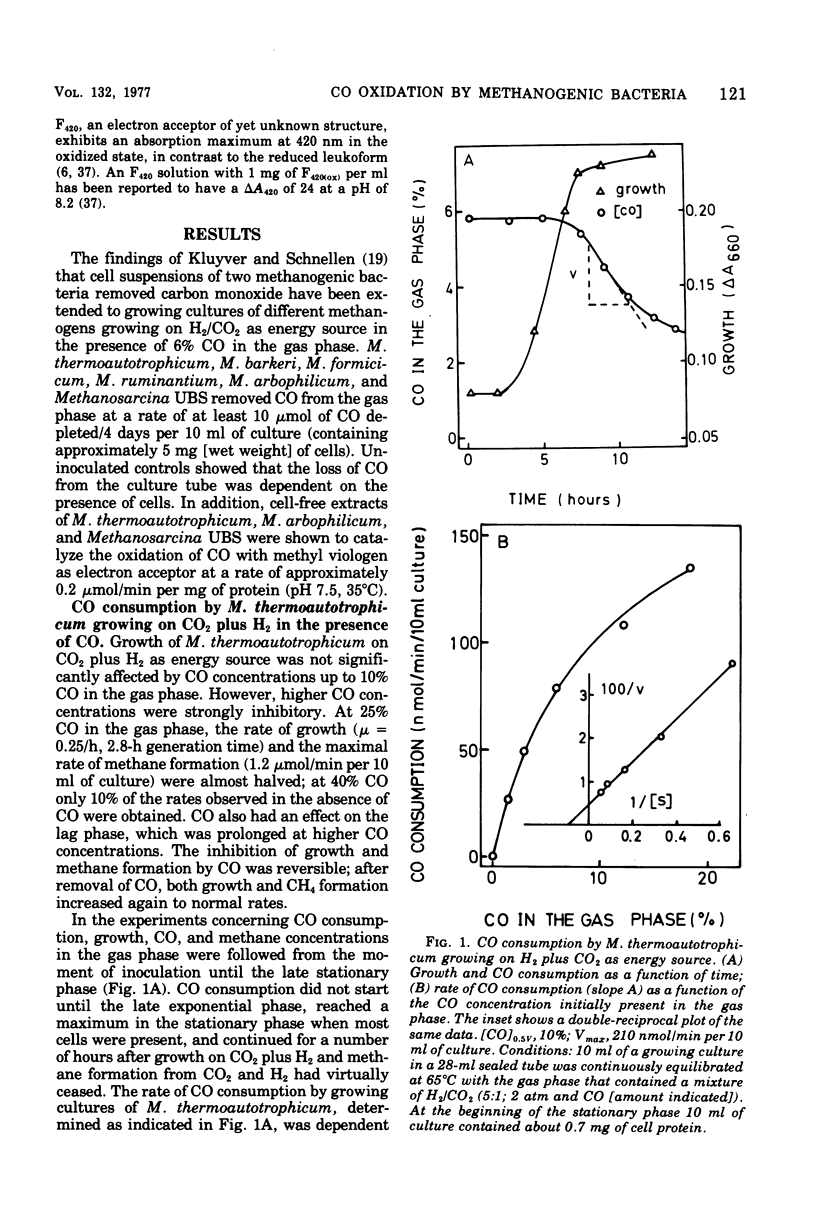
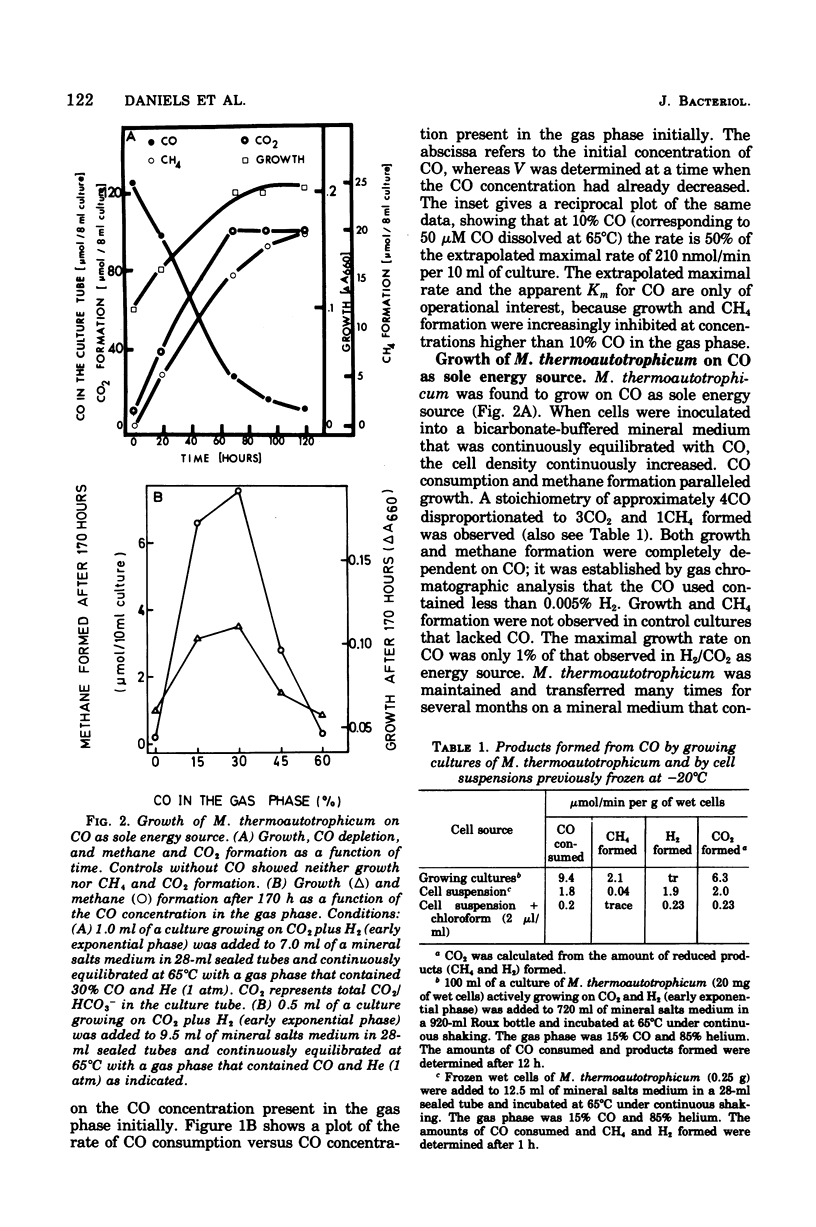
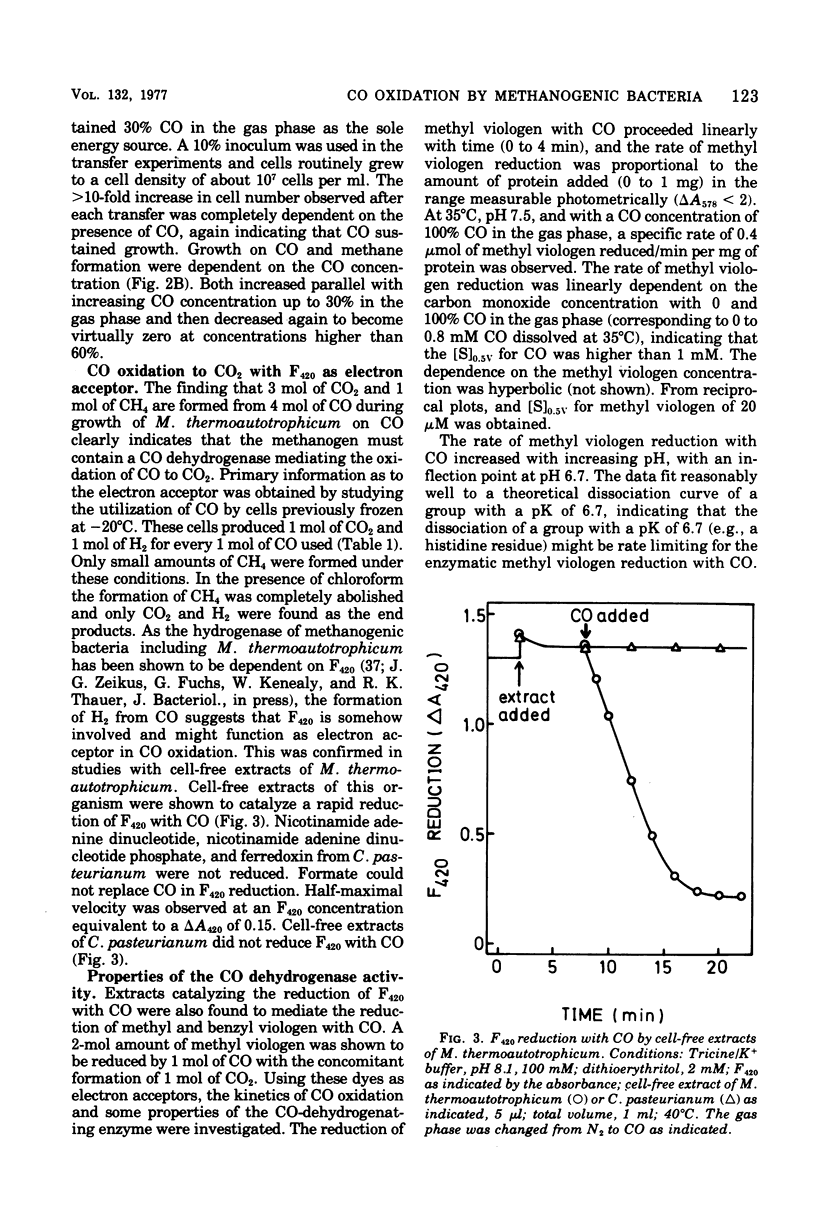
Different species of methanogenic bacteria growing on CO2 and H2 were shown to remove CO added to the gas phase. Rates up to 0.2 μmol of CO depleted/min per 10 ml of culture containing approximately 7 mg of cells (wet weight) were observed. Methanobacterium thermoautotrophicum was selected for further study based on its ability to grow rapidly on a completely mineral medium. This species used CO as the sole energy source by disproportionating CO to CO2 and CH4 according to the following equation: 4CO + 2H2O → 1CH4 + 3CO2. However, growth was slight, and the growth rate on CO was only 1% of that observed on H2/CO2. Growth only occurred with CO concentrations in the gas phase of lower than 50%. Growth on CO agrees with the finding that cell-free extracts of M. thermoautotrophicum contained both an active factor 420 (F420)-dependent hydrogenase (7.7 μmol/min per mg of protein at 35°C) and a CO-dehydrogenating enzyme (0.2 μmol/min per mg of protein at 35°C) that catalyzed the reduction of F420 with CO. The properties of the CO-dehydrogenating enzyme are described. In addition to F420, viologen dyes were effective electron acceptors for the enzyme. The apparent Km for CO was higher than 1 mM. The reaction rate increased with increasing pH and displayed an inflection point at pH 6.7. The temperature dependence of the reaction rate followed the Arrhenius equation with an activation energy (ΔH‡) of 14.1 kcal/mol (59.0 kJ/mol). The CO dehydrogenase activity was reversibly inactivated by low concentrations of cyanide (2 μM) and was very sensitive to inactivation by oxygen. Carbon monoxide dehydrogenase of M. thermoautotrophicum exhibited several characteristic properties found for the enzyme of Clostridium pasteurianum but differed mainly in that the clostridial enzyme did not utilize F420 as the electron acceptor.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T. Inhibition of rumen methanogenesis by methane analogues. J Bacteriol. 1967 Jul;94(1):171–175. doi: 10.1128/jb.94.1.171-175.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Cheeseman P., Toms-Wood A., Wolfe R. S. Isolation and properties of a fluorescent compound, factor 420 , from Methanobacterium strain M.o.H. J Bacteriol. 1972 Oct;112(1):527–531. doi: 10.1128/jb.112.1.527-531.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T. Carbon monoxide-stimulated respiration in methane-utilizing bacteria. FEBS Lett. 1974 Apr 15;41(1):94–98. doi: 10.1016/0014-5793(74)80962-2. [DOI] [PubMed] [Google Scholar]
- Fuchs G., Schnitker U., Thauer R. K. Carbon monoxide oxidation by growing cultures of Clostridium pasteurianum. Eur J Biochem. 1974 Nov 1;49(1):111–115. doi: 10.1111/j.1432-1033.1974.tb03816.x. [DOI] [PubMed] [Google Scholar]
- Hirsch P. Photosynthetic bacterium growing under carbon monoxide. Nature. 1968 Feb 10;217(5128):555–556. doi: 10.1038/217555a0. [DOI] [PubMed] [Google Scholar]
- Hubley J. H., Mitton J. R., Wilkinson J. F. The oxidation of carbon monoxide by methane-oxidizing bacteria. Arch Mikrobiol. 1974 Feb 13;95(4):365–368. doi: 10.1007/BF02451778. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McBride B. C., Wolfe R. S. A new coenzyme of methyl transfer, coenzyme M. Biochemistry. 1971 Jun 8;10(12):2317–2324. doi: 10.1021/bi00788a022. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozhevnikova A. N., Zavarzin G. A. K taksonomii CO-okisliaiushchikh gramotritsatel'nykh bakterii. Izv Akad Nauk SSSR Biol. 1974 May-Jun;(3):436–440. [PubMed] [Google Scholar]
- Postgate J. Carbon monoxide as a basis for primitive life on other planets: a comment. Nature. 1970 Jun 6;226(5249):978–978. doi: 10.1038/226978a0. [DOI] [PubMed] [Google Scholar]
- Roberton A. M., Wolfe R. S. Adenosine triphosphate pools in Methanobacterium. J Bacteriol. 1970 Apr;102(1):43–51. doi: 10.1128/jb.102.1.43-51.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanzhieva E. U., Zavarzin G. A. Bakteriia, okisliaiushchaia okis' ugleroda. Dokl Akad Nauk SSSR. 1971 Feb 1;196(4):956–958. [PubMed] [Google Scholar]
- Savel'eva N. D., Nozhevnikova A. N. Avtotrofnyi rost Seliberia carboxydohydrogena pri okislenii vodoroda i okisi ugleroda. Mikrobiologiia. 1972 Sep-Oct;41(5):813–817. [PubMed] [Google Scholar]
- Stephenson M., Stickland L. H. Hydrogenase: The bacterial formation of methane by the reduction of one-carbon compounds by molecular hydrogen. Biochem J. 1933;27(5):1517–1527. doi: 10.1042/bj0271517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Fuchs G., Käufer B., Schnitker U. Carbon-monoxide oxidation in cell-free extracts of Clostridium pasteurianum. Eur J Biochem. 1974 Jun 15;45(2):343–349. doi: 10.1111/j.1432-1033.1974.tb03559.x. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng S. F., Wolfe R. S., Bryant M. P. Factor 420-dependent pyridine nucleotide-linked hydrogenase system of Methanobacterium ruminantium. J Bacteriol. 1975 Jan;121(1):184–191. doi: 10.1128/jb.121.1.184-191.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzing S. F., Bryant M. P., Wolfe R. S. Factor 420-dependent pyridine nucleotide-linked formate metabolism of Methanobacterium ruminantium. J Bacteriol. 1975 Jan;121(1):192–196. doi: 10.1128/jb.121.1.192-196.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L. Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3298–3302. doi: 10.1073/pnas.73.9.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Wolfe R. S. Microbial formation of methane. Adv Microb Physiol. 1971;6:107–146. doi: 10.1016/s0065-2911(08)60068-5. [DOI] [PubMed] [Google Scholar]
- YAGI T. Enzymic oxidation of carbon monoxide. Biochim Biophys Acta. 1958 Oct;30(1):194–195. doi: 10.1016/0006-3002(58)90263-4. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Henning D. L. Methanobacterium arbophilicum sp.nov. An obligate anaerobe isolated from wetwood of living trees. Antonie Van Leeuwenhoek. 1975;41(4):543–552. doi: 10.1007/BF02565096. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



