Abstract
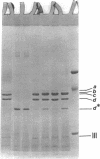
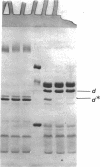
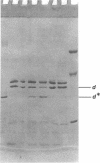
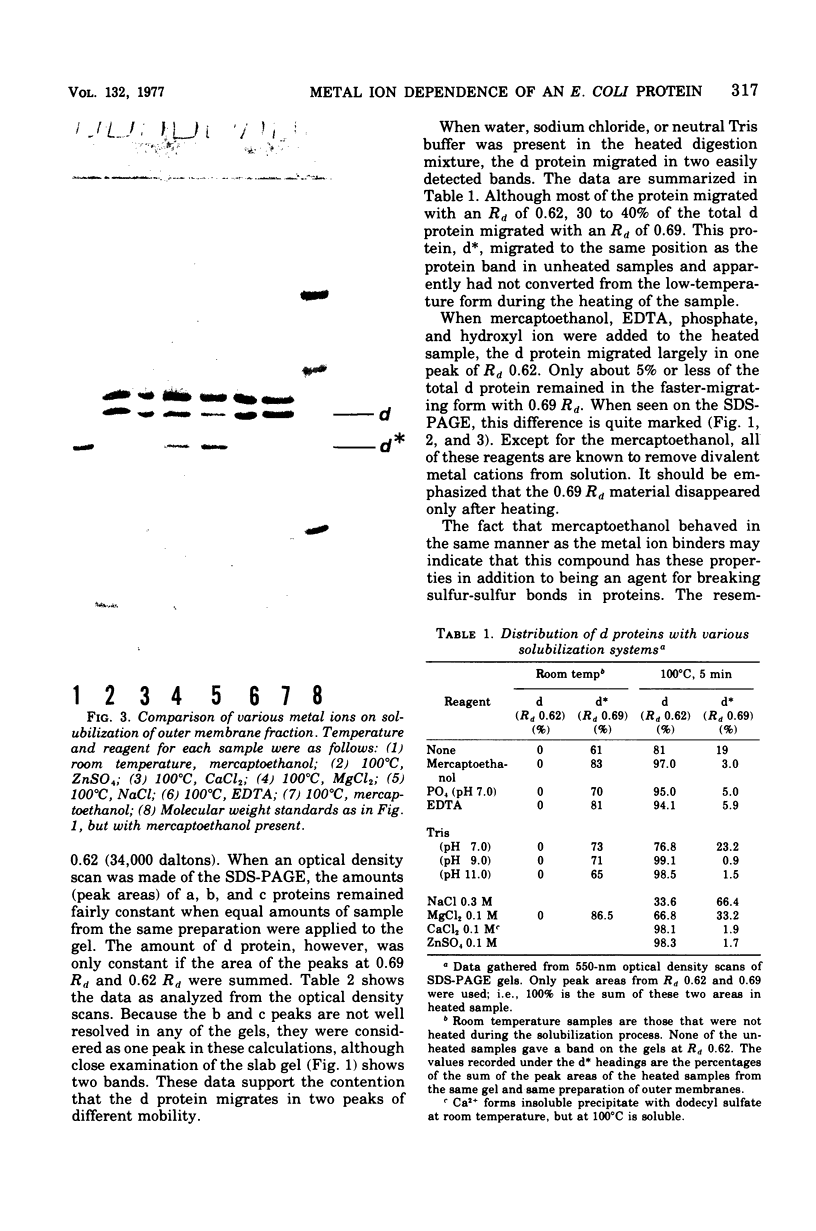
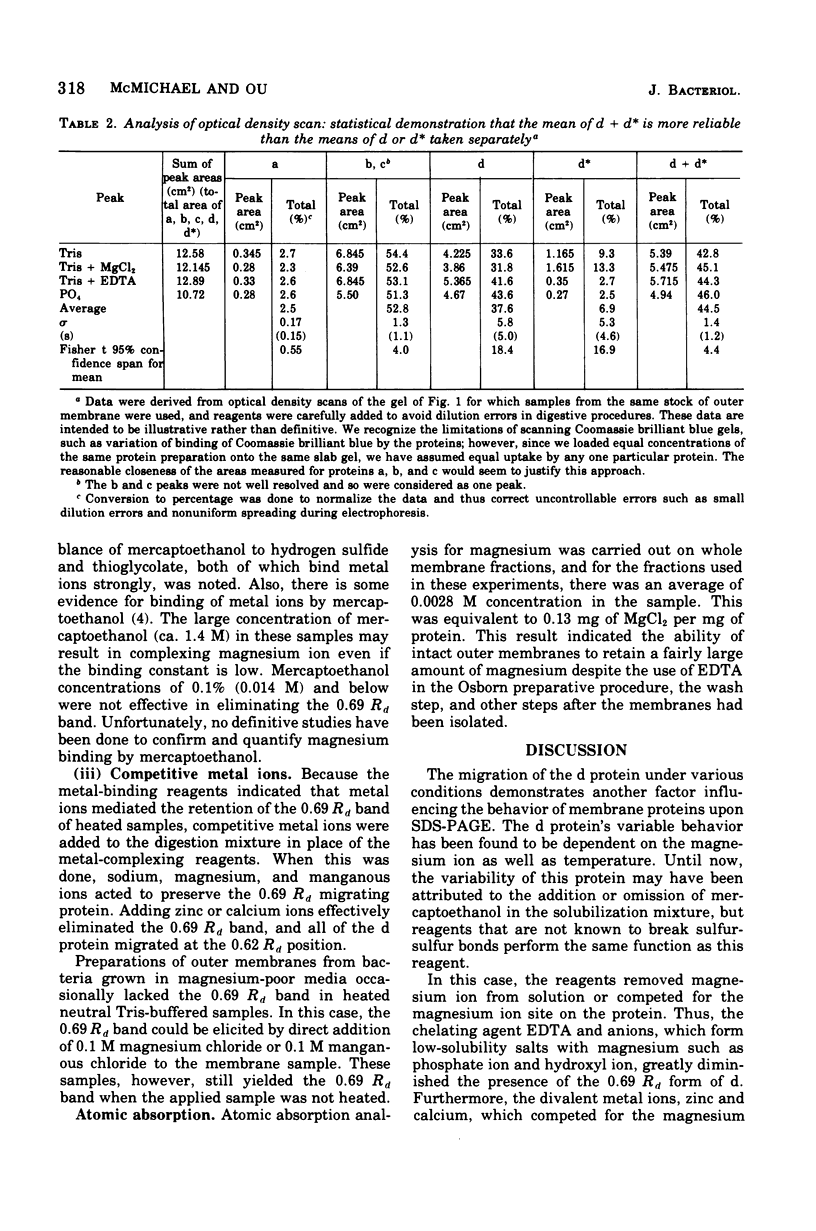
One heat-modifiable protein of Escherichia coli outer membrane does not completely change to the high-temperature form in the presence of magnesium ion in sodium dodecyl sulfate solution. When the metal ion complexing reagents ethylenediaminetetraacetic acid, phosphate ion, hydroxyl ion, or the competitive cations Zn2+ or Ca2+ are added to the sodium dodecyl sulfate-solubilized sample of outer membrane, and then the sample is heated to 100 degrees C and recooled to room temperature, the protein is almost completely converted to the high-temperature form. In control samples, or if sodium chloride, magnesium chloride, or manganous chloride are added to these samples and treated the same way, a large amount of the low-temperature form of the protein is preserved. beta-Mercaptoethanol additions gave the same results as the metal ion complexing reagents and may owe its activity in these solutions to metal-binding activity and not to its role as a reducing reagent. We concluded that magnesium ion may be involved with stabilization of the low-temperature form of the protein either by directly binding the magnesium or by mediating interaction with other components of the membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Bassford P. J., Jr, Diedrich D. L., Schnaitman C. L., Reeves P. Outer membrane proteins of Escherichia coli. VI. Protein alteration in bacteriophage-resistant mutants. J Bacteriol. 1977 Aug;131(2):608–622. doi: 10.1128/jb.131.2.608-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J., Barrett C. Characterization of Escherichia coli mutants tolerant to bacteriocin JF246: two new classes of tolerant mutants. J Bacteriol. 1973 Nov;116(2):885–892. doi: 10.1128/jb.116.2.885-892.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten W., Hindennach I., Henning U. The major proteins of the Escherichia coli outer cell envelope membrane. Characterization of proteins II* and III, comparison of all proteins. Eur J Biochem. 1975 Nov 1;59(1):215–221. doi: 10.1111/j.1432-1033.1975.tb02444.x. [DOI] [PubMed] [Google Scholar]
- Good N. E., Izawa S. Hydrogen ion buffers. Methods Enzymol. 1972;24:53–68. doi: 10.1016/0076-6879(72)24054-x. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Hanstein W. G. Solubilization of particulate proteins and nonelectrolytes by chaotropic agents. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1129–1136. doi: 10.1073/pnas.62.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning U., Hindennach I., Haller I. The major proteins of the Escherichia coli outer cell envelope membrane: evidence for the structural gene of protein II. FEBS Lett. 1976 Jan 1;61(1):46–48. doi: 10.1016/0014-5793(76)80168-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Manning P. A., Reeves P. Outer membrane of Escherichia coli K-12: differentiation of proteins 3A and 3B on acrylamide gels and further characterization of con (tolG) mutants. J Bacteriol. 1976 Sep;127(3):1070–1079. doi: 10.1128/jb.127.3.1070-1079.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T., Mizushima S. Separation by density gradient centrifugation of two types of membranes from spheroplast membrane of Escherichia coli K12. Biochim Biophys Acta. 1968 Jan 3;150(1):159–161. doi: 10.1016/0005-2736(68)90020-5. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Ou J. T. Effect of Zn2+ on bacterial conjugation: increase in ability of F- cells to form mating pairs. J Bacteriol. 1973 Aug;115(2):648–654. doi: 10.1128/jb.115.2.648-654.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithmeier R. A., Bragg P. D. Molecular characterization of a heat-modifiable protein from the outer membrane of Escherichia coli. Arch Biochem Biophys. 1977 Jan 30;178(2):527–534. doi: 10.1016/0003-9861(77)90223-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. 3. Evidence that the major protein of Escherichia coli O111 outer membrane consists of four distinct polypeptide species. J Bacteriol. 1974 May;118(2):442–453. doi: 10.1128/jb.118.2.442-453.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Clark D. Magnesium transport in Escherichia coli. J Biol Chem. 1971 Feb 10;246(3):569–576. [PubMed] [Google Scholar]
- Tomizawa J. GENETIC STRUCTURE OF RECOMBINANT CHROMOSOMES FORMED AFTER MATING IN ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1960 Jan;46(1):91–101. doi: 10.1073/pnas.46.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VONHIPPEL P. H., WONG K. Y. NEUTRAL SALTS: THE GENERALITY OF THEIR EFFECTS ON THE STABILITY OF MACROMOLECULAR CONFORMATIONS. Science. 1964 Aug 7;145(3632):577–580. doi: 10.1126/science.145.3632.577. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]