Abstract
The membrane-bound enzyme ferrochelatase (protoheme ferro-lyase, EC 4.99.1.1) was purified from isolated membrane fragments of Spirillum itersonii approximately 490-fold. Purification was achieved by solubilization with chaotropic salts followed by ammonium sulfate fractionation, diethylaminoethyl-cellulose chromatography, and gel filtration on Sephadex G-200. The purified enzyme has an apparent minimum molecular weight of approximately 50,000, as determined by gel filtration in the presence of 0.1% Brij 35 and 1 mM dithiothreitol but forms high-molecular-weight aggregates in the absence of detergent. Purified ferrochelatase is strongly stimulated in the presence of copper. The apparent Km for Fe2+ is 20 micrometer in the absence of copper and 9.5 micrometer in the presence of 20 micrometer CuCl2. The apparent Km for protoporphyrin is 50 micrometer, and it is unaltered by copper. Ferrochelatase has a single pH optimum of 7.50, and it is inhibited 50% by 20 micrometer heme. Certain divalent cations and sulfhydryl reagents also inhibit the enzyme.
Full text
PDF


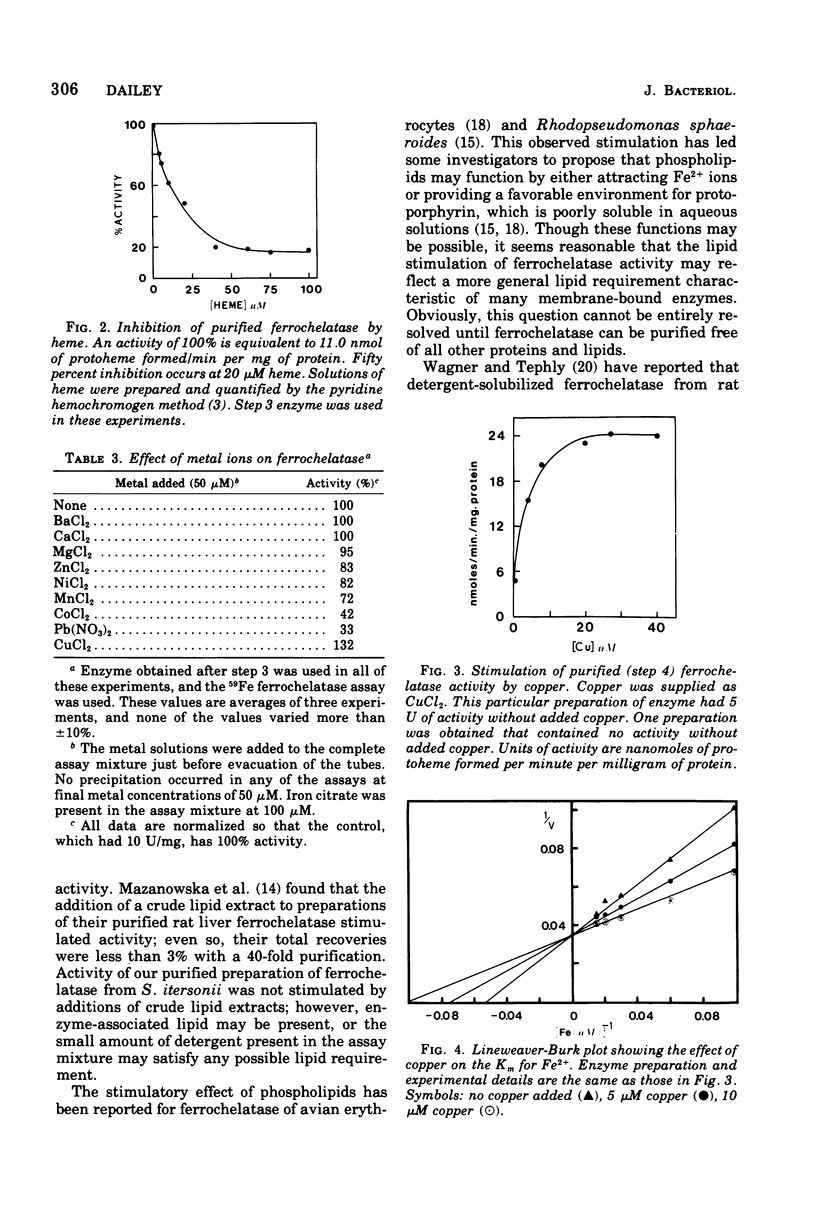

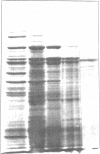
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes R., Connelly J. L., Jones O. T. The utilization of iron and its complexes by mammalian mitochondria. Biochem J. 1972 Aug;128(5):1043–1055. doi: 10.1042/bj1281043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D., Rittenberg B., Lascelles J. Cytochrome synthesis and its regulation in Spirillum itersonii. J Bacteriol. 1967 Nov;94(5):1648–1655. doi: 10.1128/jb.94.5.1648-1655.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A., Jr, Lascelles J. Ferrochelatase activity in wild-type and mutant strains of Spirillum itersonii. Solubilization with chaotropic reagents. Arch Biochem Biophys. 1974 Feb;160(2):523–529. doi: 10.1016/0003-9861(74)90429-9. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Jr, Lascelles J. Reduction of iron and synthesis of protoheme by Spirillum itersonii and other organisms. J Bacteriol. 1977 Feb;129(2):815–820. doi: 10.1128/jb.129.2.815-820.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap W. M., James G. W., 3rd, Hume D. M. Anemia and neutropenia caused by copper deficiency. Ann Intern Med. 1974 Apr;80(4):470–476. doi: 10.7326/0003-4819-80-4-470. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Jr, Levinthal C., Reeder R. H. Analysis of C14-labeled proteins by disc electrophoresis. Biochem Biophys Res Commun. 1965 Aug 16;20(4):393–399. doi: 10.1016/0006-291x(65)90589-9. [DOI] [PubMed] [Google Scholar]
- JOHNSON A., JONES O. G. ENZYMIC FORMATION OF HAEMS AND OTHER METALLOPORPHYRINS. Biochim Biophys Acta. 1964 Oct 9;93:171–173. doi: 10.1016/0304-4165(64)90273-9. [DOI] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. Ferrochelatase of Rhodopseudomonas spheroides. Biochem J. 1970 Sep;119(3):453–462. doi: 10.1042/bj1190453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. The structural organization of haem synthesis in rat liver mitochondria. Biochem J. 1969 Jul;113(3):507–514. doi: 10.1042/bj1130507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones O. T. Ferrochelatase of spinach chloroplasts. Biochem J. 1968 Mar;107(1):113–119. doi: 10.1042/bj1070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mazanowska A. M., Neuberger A., Tait G. H. Effect of lipids and organic solvents on the enzymic formation of zinc protoporphyrin and haem. Biochem J. 1966 Jan;98(1):117–127. doi: 10.1042/bj0980117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazanowska A., Dancewicz A. M., Malinowska T., Kowalski E. Chelation of iron and zinc by protoporphyrin catalyzed by mitochondrial preparations. Eur J Biochem. 1969 Feb;7(4):583–587. doi: 10.1111/j.1432-1033.1969.tb19646.x. [DOI] [PubMed] [Google Scholar]
- Porra R. J., Langman L., Young I. G., Gibson F. The role of ferric enterochelin esterase in enterochelin-mediated iron transport and ferrochelatase activity in Escherichia coli. Arch Biochem Biophys. 1972 Nov;153(1):74–78. doi: 10.1016/0003-9861(72)90422-5. [DOI] [PubMed] [Google Scholar]
- Reid M. S., Bieleski R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Anal Biochem. 1968 Mar;22(3):374–381. doi: 10.1016/0003-2697(68)90278-9. [DOI] [PubMed] [Google Scholar]
- Sawada H., Takeshita M., Sugita Y., Yoneyama Y. Effect of lipid on protoheme ferro-lyase. Biochim Biophys Acta. 1969 Mar 18;178(1):145–155. doi: 10.1016/0005-2744(69)90141-7. [DOI] [PubMed] [Google Scholar]
- Tait G. H. Aminolaevulinate synthetase of Micrococcus denitrificans. Purification and properties of the enzyme, and the effect of growth conditions on the enzyme activity in cells. Biochem J. 1973 Feb;131(2):389–403. doi: 10.1042/bj1310389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G. S., Tephly T. R. A possible role of copper in the regulation of heme biosynthesis through ferrochelatase. Adv Exp Med Biol. 1975;58(00):343–354. doi: 10.1007/978-1-4615-9026-2_24. [DOI] [PubMed] [Google Scholar]
- YONEYAMA Y., OHYAMA H., SUGITA Y., YOSHIKAWA H. Iron-chelating enzyme from duck erythrocytes. Biochim Biophys Acta. 1962 Aug 13;62:261–268. doi: 10.1016/0006-3002(62)90039-2. [DOI] [PubMed] [Google Scholar]
- Yoneyama Y., Tamai A., Yasuda T., Yoshikawa H. Iron-chelating enzyme from rat liver. Biochim Biophys Acta. 1965 Jul 29;105(1):100–105. doi: 10.1016/s0926-6593(65)80178-3. [DOI] [PubMed] [Google Scholar]