Abstract
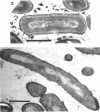
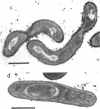
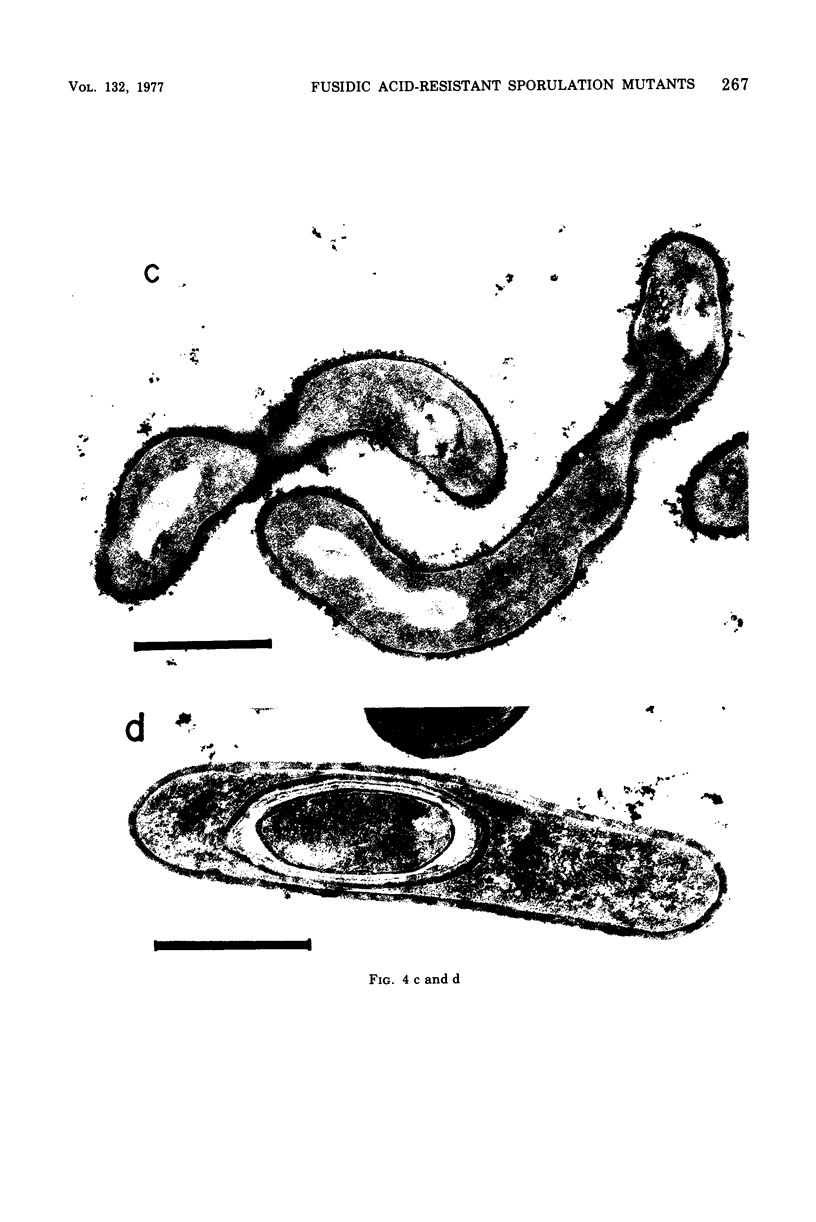
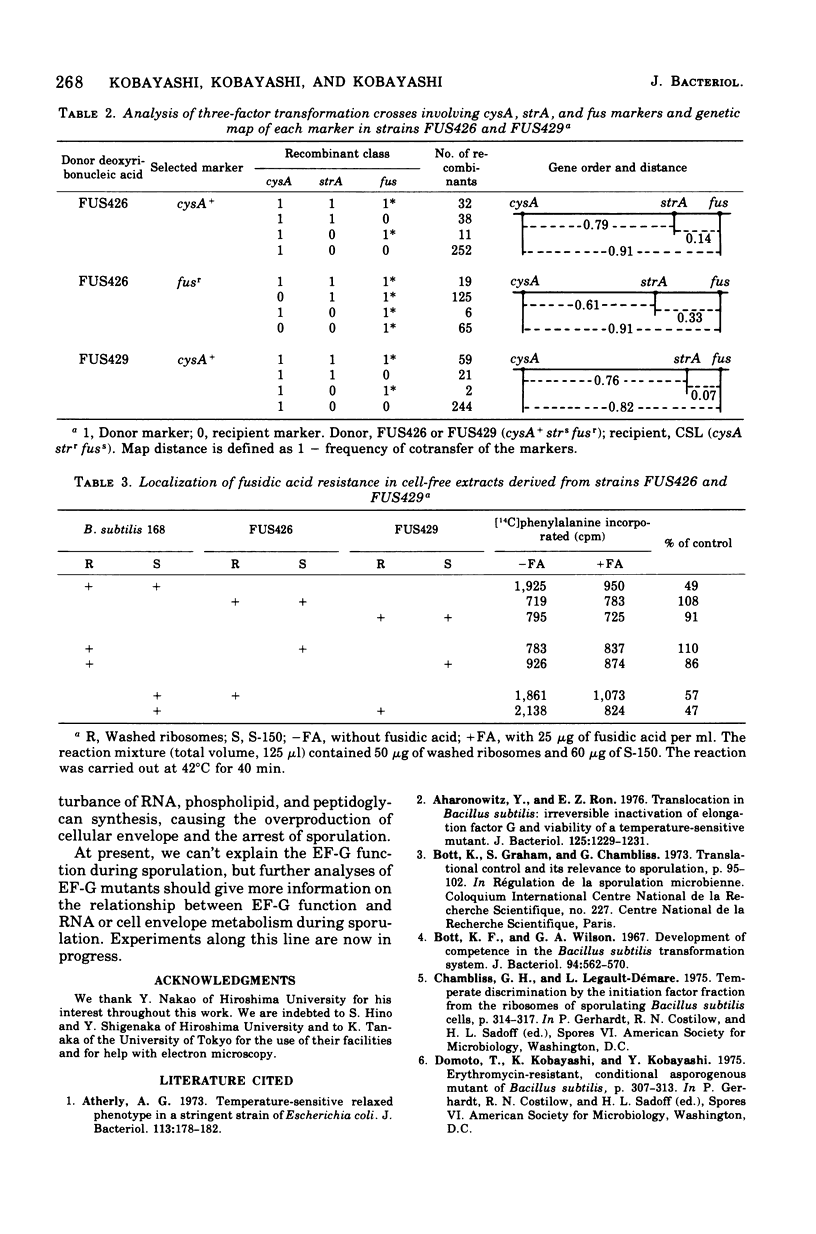
Fusidic acid-resistant, sporulation-defective mutants were isolated from Bacillus subtilis 168 thy trp. About two-thirds of the fusidic acid-resistant (fusr) mutants were defective in sporulation ability and fell into three classes with respect to sporulation character. The representative mutants FUS426 and FUS429 were characterized in detail. FUS426 [fusr spo (Ts)], a temperature-sensitive sporulation mutant, grew well at 30 and 42 degrees C but did not sporulate at 42 degrees C. FUS429 [fusr spo (Con)], conditional sporulation mutant, grew and sporulated normally in the absence of fusidic acid, but its sporulation and growth rates decreased in the presence of fusidic acid, depending on the concentration of the drug. Although electron microscopic observation showed that both mutants were blocked at stage I of sporulation, the physiological analyses indicate that these mutants belong to the SpoOB class. Both mutants formed a thickened cell wall as compared with that of the parental strain. Genetic and in vitro protein synthesis analyses led to the conclusion that the sporulation-defective character of mutants FUS426 and FUS429 resulted from an alteration in elongation factor G caused by a single lesion in the fus locus. The possible role of elongation factor G in sporulation is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonowitz Y., Ron E. Z. Translocation in Bacillus subtilis: irreversible inactivation of elongation factor G and viability of a temperature- sensitive mutant. J Bacteriol. 1976 Mar;125(3):1229–1231. doi: 10.1128/jb.125.3.1229-1231.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherly A. G. Temperature-sensitive relaxed Phenotype in a stringent strain of Escherichia coli. J Bacteriol. 1973 Jan;113(1):178–182. doi: 10.1128/jb.113.1.178-182.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott K. F., Wilson G. A. Development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1967 Sep;94(3):562–570. doi: 10.1128/jb.94.3.562-570.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P., Bergmann R. Alteration of the ribosomal fraction of Bacillus subtilis during sporulation. Biochim Biophys Acta. 1973 Feb 23;299(1):136–141. doi: 10.1016/0005-2787(73)90404-8. [DOI] [PubMed] [Google Scholar]
- Goldthwaite C., Smith I. Genetic mapping of aminoglycoside and fusidic acid resistant mutations in Bacillus subtilis. Mol Gen Genet. 1972;114(3):181–189. doi: 10.1007/BF01788887. [DOI] [PubMed] [Google Scholar]
- Graham R. S., Bott K. F. Antibiotic-resistant mutants of Bacillus subtilis conditional for sporulation. Mol Gen Genet. 1975;137(3):227–237. doi: 10.1007/BF00333018. [DOI] [PubMed] [Google Scholar]
- Guha S., Szulmajster J. Effect of fusidic acid on sporulation of Bacillus subtilis. FEBS Lett. 1974 Jan 15;38(3):315–319. doi: 10.1016/0014-5793(74)80081-5. [DOI] [PubMed] [Google Scholar]
- Harford N., Sueoka N. Chromosomal location of antibiotic resistance markers in Bacillus subtilis. J Mol Biol. 1970 Jul 28;51(2):267–286. doi: 10.1016/0022-2836(70)90142-7. [DOI] [PubMed] [Google Scholar]
- Hitchins A. D., Slepecky R. A. Bacterial sporulation as a modified procaryotic cell division. Nature. 1969 Aug 23;223(5208):804–807. doi: 10.1038/223804a0. [DOI] [PubMed] [Google Scholar]
- Ishiguro E. E., Ramey W. D. Stringent control of peptidoglycan biosynthesis in Escherichia coli K-12. J Bacteriol. 1976 Sep;127(3):1119–1126. doi: 10.1128/jb.127.3.1119-1126.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Muto A., Osawa S. Control of stable RNA synthesis in a temperature-sensitive mutant of elongation factor G of Bacillus subtilis. Mol Gen Genet. 1974 May 31;130(3):203–214. doi: 10.1007/BF00268800. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Schlessinger D. G factor mutants of Escherichia coli: map location and properties. Biochem Biophys Res Commun. 1971 Feb 5;42(3):441–444. doi: 10.1016/0006-291x(71)90390-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Legault-Demare L., Chambliss G. H. Natural messenger ribonucleic acid-directed cell-free protein-synthesizing system of Bacillus subtilis. J Bacteriol. 1974 Dec;120(3):1300–1307. doi: 10.1128/jb.120.3.1300-1307.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. Sporulation-specific translational discrimination in Bacillus subtilis. J Mol Biol. 1974 Jul 15;86(4):855–863. doi: 10.1016/0022-2836(74)90358-1. [DOI] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Pizer L. I. Regulation of phospholipid synthesis in Escherichia coli by guanosine tetraphosphate. J Bacteriol. 1973 Oct;116(1):355–366. doi: 10.1128/jb.116.1.355-366.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y., Lipmann F. Comparison of guanosine triphosphate split and polypeptide synthesis with a purified E. coli system. Proc Natl Acad Sci U S A. 1966 Jan;55(1):212–219. doi: 10.1073/pnas.55.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel D. W., Gilvarg C. Timing of mucopeptide and phospholipid synthesis in sporulating Bacillus megaterium. J Biol Chem. 1971 Jun 10;246(11):3720–3724. [PubMed] [Google Scholar]
- Rabbani E., Srinivasan P. R. Role of the translocation factor G in the regulation of ribonucleic acid synthesis. J Bacteriol. 1973 Mar;113(3):1177–1183. doi: 10.1128/jb.113.3.1177-1183.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Sokawa Y., Nakao E., Kaziro Y. On the nature of the control by RC gene in e. coli: amino acid-dependent control of lipid synthesis. Biochem Biophys Res Commun. 1968 Oct 10;33(1):108–112. doi: 10.1016/0006-291x(68)90263-5. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Kawano G., Kinoshita T. Chromosomal location of a fusidic acid resistant marker in Escherichia coli. Biochem Biophys Res Commun. 1971 Feb 5;42(3):564–567. doi: 10.1016/0006-291x(71)90408-6. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Bott K. F. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1968 Apr;95(4):1439–1449. doi: 10.1128/jb.95.4.1439-1449.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]