Abstract
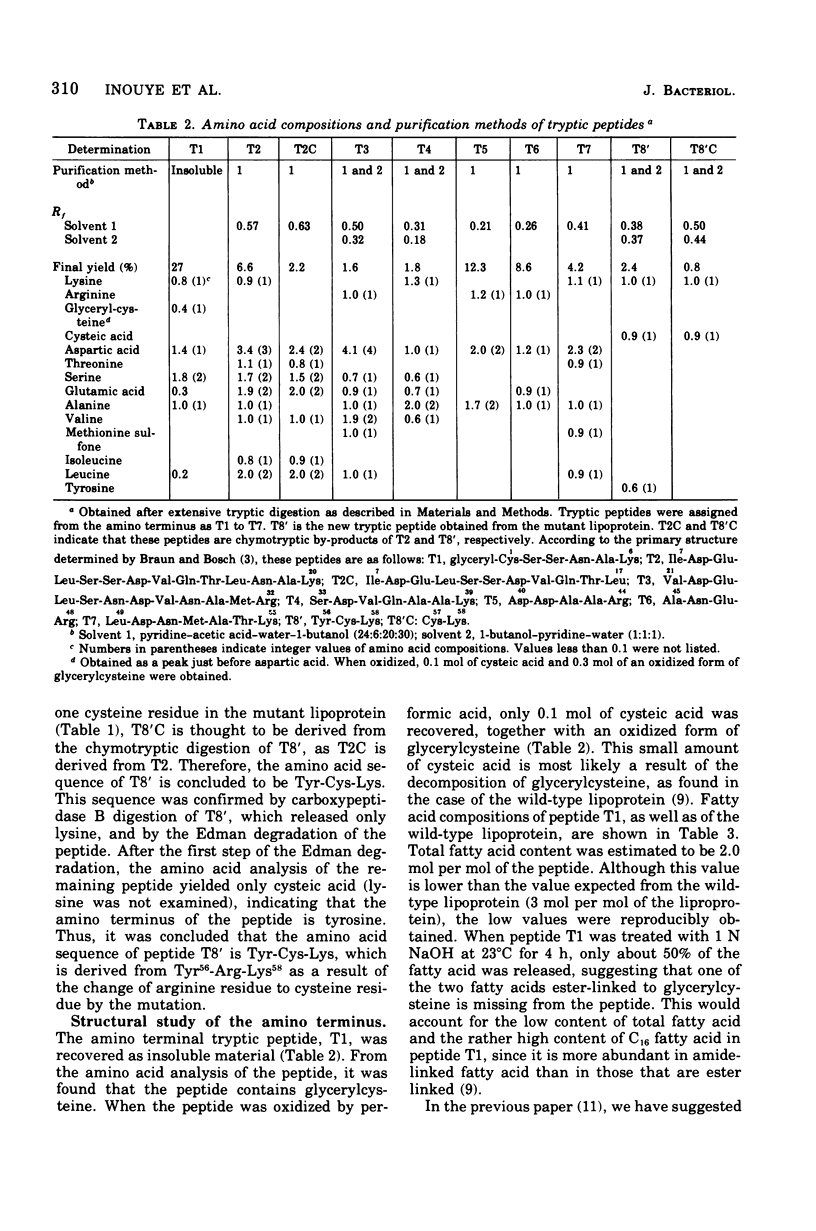
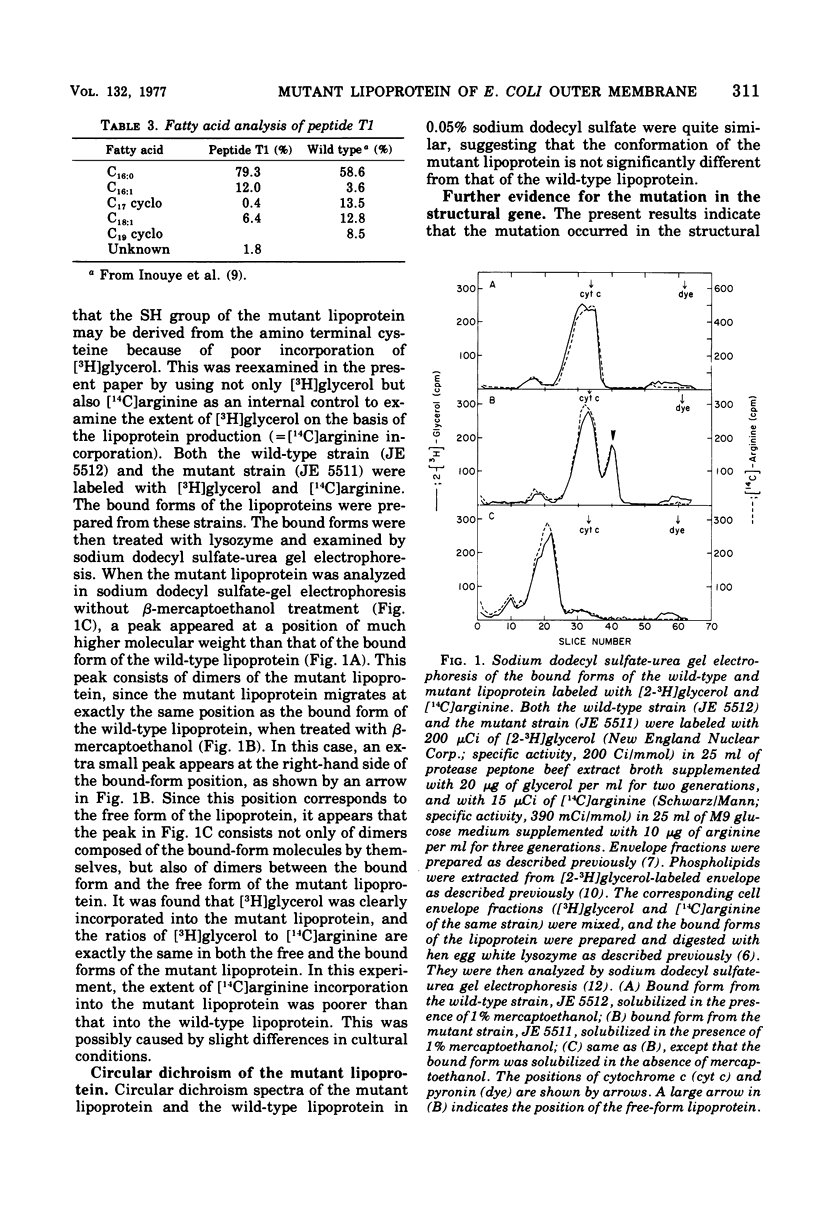
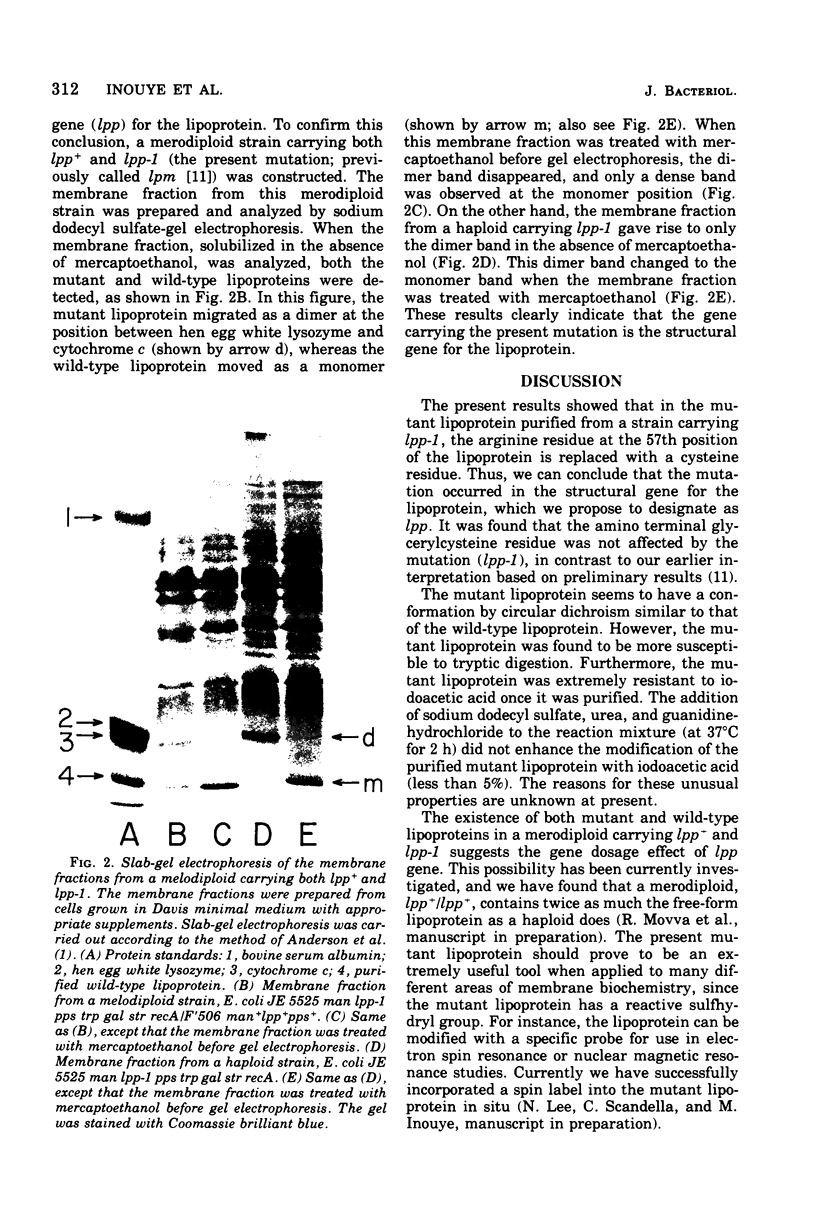
The primary structure of a mutant lipoprotein of the outer membrane of Escherichia coli was investigated. This mutant was previously described as a mutant that forms a dimer of the lipoprotein by an S-S bridge (H. Suzuki et al., J. Bacteriol. 127:1494-1501, 1976). The amino acid analysis of the mutant lipoprotein revealed that the mutant lipoprotein had an extra cysteine residue, with concomitant loss of an arginine residue. From the analysis of the mutant lipoprotein revealed that the mutant lipoprotein had an extra cysteine residue, with concomitant loss of an arginine residue. From the analysis of tryptic peptides, it was found that the arginine residue at position 57 was replaced with a cysteine residue. The amino terminal structure of the mutant lipoprotein was found to be glycerylcysteine, as in the case of the wild-type lipoprotein. The present results show that the mutation that was previously determined to map at 36.5 min on the E. coli chromosome occurred in the structure gene (lpp) for the lipoprotein. This was further confirmed by the fact that a merodiploid carrying both lpp+ and lpp produces not only the wild-type lipoprotein but also the mutant lipoprotein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Bosch V. Repetitive sequences in the murein-lipoprotein of the cell wall of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):970–974. doi: 10.1073/pnas.69.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim Biophys Acta. 1975 Oct 31;415(3):335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Inouye M., Arnheim N., Sternglanz R. Bacteriophage T7 lysozyme is an N-acetylmuramyl-L-alanine amidase. J Biol Chem. 1973 Oct 25;248(20):7247–7252. [PubMed] [Google Scholar]
- Inouye M., Guthrie J. P. A mutation which changes a membrane protein of E. coli. Proc Natl Acad Sci U S A. 1969 Nov;64(3):957–961. doi: 10.1073/pnas.64.3.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Okada Y., Tsugita A. The amino acid sequence of T4 phage lysozyme. I. Tryptic digestion. J Biol Chem. 1970 Jul 25;245(14):3439–3454. [PubMed] [Google Scholar]
- Inoyye S., Takeishi K., Lee N., DeMartini M., Hirashima A., Inouye M. Lipoprotein from the outer membrane of Escherichia coli: purification, paracrystallization, and some properties of its free form. J Bacteriol. 1976 Jul;127(1):555–563. doi: 10.1128/jb.127.1.555-563.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J., Wu H. C. Biosynthesis and assembly of envelope lipoprotein in a glycerol-requiring mutant of Salmonella typhimurium. J Bacteriol. 1976 Mar;125(3):892–904. doi: 10.1128/jb.125.3.892-904.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Iketani H., Campisi J., Hirashima A. Novel mutation that causes a structural change in a lipoprotein in the outer membrane of Escherichia coli. J Bacteriol. 1976 Sep;127(3):1494–1501. doi: 10.1128/jb.127.3.1494-1501.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]