Abstract
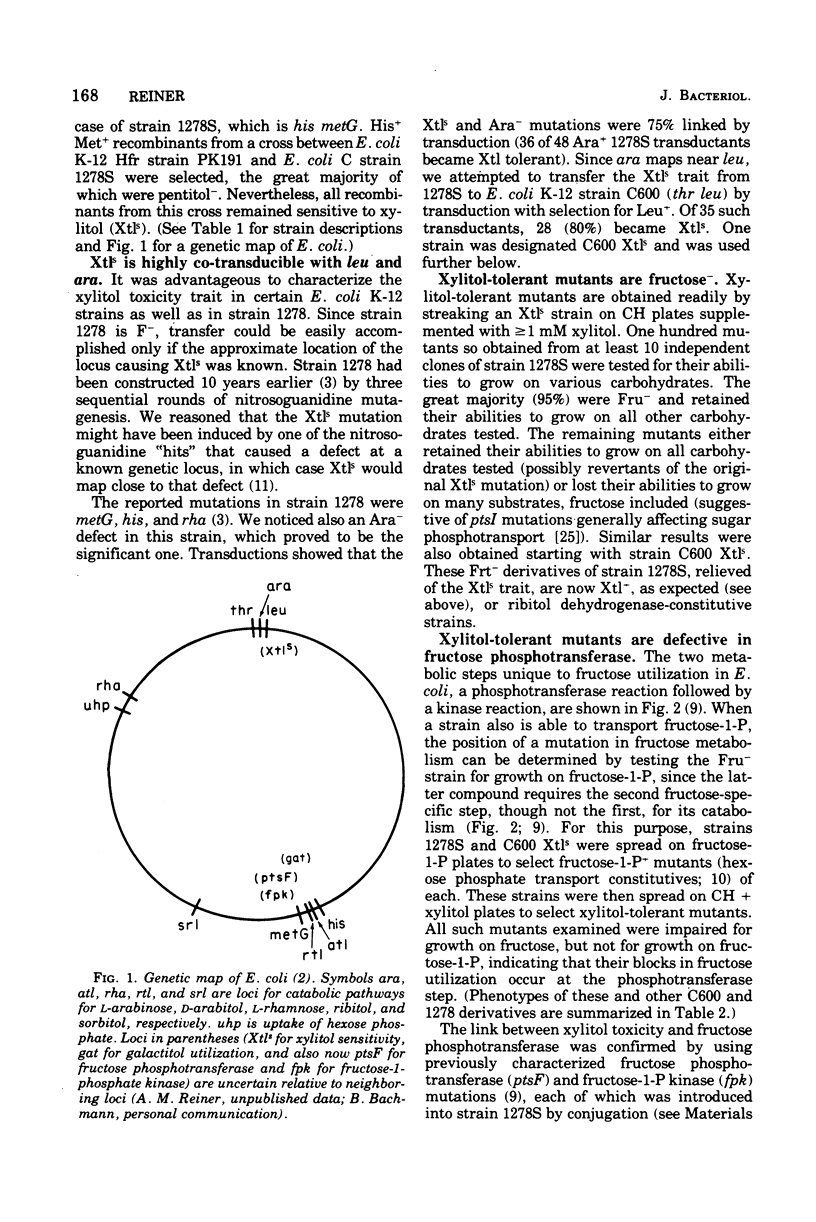
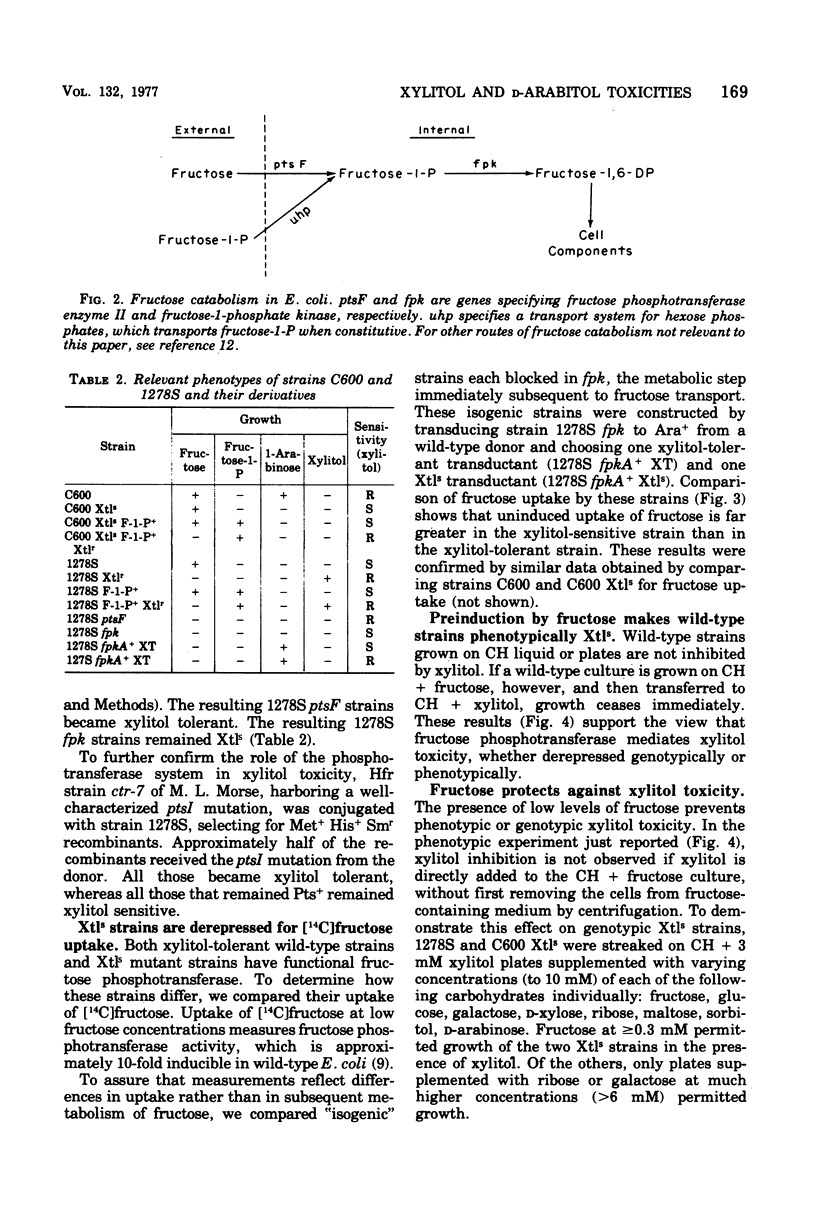
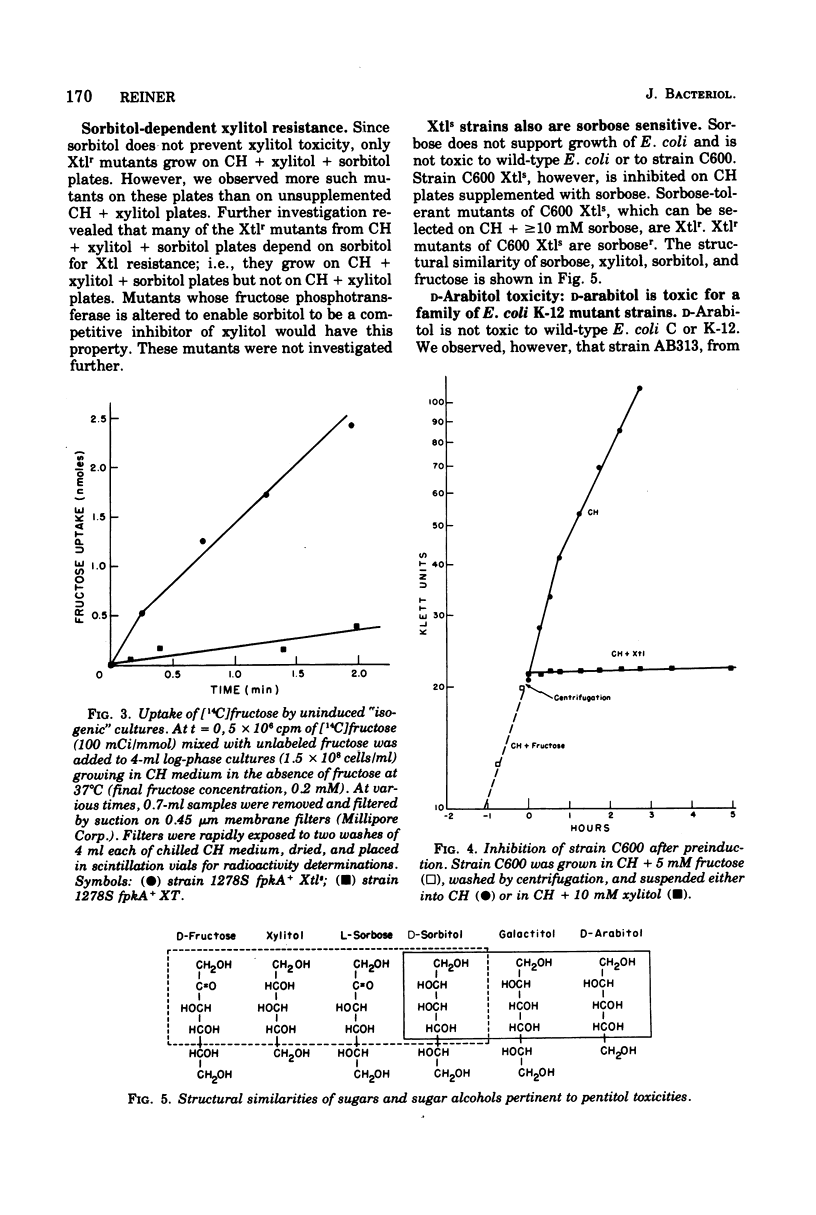
d-Arabitol was observed to be toxic to many laboratory strains of Escherichia coli K-12, and xylitol was found to be toxic to an existing E. coli C mutant strain. Fructose-specific components of the phosphoenolpyruvate:sugar phosphotransferase system are required for xylitol toxicity. Selection for xylitol resistance results in Fru− strains blocked in fructose phosphotransferase. Introduction of the ptsF or ptsI mutation into a xylitol-sensitive strain eliminates sensitivity. [14C]fructose uptake experiments imply that the mutation to xylitol sensitivity, which is co-transducible with ara and leu, results in derepression of normally inducible fructose phosphotransferase. Wild-type strains also become xylitol sensitive if induced by (and then removed from) fructose. Xylitol toxicity is prevented by fructose in both wild-type and mutant strains. Circumstances causing xylitol, a new food additive, to become toxic to an otherwise insensitive wild-type organism have not been reported previously. The d-arabitol-sensitive laboratory strains are galactitol (dulcitol) utilizers, although most other strains are not. Selection for d-arabitol resistance results in Gat− strains blocked in a constitutive galactitol-specific component of the phosphotransferase system. A mutation causing d-arabitol sensitivity occurred many years ago in AB284, the parent of AB311, AB312, AB313, and many other strains. d-Arabitol sensitivity also occurs in sorbitol-constitutive strains and is shown, like the previous two instances of pentitol toxicities, to result from a constitutive phosphotransferase, which is blocked in mutants selected for resistance.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calendar R., Lindahl G. Attachment of prophage P2: gene order at different host chromosomal sites. Virology. 1969 Dec;39(4):867–881. doi: 10.1016/0042-6822(69)90023-3. [DOI] [PubMed] [Google Scholar]
- Charnetzky W. T., Mortlock R. P. Ribitol catabolic pathway in Klebsiella aerogenes. J Bacteriol. 1974 Jul;119(1):162–169. doi: 10.1128/jb.119.1.162-169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaro C. Genetics of the bacterial phosphoenolpyruvate: glycose phosphotransferase system. Annu Rev Genet. 1976;10:341–359. doi: 10.1146/annurev.ge.10.120176.002013. [DOI] [PubMed] [Google Scholar]
- Curtis S. J., Epstein W. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J Bacteriol. 1975 Jun;122(3):1189–1199. doi: 10.1128/jb.122.3.1189-1199.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., ANDERSON R. L., WEINBERG R., LEE N., HOFFEE P., HUTTENHAUER G., BOYER H. L-Arabinose-sensitive, L-ribulose 5-phosphate 4-epimerase-deficient mutants of Escherichia coli. J Bacteriol. 1962 Jul;84:137–146. doi: 10.1128/jb.84.1.137-146.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci T., Kornberg H. L., Smith Janet. Isolation and properties of a regulatory mutant in the hexose phosphate transport system of Escherichia coli. FEBS Lett. 1971 Mar 5;13(3):133–136. doi: 10.1016/0014-5793(71)80218-1. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Kornberg H. L. The role of phosphotransferase-mediated syntheses of fructose 1-phosphate and fructose 6-phosphate in the growth of Escherichia coli on fructose. Proc R Soc Lond B Biol Sci. 1974 Sep 17;187(1087):105–119. doi: 10.1098/rspb.1974.0065. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Kornberg H. L. The utilization of fructose by Escherichia coli. Properties of a mutant defective in fructose 1-phosphate kinase activity. Biochem J. 1973 Feb;132(2):341–347. doi: 10.1042/bj1320341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Kornberg H. L. Uptake of fructose by the sorbitol phosphotransferase of Escherichia coli K12. J Gen Microbiol. 1976 Oct;96(2):383–391. doi: 10.1099/00221287-96-2-383. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L. Genetics in the study of carbohydrate transport by bacteria. Sixth Griffith Memorial Lecture. J Gen Microbiol. 1976 Sep;96(1):1–16. doi: 10.1099/00221287-96-1-1. [DOI] [PubMed] [Google Scholar]
- LERNER S. A., WU T. T., LIN E. C. EVOLUTION OF A CATABOLIC PATHWAY IN BACTERIA. Science. 1964 Dec 4;146(3649):1313–1315. doi: 10.1126/science.146.3649.1313. [DOI] [PubMed] [Google Scholar]
- Lengeler J. Analysis of mutations affecting the dissmilation of galactitol (dulcitol) in Escherichia coli K 12. Mol Gen Genet. 1977 Mar 28;152(1):83–91. doi: 10.1007/BF00264944. [DOI] [PubMed] [Google Scholar]
- Lengeler J., Lin E. C. Reversal of the mannitol-sorbitol diauxie in Escherichia coli. J Bacteriol. 1972 Nov;112(2):840–848. doi: 10.1128/jb.112.2.840-848.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler J. Mutations affecting transport of the hexitols D-mannitol, D-glucitol, and galactitol in Escherichia coli K-12: isolation and mapping. J Bacteriol. 1975 Oct;124(1):26–38. doi: 10.1128/jb.124.1.26-38.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Parke D. Evolution of catabolic pathways. Biochem Soc Trans. 1976;4(3):468–472. doi: 10.1042/bst0040468. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Genes for ribitol and D-arabitol catabolism in Escherichia coli: their loci in C strains and absence in K-12 and B strains. J Bacteriol. 1975 Aug;123(2):530–536. doi: 10.1128/jb.123.2.530-536.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Burleigh B. D., Jr, Hartley B. S. Gene duplication in experimental enzyme evolution. Nature. 1974 Sep 20;251(5472):200–204. doi: 10.1038/251200a0. [DOI] [PubMed] [Google Scholar]
- Scheinin A., Mäkinen K. K., Ylitalo K. Turku sugar studies. I. An intermediate report on the effect of sucrose, fructose and xylitol diets on the caries incidence in man. Acta Odontol Scand. 1974;32(6):383–412. doi: 10.3109/00016357409026549. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Roseman S., Saier M. H., Jr Sugar transport. Properties of mutant bacteria defective in proteins of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1976 Nov 10;251(21):6584–6597. [PubMed] [Google Scholar]
- Solomon E., Lin E. C. Mutations affecting the dissimilation of mannitol by Escherichia coli K-12. J Bacteriol. 1972 Aug;111(2):566–574. doi: 10.1128/jb.111.2.566-574.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolinsky M. B., Wiesmeyer H., Kalckar H. M., Jordan E. HEREDITARY DEFECTS IN GALACTOSE METABOLISM IN ESCHERICHIA COLI MUTANTS, II. GALACTOSE-INDUCED SENSITIVITY. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1786–1791. doi: 10.1073/pnas.45.12.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]



