Abstract
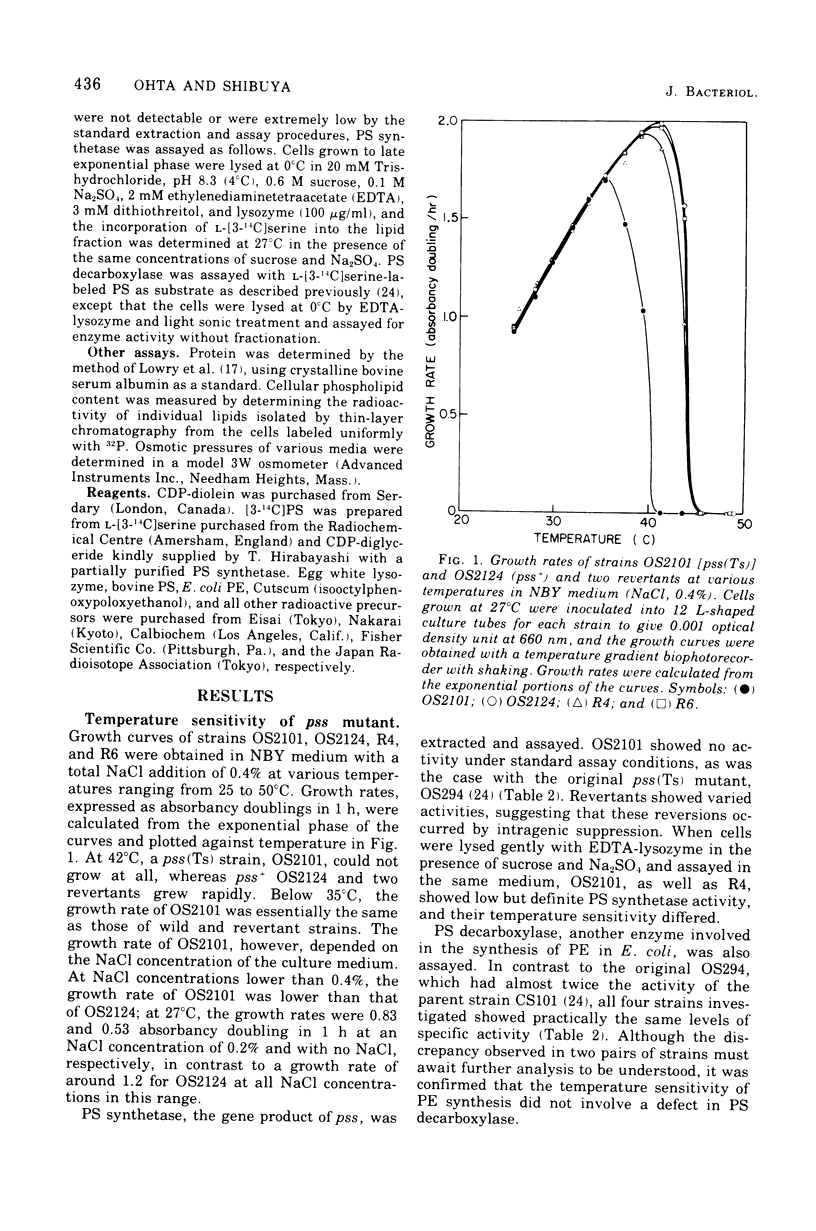
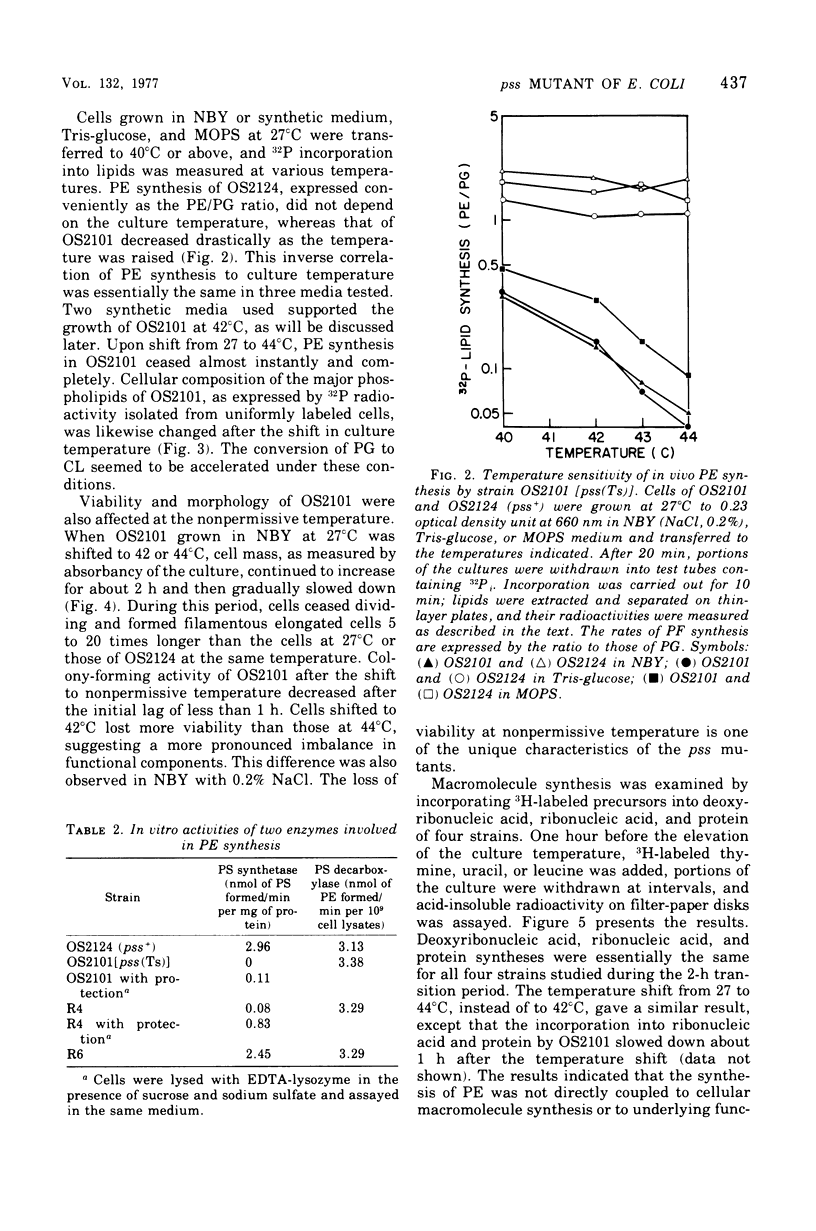
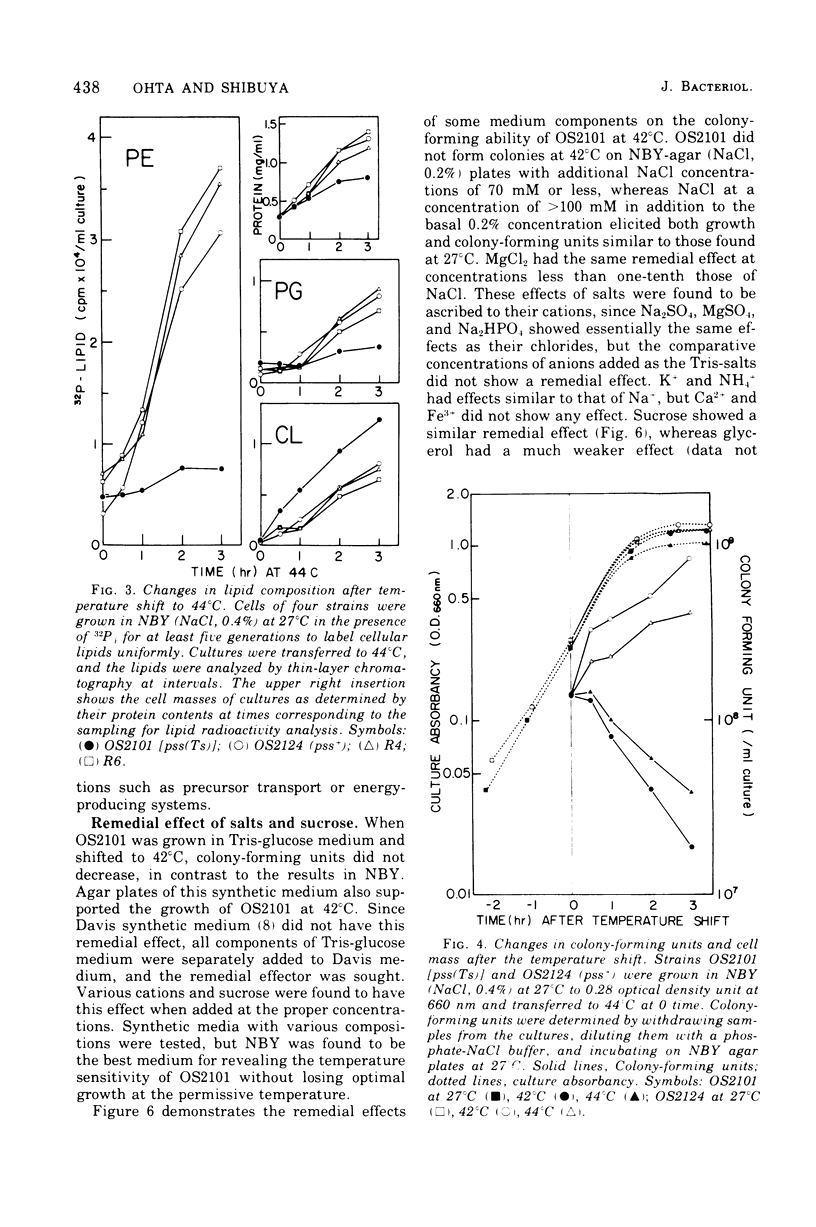
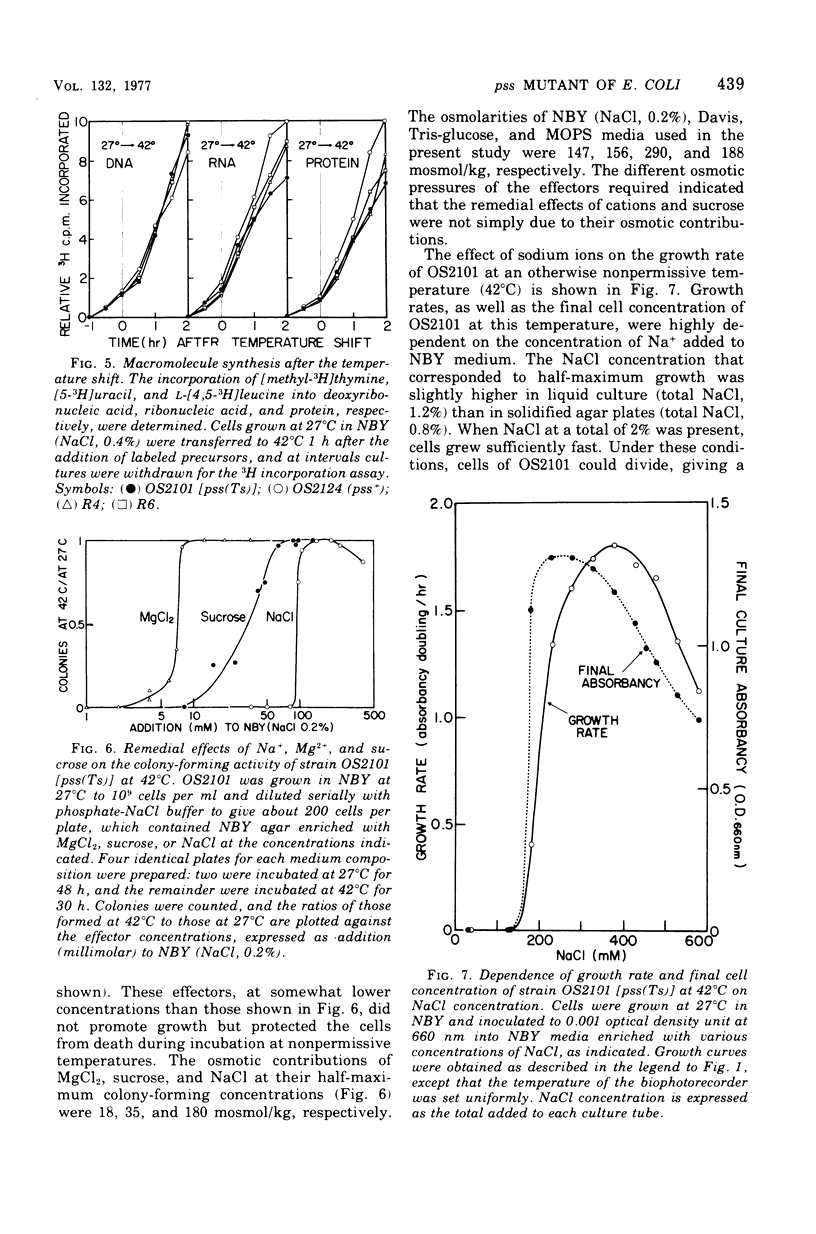
A pair of putatively isogenic pss(Ts) and pss+ (phosphatidylserine synthetase structural gene) strains was constructed and analyzed, together with the revertants, for the physiological consequences of cessation of the optimal synthesis of phosphatidylethanolamine (PE). Their in vivo and in vitro abilities to synthetize PE and the growth rates at different temperatures were determined. The rate of PE synthesis by OS2101 pss(Ts) was inversely related to the culture temperature. OS2101 in a low-salt broth medium stopped division and formed filamentous cells with declining viability upon the elevation of culture temperature from 27 to 42 or 44 degrees C, whereas the syntheses of deoxyribonucleic acid, ribonucleic acid, and protein were not affected. Proper concentrations of cations such as Na+, K+, NH4+, and Mg2+ or of sucrose could remedy the division and growth of OS2101 at the restrictive temperature without restoring normal PE synthesis. A remedial effect other than osmotic protection of these effectors and an adaptive regulatory mechanism for PE formation are suggested.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akamatsu Y. Osmotic stabilization of unsaturated fatty acid auxotrophs of Escherichia coli. J Biochem. 1974 Sep;76(3):553–561. doi: 10.1093/oxfordjournals.jbchem.a130599. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsky A. Z., Armstrong J. B. Osmotic reversal of temperature sensitivity in Escherichia coli. J Bacteriol. 1973 Jan;113(1):76–81. doi: 10.1128/jb.113.1.76-81.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman J. H., Steenbakkers J. F. Effect of the osmotic pressure of the growth medium on fabB mutants of Escherichia coli. J Bacteriol. 1974 Mar;117(3):971–977. doi: 10.1128/jb.117.3.971-977.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr A new method for selection of Escherichia coli mutants defective in membrane lipid synthesis. Nat New Biol. 1972 Nov 1;240(96):21–22. doi: 10.1038/newbio240021a0. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr Phospholipid alterations during growth of Escherichia coli. J Bacteriol. 1968 Jun;95(6):2054–2061. doi: 10.1128/jb.95.6.2054-2061.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- Egan A. F., Russell R. R. Conditional mutations affecting the cell envelope of Escherichia coli K-12. Genet Res. 1973 Apr;21(2):139–152. doi: 10.1017/s001667230001332x. [DOI] [PubMed] [Google Scholar]
- Hawrot E., Kennedy E. P. Biogenesis of membrane lipids: mutants of Escherichia coli with temperature-sensitive phosphatidylserine decarboxylase. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1112–1116. doi: 10.1073/pnas.72.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg C. B., Kennedy E. P. Mechanism of the enzymatic synthesis of cardiolipin in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Mar;69(3):648–651. doi: 10.1073/pnas.69.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K., Imagawa T., Amano T. Temperature-sensitive mutants of Escherichia coli B which can grow in high-osmotic medium at the nonpermissive temperature. Biken J. 1974 Dec;17(4):149–159. [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. I. METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI B. J Biol Chem. 1963 Sep;238:2919–2922. [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. II. BIOSYNTHESIS OF PHOSPHOLIPIDS IN ESCHERICHIA COLI. J Biol Chem. 1964 Jun;239:1720–1726. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larson T. J., Dowhan W. Ribosomal-associated phosphatidylserine synthetase from Escherichia coli: purification by substrate-specific elution from phosphocellulose using cytidine 5'-diphospho-1,2-diacyl-sn-glycerol. Biochemistry. 1976 Nov 30;15(24):5212–5218. doi: 10.1021/bi00669a003. [DOI] [PubMed] [Google Scholar]
- Lusk J. E., Kennedy E. P. Altered phospholipid metabolism in a sodium-sensitive mutant of Escherichia coli. J Bacteriol. 1972 Mar;109(3):1034–1046. doi: 10.1128/jb.109.3.1034-1046.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel G., Di Savino D., Starka J. Phospholipid composition and phenotypic correction of an envC division mutant of Escherichia coli. J Bacteriol. 1977 Jan;129(1):145–150. doi: 10.1128/jb.129.1.145-150.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn W. D., Tropp B. E. Effects of phenethyl alcohol on phospholipid metabolism in Escherichia coli. J Bacteriol. 1972 Jan;109(1):162–168. doi: 10.1128/jb.109.1.162-168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota A., Shibuya I., Maruo B., Ishinaga M., Kito M. An extremely labile phosphatidylserine synthetase of an Escherichia coli mutant with the temperature-sensitive formation of phosphatidylethanolamine. Biochim Biophys Acta. 1974 Jun 26;348(3):449–454. [PubMed] [Google Scholar]
- Raetz C. R. Isolation of Escherichia coli mutants defective in enzymes of membrane lipid synthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2274–2278. doi: 10.1073/pnas.72.6.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Phosphatidylserine synthetase mutants of Escherichia coli. Genetic mapping and membrane phospholipid composition. J Biol Chem. 1976 Jun 10;251(11):3242–3249. [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Effet des sels et autres composés sur le phénotype de mutants thermosensibles de Escherichia coli. Ann Microbiol (Paris) 1973 Jan;124(1):29–43. [PubMed] [Google Scholar]
- Russell R. R. Temperature-sensitive osmotic remedial mutants of Escherichia coli. J Bacteriol. 1972 Nov;112(2):661–665. doi: 10.1128/jb.112.2.661-665.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert D. F. Genetic modification of membrane lipid. Annu Rev Biochem. 1975;44:315–339. doi: 10.1146/annurev.bi.44.070175.001531. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., de Kruyff B., Ververgaert P. H., Tocanne J. F., van Deenen L. L. The influence of pH, Ca2+ and protein on the thermotropic behaviour of the negatively charged phospholipid, phosphatidylglycerol. Biochim Biophys Acta. 1974 Mar 29;339(3):432–437. doi: 10.1016/0005-2736(74)90171-0. [DOI] [PubMed] [Google Scholar]



