Abstract
Late during sporulation, Bacillus subtilis produces glucose dehydrogenase (GlcDH; EC 1.1.1.47), which can react with D-glucose or 2-deoxy-D-glucose and can use nicotinamide adenine dinucleotide (NAD) or nicotinamide adenine dinucleotide phosphate (NADP) as a cofactor. This enzyme is found mainly in the forespore compartment and is present in spores; it is probably made exclusively in the forespore. The properties of GlcDH were determined both in crude cell extracts and after purification. The enzyme is stable at pH 6.5 but labile at pH 8 or higher; the pH optimum of enzyme activity is 8. After inactivation at pH 8, the activity can be recovered in crude extracts, but not in solutions of the purified enzyme, by incubation with 3 M KCl and 5 mM NAD or NADP. As determined by gel filtration, enzymatically active GlcDH has a molecular weight of about 115,000 (if the enzyme is assumed to be globular). GlcDH is distinct from a catabolite-repressible inositol dehydrogenase (EC 1.1.1.18), which can also react with D-glucose, requires specifically NAD as a cofactor, and has an electrophoretic mobility different from that of GlcDH.
Full text
PDF


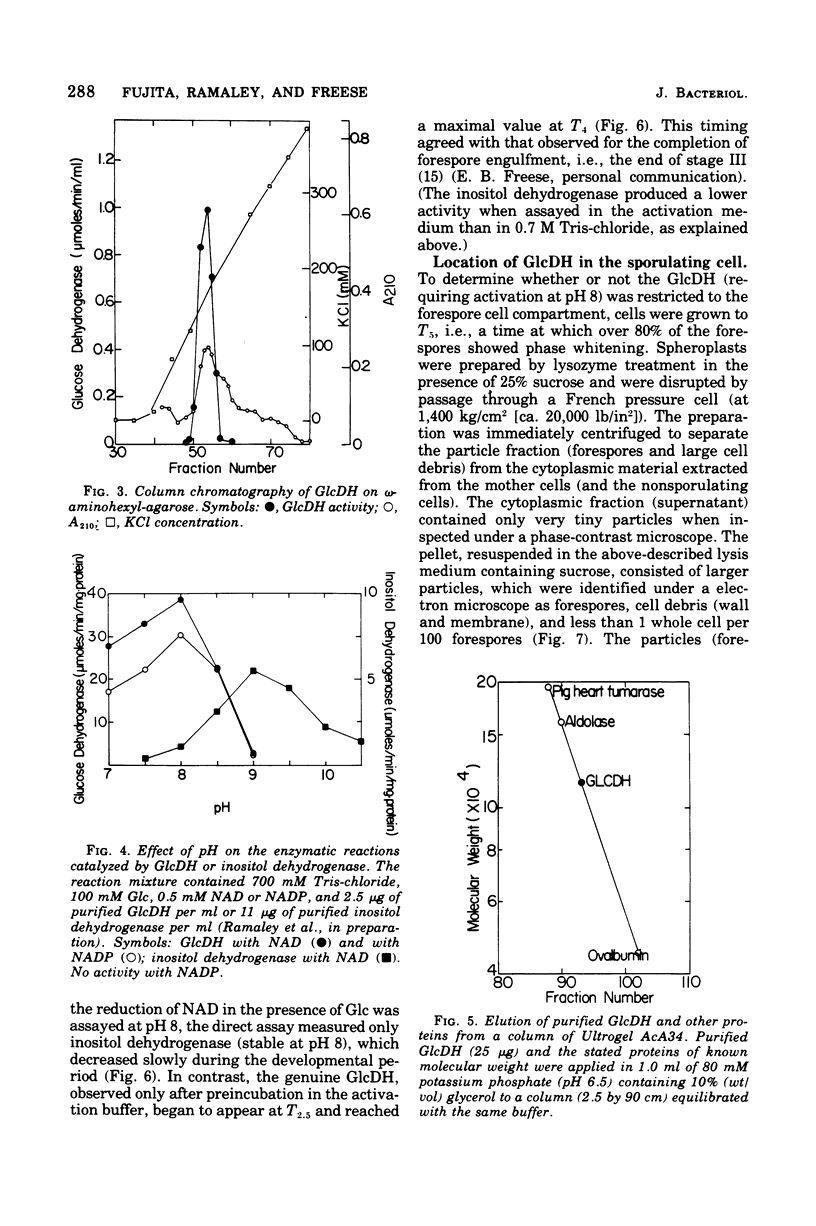
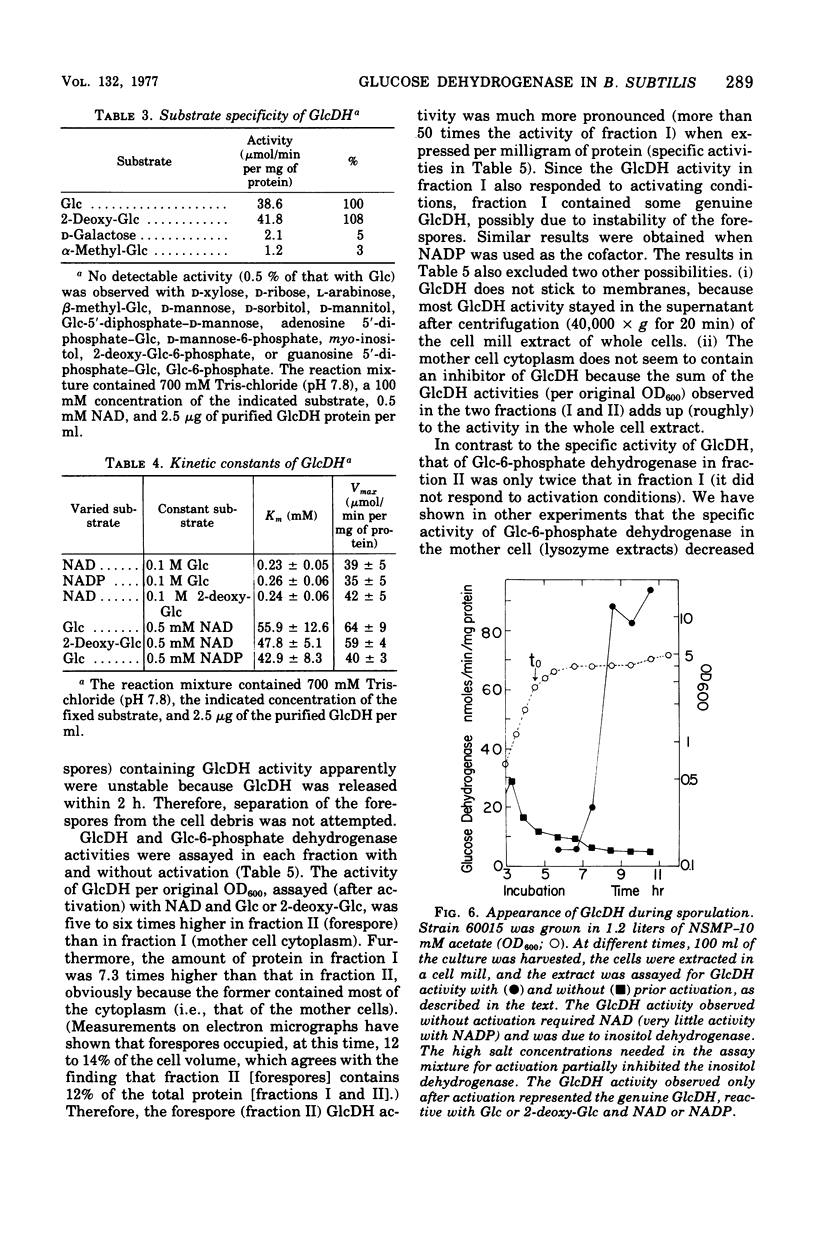
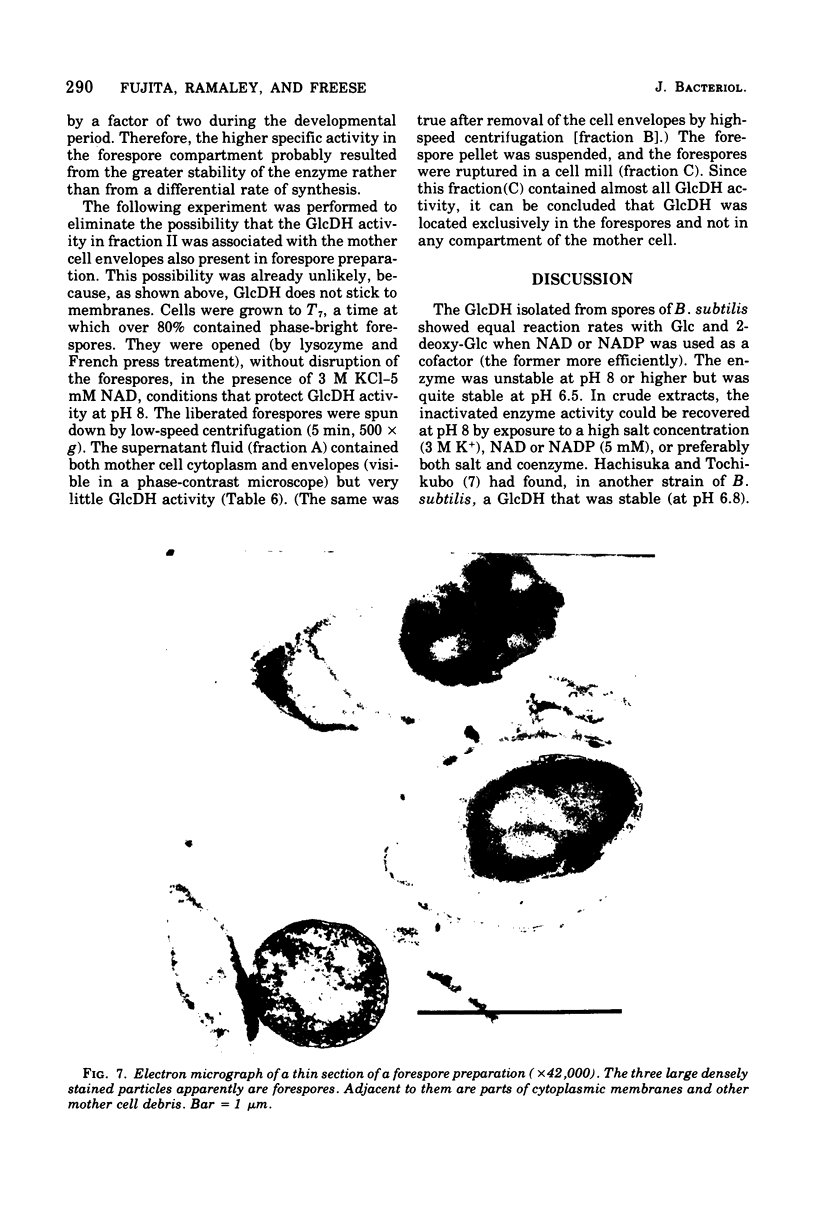



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACH J. A., SADOFF H. L. Aerobic sporulating bacteria. I. Glucose dehydrogenase of Bacillus cereus. J Bacteriol. 1962 Apr;83:699–707. doi: 10.1128/jb.83.4.699-707.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banauch D., Brümmer W., Ebeling W., Metz H., Rindfrey H., Lang H., Leybold K., Rick W., Staudinger H. J. Eine Glucose-Dehydrogenase für die Glucose-Bestimmung in Körperflüssigkeiten. Z Klin Chem Klin Biochem. 1975 Mar;13(3):101–107. [PubMed] [Google Scholar]
- Bott K. F. Acrylamide gel electrophoresis of intracellular proteins during early stages of sporulation in Bacillus subtilis. J Bacteriol. 1971 Nov;108(2):720–732. doi: 10.1128/jb.108.2.720-732.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E. B., Cole R. M., Klofat W., Freese E. Growth, sporulation, and enzyme defects of glucosamine mutants of Bacillus subtilis. J Bacteriol. 1970 Mar;101(3):1046–1062. doi: 10.1128/jb.101.3.1046-1062.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Klofat W., Galliers E. Commitment to sporulation and induction of glucose-phosphoenolpyruvate-transferase. Biochim Biophys Acta. 1970 Nov 24;222(2):265–289. doi: 10.1016/0304-4165(70)90115-7. [DOI] [PubMed] [Google Scholar]
- Hachisuka Y., Tochikubo K. Reactivative action of ethylenediaminetetraacetic acid or dipicolinic acid on inactive glucose dehydrogenase obtained from heated spores of Bacillus subtilis. J Bacteriol. 1971 Aug;107(2):442–447. doi: 10.1128/jb.107.2.442-447.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pauly H. E., Pfleiderer G. D-glucose dehydrogenase from Bacillus megaterium M 1286: purification, properties and structure. Hoppe Seylers Z Physiol Chem. 1975 Oct;356(10):1613–1623. doi: 10.1515/bchm2.1975.356.2.1613. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad C., Diesterhaft M., Freese E. Initiation of spore germination in glycolytic mutants of Bacillus subtilis. J Bacteriol. 1972 Apr;110(1):321–328. doi: 10.1128/jb.110.1.321-328.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad C. Initiation of spore germination in Bacillus subtilis: relationship to inhibition of L-alanine metabolism. J Bacteriol. 1974 Sep;119(3):805–810. doi: 10.1128/jb.119.3.805-810.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A. ETUDE MORPHOLOGIQUE DE LA SPORULATION DE BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1965 Jan;108:40–60. [PubMed] [Google Scholar]
- Shaltiel S., Er-El Z. Hydrophobic chromatography: use for purification of glycogen synthetase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):778–781. doi: 10.1073/pnas.70.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S. C. Sporulation in Bacillus subtilis. Biochemical changes. Biochem J. 1968 Oct;109(5):811–818. doi: 10.1042/bj1090811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax R., Freese E., Cashel M. Separation of two functional roles of L-alanine in the initiation of Bacillus subtilis spore germination. J Bacteriol. 1967 Sep;94(3):522–529. doi: 10.1128/jb.94.3.522-529.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]