Abstract
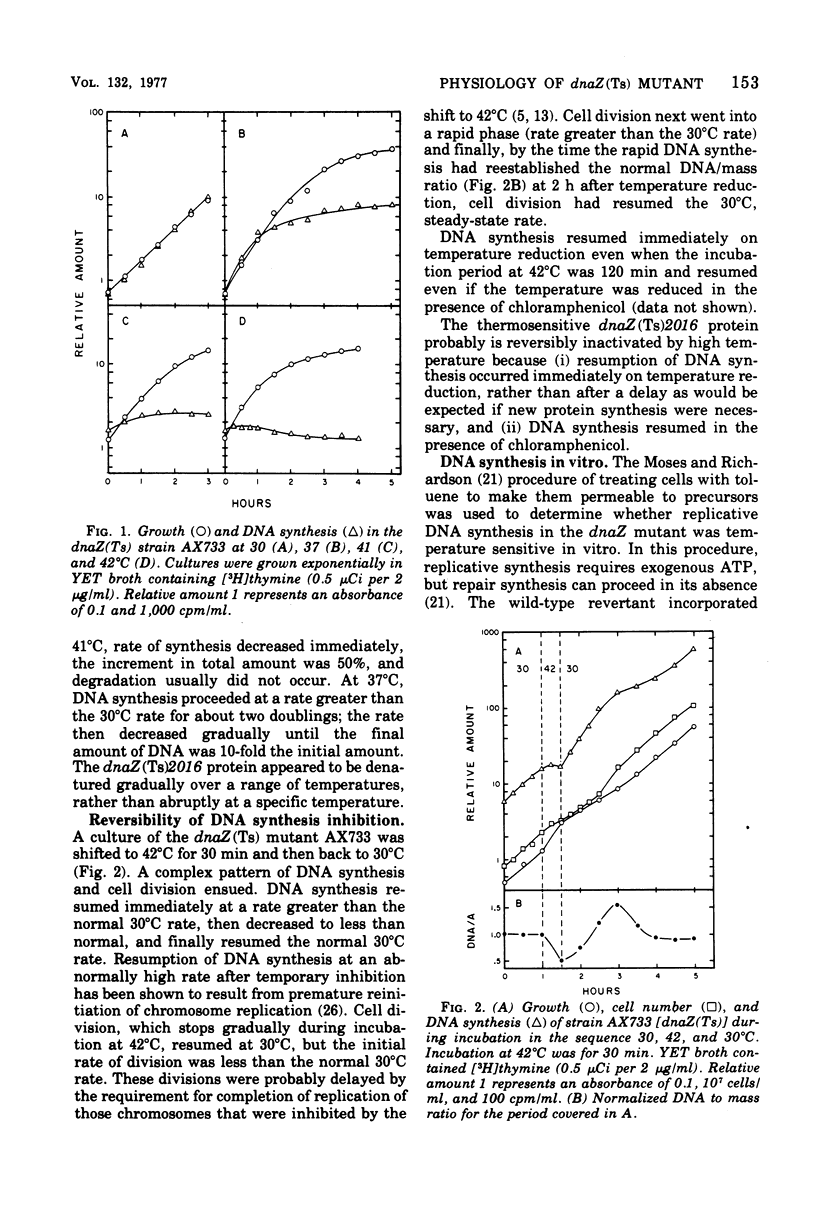
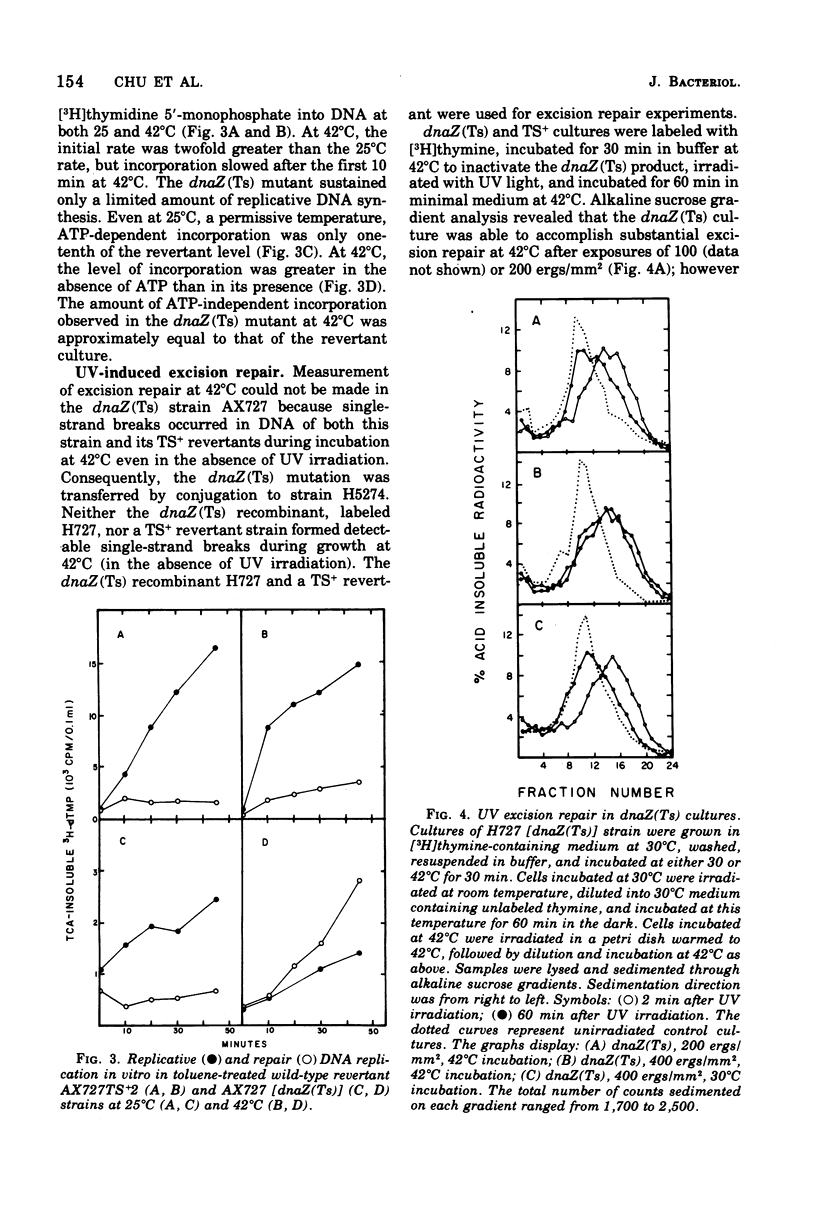
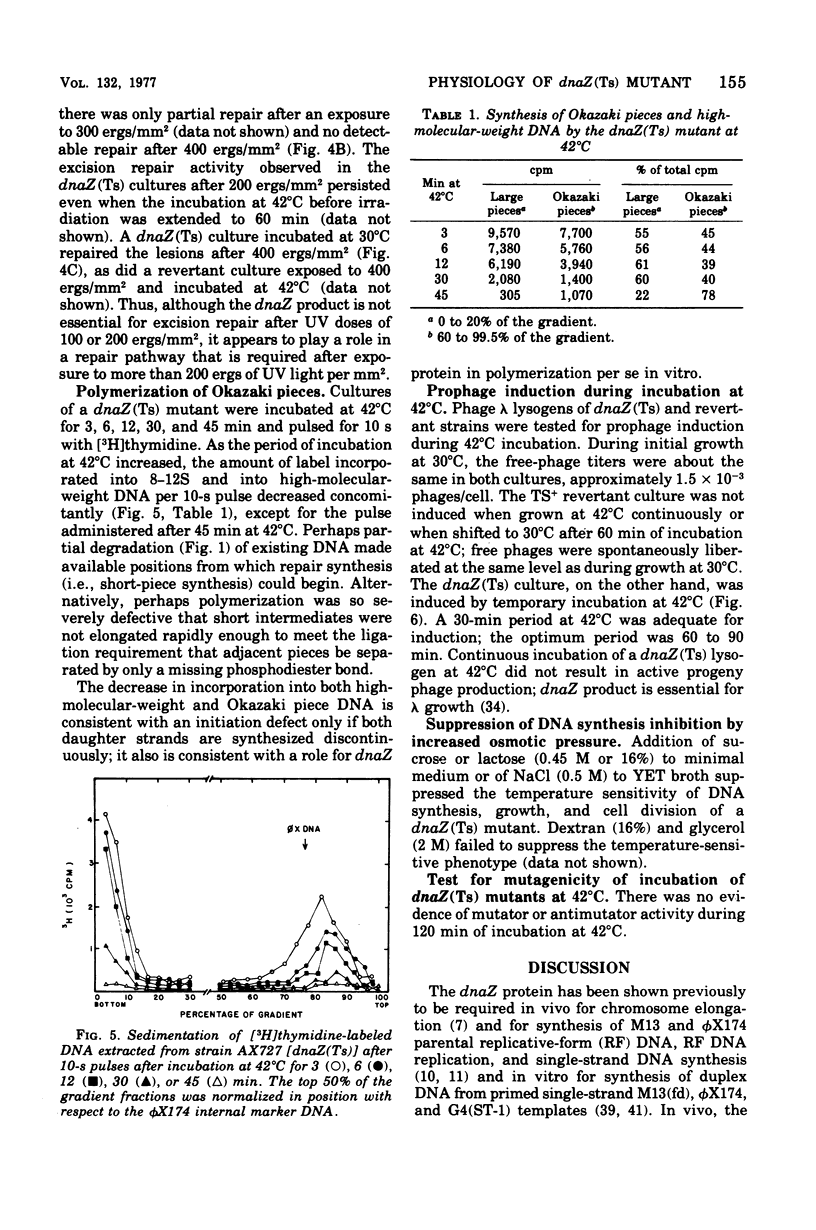
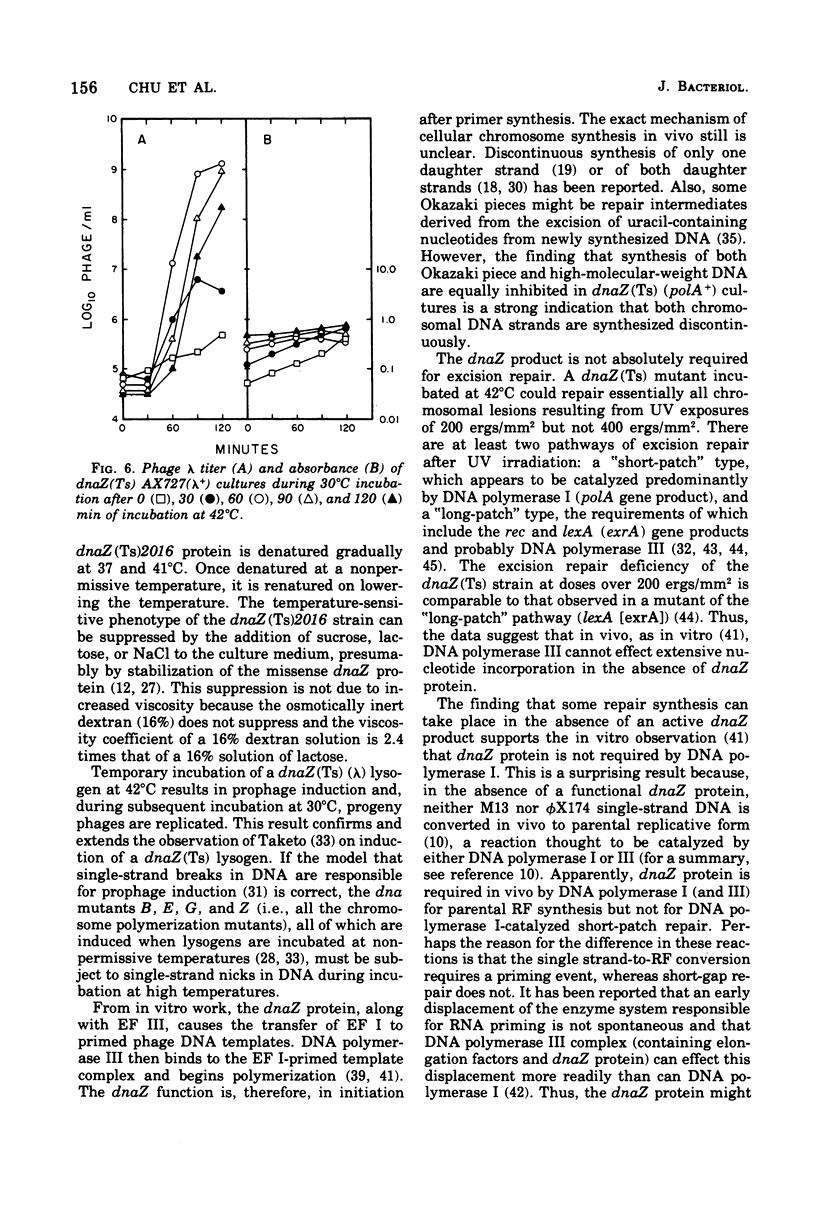
The physiological effects of incubation at nonpermissive temperatures of Escherichia coli mutants that carry a temperature-sensitive dnaZ allele [dnaZ(Ts)2016] were examined. The temperature at which the dnaZ(Ts) protein becomes inactivated in vivo was investigated by measurements of deoxyribonucleic acid (DNA) synthesis at temperatures intermediate between permissive and nonpermissive. DNA synthesis inhibition was reversible by reducing the temperature of cultures from 42 to 30°C; DNA synthesis resumed immediately after temperature reduction and occurred even in the presence of chloramphenicol. Inasmuch as DNA synthesis could be resumed in the absence of protein synthesis, we concluded that the protein product of the dnaZ allele (Ts)2016 is renaturable. Cell division, also inhibited by 42°C incubation, resumed after temperature reduction, but the length of time required for resumption depended on the duration of the period at 42°C. Replicative synthesis of cellular DNA, examined in vitro in toluene-permeabilized cells, was temperature sensitive. Excision repair of ultraviolet light-induced DNA lesions was partially inhibited in dnaZ(Ts) cells at 42°C. The dnaZ+ product participated in the synthesis of both Okazaki piece (8–12S) and high-molecular-weight DNA. During incubation of dnaZ(Ts)(λ) lysogens at 42°C, prophage induction occurred, and progeny phage were produced during subsequent incubation at 30°C. The temperature sensitivity of both DNA synthesis and cell division in the dnaZ(Ts)2016 mutant was suppressed by high concentrations of sucrose, lactose, or NaCl. Incubation at 42°C was neither mutagenic nor antimutagenic for the dnaZ(Ts) mutant.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyersmann D., Messer W., Schlicht M. Mutants of Escherichia coli B-r defective in deoxyribonucleic acid initiation: dnaI, a new gene for replication. J Bacteriol. 1974 Jun;118(3):783–789. doi: 10.1128/jb.118.3.783-789.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown L. R., Dowell C. E. Replication of coliphage M-13. I. Effects on host cells after synchronized infection. J Virol. 1968 Nov;2(11):1290–1295. doi: 10.1128/jvi.2.11.1290-1295.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl P. L. Escherichia coli mutants with temperature-sensitive synthesis of DNA. Mol Gen Genet. 1970;109(2):107–122. doi: 10.1007/BF00269647. [DOI] [PubMed] [Google Scholar]
- Caster J. H. Selection of thymine-requiring strains from Escherichia coli on solid medium. J Bacteriol. 1967 Nov;94(5):1804–1804. doi: 10.1128/jb.94.5.1804-.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. J. Regulation of deoxyribonucleic acid replication and cell division in Escherichia coli B-r. J Bacteriol. 1968 Oct;96(4):1214–1224. doi: 10.1128/jb.96.4.1214-1224.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangman W. L., Novick A. Characterization of two bacterial mutants with temperature-sensitive synthesis of DNA. Genetics. 1968 Sep;60(1):1–17. doi: 10.1093/genetics/60.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C. C., Allen J. S., Gustafson R. A., Allen R. G., Walker J. R. Bacterial cell division regulation: characterization of the dnaH locus of Escherichia coli. J Bacteriol. 1974 Aug;119(2):443–449. doi: 10.1128/jb.119.2.443-449.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTHRIE G. D., SINSHEIMER R. L. Observations on the infection of bacterial protoplasts with the deoxyribonucleic acid of bacteriophage phi X174. Biochim Biophys Acta. 1963 Jun 25;72:290–297. [PubMed] [Google Scholar]
- Gefter M. L., Hirota Y., Kornberg T., Wechsler J. A., Barnoux C. Analysis of DNA polymerases II and 3 in mutants of Escherichia coli thermosensitive for DNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3150–3153. doi: 10.1073/pnas.68.12.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWTHORNE D. C., FRIIS J. OSMOTIC-REMEDIAL MUTANTS. A NEW CLASSIFICATION FOR NUTRITIONAL MUTANTS IN YEAST. Genetics. 1964 Nov;50:829–839. doi: 10.1093/genetics/50.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldenwang W. G., Walker J. R. Inhibition of bacteriophage M13 and phix174 duplex DNA replication and single-strand synthesis in temperature-sensitive dnaZ mutants of Escherichia coli. J Virol. 1977 Apr;22(1):23–30. doi: 10.1128/jvi.22.1.23-30.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldenwang W. G., Walker J. R. Parental RF formation on phages phiX174 and M13 requires the dnaZ gene product of Escherichia coli. Biochem Biophys Res Commun. 1976 Jun 7;70(3):932–938. doi: 10.1016/0006-291x(76)90681-1. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E., Pierucci O. Cell division during inhibition of deoxyribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1968 May;95(5):1627–1633. doi: 10.1128/jb.95.5.1627-1633.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Jacobson M. K., Lark K. G. DNA replication in Escherichia coli: evidence for two classes of small deoxyribonucleotide chains. J Mol Biol. 1973 Feb 5;73(4):371–396. doi: 10.1016/0022-2836(73)90088-0. [DOI] [PubMed] [Google Scholar]
- Kuempel P. L. Temperature-sensitive initiation of chromosome replication in a mutant of Escherichia coli. J Bacteriol. 1969 Dec;100(3):1302–1310. doi: 10.1128/jb.100.3.1302-1310.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa Y., Okazaki R. Mechanism of DNA chain growth. XIII. Evidence for discontinuous replication of both strands of P2 phage DNA. J Mol Biol. 1975 May 15;94(2):229–241. doi: 10.1016/0022-2836(75)90080-7. [DOI] [PubMed] [Google Scholar]
- Louarn J. M., Bird R. E. Size distribution and molecular polarity of newly replicated DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Feb;71(2):329–333. doi: 10.1073/pnas.71.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANTEN A., ROWLEY D. Genetic analysis of valine inhibition in the K12 strain of Bacterium coli. J Gen Microbiol. 1953 Oct;9(2):226–233. doi: 10.1099/00221287-9-2-226. [DOI] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsslein V., Otto B., Bonhoeffer F., Schaller H. Function of DNA polymerase 3 in DNA replication. Nat New Biol. 1971 Dec 29;234(52):285–286. doi: 10.1038/newbio234285a0. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRITCHARD R. H., LARK K. G. INDUCTION OF REPLICATION BY THYMINE STARVATION AT THE CHROMOSOME ORIGIN IN ESCHERICHIA COLI. J Mol Biol. 1964 Aug;9:288–307. doi: 10.1016/s0022-2836(64)80208-4. [DOI] [PubMed] [Google Scholar]
- Pisetsky D., Berkower I., Wickner R., Hurwitz J. Role of ATP in DNA synthesis in Escherichia coli. J Mol Biol. 1972 Nov 28;71(3):557–571. doi: 10.1016/s0022-2836(72)80023-8. [DOI] [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Effet des sels et autres composés sur le phénotype de mutants thermosensibles de Escherichia coli. Ann Microbiol (Paris) 1973 Jan;124(1):29–43. [PubMed] [Google Scholar]
- Schuster H., Beyersmann D., Mikolajczyk M., Schlicht M. Prophage induction by high temperature in thermosensitive dna mutants lysogenic for bacteriophage lambda. J Virol. 1973 Jun;11(6):879–885. doi: 10.1128/jvi.11.6.879-885.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinsheimer R. L. Bacteriophage phi-X174 and related viruses. Prog Nucleic Acid Res Mol Biol. 1968;8:115–169. [PubMed] [Google Scholar]
- Sternglanz R., Wang H. F., Donegan J. J. Evidence that both growing DNA chains at a replication fork are synthesized discontinuously. Biochemistry. 1976 May 4;15(9):1838–1843. doi: 10.1021/bi00654a008. [DOI] [PubMed] [Google Scholar]
- Sussman R., Zeev H. B. Proposed mechanism of bacteriophage lambda induction: acquisition of binding sites for lambda repressor by DNA of the host. Proc Natl Acad Sci U S A. 1975 May;72(5):1973–1976. doi: 10.1073/pnas.72.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait R. C., Harris A. L., Smith D. W. DNA repair in Escherichia coli mutants deficient in DNA polymerases I, II and-or 3. Proc Natl Acad Sci U S A. 1974 Mar;71(3):675–679. doi: 10.1073/pnas.71.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketo A. Inducibility of lambda phage development in Escherichia coli mutants thermosensitive for DNA replication. Z Naturforsch C. 1975 Nov-Dec;30(6):800–803. doi: 10.1515/znc-1975-11-1219. [DOI] [PubMed] [Google Scholar]
- Truitt C. L., Walker J. R. Growth of phages lambda, phiX174, and Ml3 requires the dnaZ (previously dnaH) gene product of Escherichia coli. Biochem Biophys Res Commun. 1974 Dec 11;61(3):1036–1042. doi: 10.1016/0006-291x(74)90259-9. [DOI] [PubMed] [Google Scholar]
- Wada C., Yura T. Phenethyl alcohol resistance in Escherichia coli. 3. A temperature-sensitive mutation(dnaP) affecting DNA replication. Genetics. 1974 Jun;77(2):199–220. doi: 10.1093/genetics/77.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R., Henson J. M., Lee C. S. Isolation and characterization of plaque-forming lambdadnaZ+ transducing bacteriophages. J Bacteriol. 1977 Apr;130(1):354–365. doi: 10.1128/jb.130.1.354-365.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Interaction of Escherichia coli dnaB and dnaC(D) gene products in vitro. Proc Natl Acad Sci U S A. 1975 Mar;72(3):921–925. doi: 10.1073/pnas.72.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Involvement of escherichia coli dnaZ gene product in DNA elongation in vitro. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1053–1057. doi: 10.1073/pnas.73.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S. Mechanism of DNA elongation catalyzed by Escherichia coli DNA polymerase III, dnaZ protein, and DNA elongation factors I and III. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3511–3515. doi: 10.1073/pnas.73.10.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Schekman R., Geider K., Kornberg A. A new form of DNA polymerase 3 and a copolymerase replicate a long, single-stranded primer-template. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1764–1767. doi: 10.1073/pnas.70.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngs D. A., Smith K. C. Evidence for the control by exrA and polA genes of two branches of the uvr gene-dependent excision repair pathway in Escherichia coli K-12. J Bacteriol. 1973 Oct;116(1):175–182. doi: 10.1128/jb.116.1.175-182.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



