Abstract
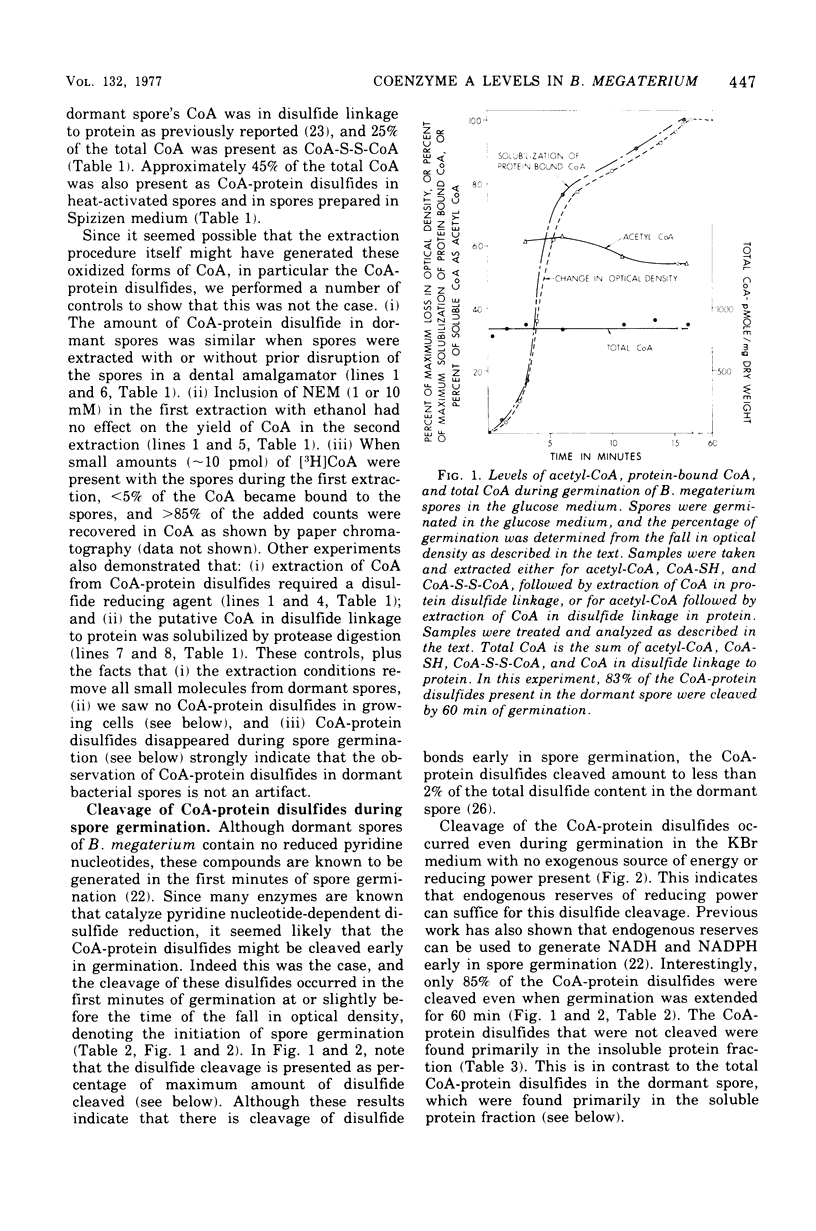
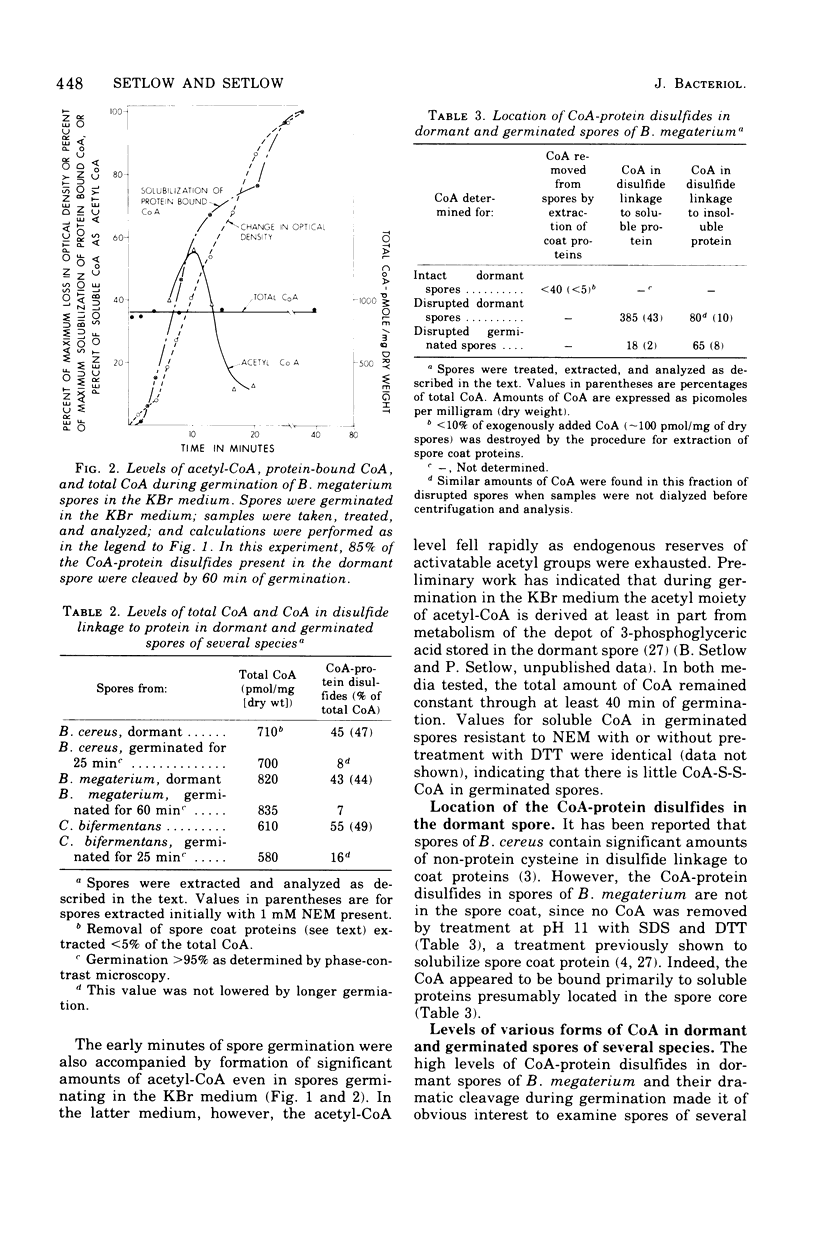
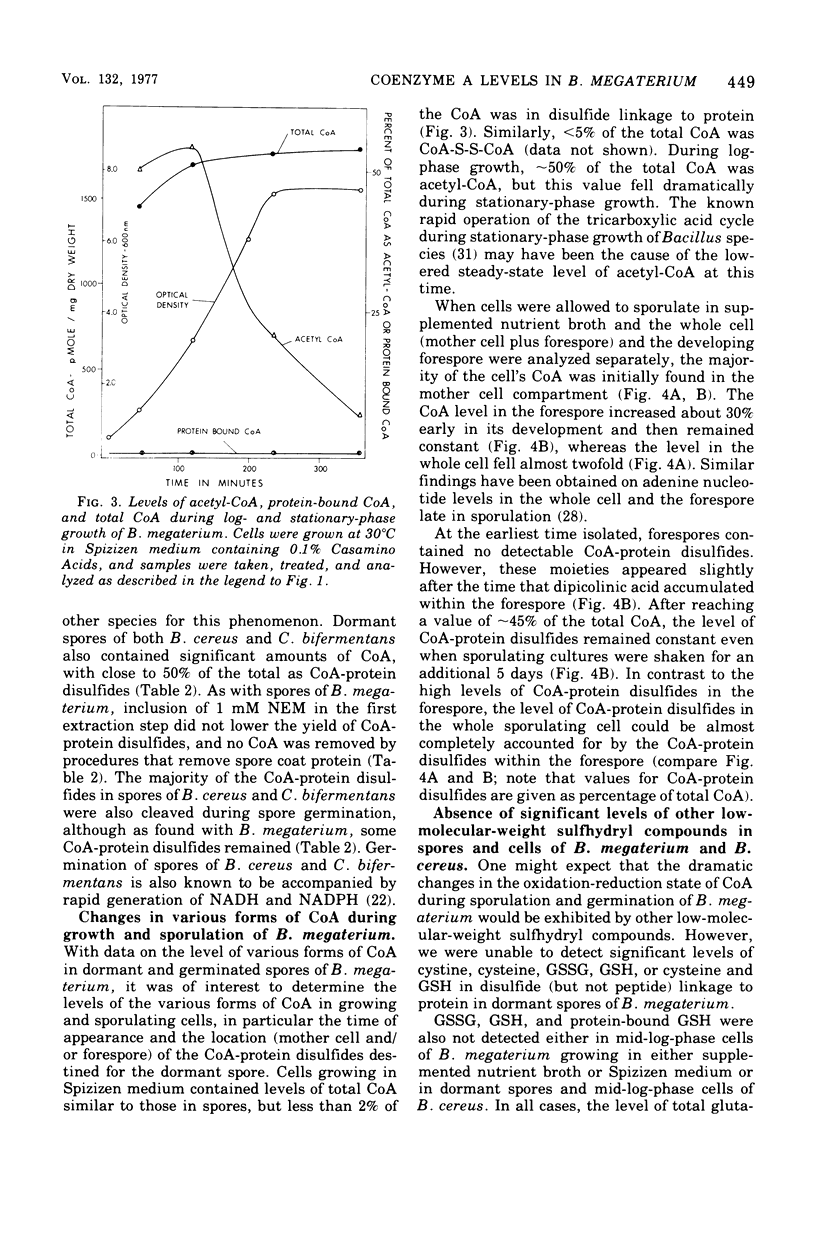
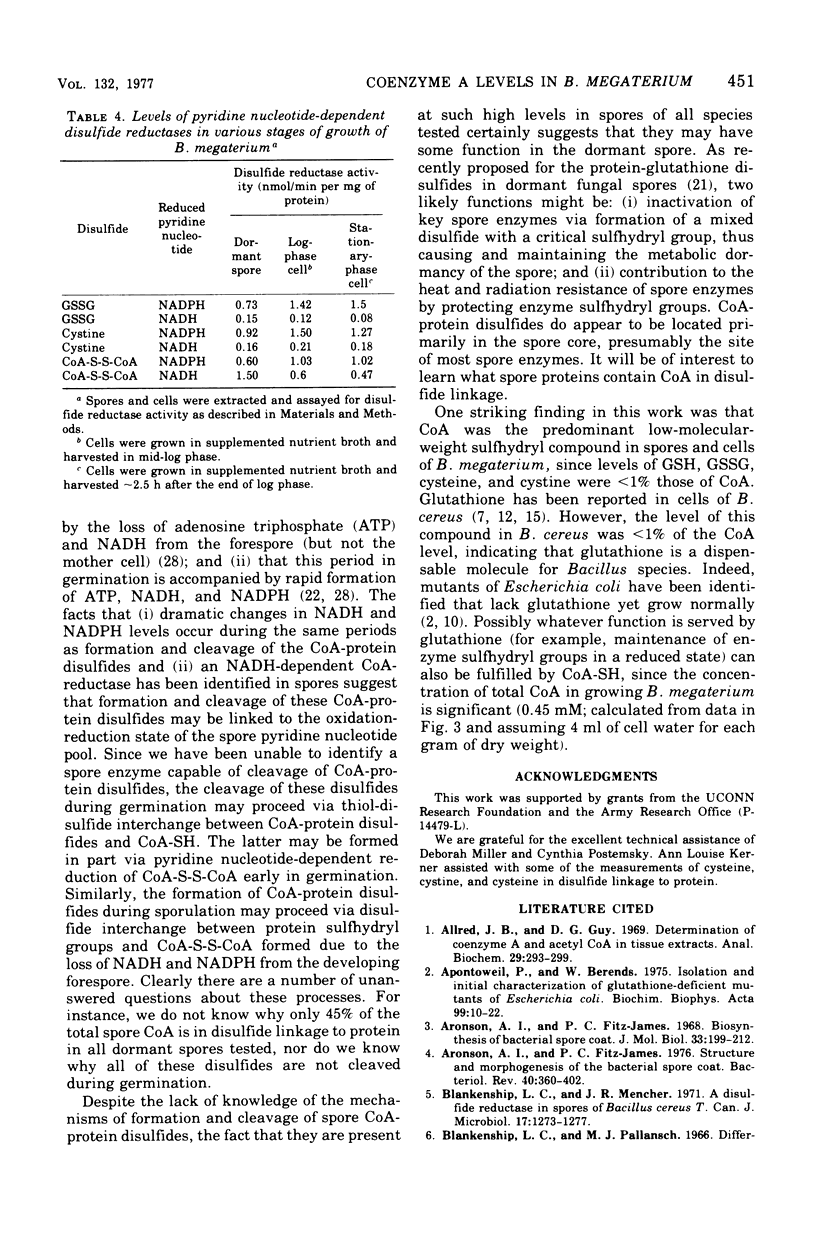
Dormant spores of Bacillus megaterium were found to contain approximately 850 pmol of coenzyme A (CoA) per milligram of dry weight. Of this total, less than 1.5% was acetyl-CoA, 25% was CoA-disulfide, 43% was in disulfide linkage to protein, and the remainder was the free thiol. Dormand spores of Bacillus cereus and Clostridium bifermentans contained 700 and 600 pmol of CoA per milligram of dry weight, respectively; in both species approximately 45% of the CoA 45% of the CoA was in disulfide linkage to protein. During germination of spores of all three species, greater than 75% of the CoA-protein disulfides were cleaved. In B. megaterium, cleavage of these disulfides during spore germination did not require exogenous metabolites and occurred at about the same time as the initiation of germination. Much of the CoA was converted to acetyl-CoA at this time. Dormant spores also contained reduced nicotinamide adenine dinucleotide-dependent CoA-disulfide reductase at levels higher than those in other stages of growth. The level of total CoA in the growing cells was two- to three-fold higher than in spores. This level remained constant throughout growth and sporulation, but less than 2% of the total cellular CoA was in disulfide linkage to protein until late in sporulation. The CoA-protein disulfides accumulated exclusively within the developing spore at about the time when dipicolinic acid was accumulated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred J. B., Guy D. G. Determination of coenzyme A and acetyl CoA in tissue extracts. Anal Biochem. 1969 May;29(2):293–299. doi: 10.1016/0003-2697(69)90312-1. [DOI] [PubMed] [Google Scholar]
- Apontoweil P., Berends W. Isolation and initial characterization of glutathione-deficient mutants of Escherichia coli K 12. Biochim Biophys Acta. 1975 Jul 14;399(1):10–22. doi: 10.1016/0304-4165(75)90206-8. [DOI] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. C. Biosynthesis of bacterial spore coats. J Mol Biol. 1968 Apr 14;33(1):199–212. doi: 10.1016/0022-2836(68)90288-x. [DOI] [PubMed] [Google Scholar]
- Aronson A. I., Fitz-James P. Structure and morphogenesis of the bacterial spore coat. Bacteriol Rev. 1976 Jun;40(2):360–402. doi: 10.1128/br.40.2.360-402.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship L. C., Mencher J. R. A disulfide reductase in spores of Bacillus cereus T. Can J Microbiol. 1971 Oct;17(10):1273–1277. doi: 10.1139/m71-204. [DOI] [PubMed] [Google Scholar]
- Blankenship L. C., Pallansch M. J. Differential analysis of sulfhydryl and disulfide groups of intact spores. J Bacteriol. 1966 Dec;92(6):1615–1617. doi: 10.1128/jb.92.6.1615-1617.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCH B. D., HALVORSON H. Intermediate metabolism of aerobic spores. I. Activation of glucose oxidation in spores of Bacillus cereus var terminalis. J Bacteriol. 1957 Apr;73(4):470–476. doi: 10.1128/jb.73.4.470-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. M., Aronson A. I., Holt S. C. Role of glutathione in the morphogenesis of the bacterial spore coat. J Bacteriol. 1973 Mar;113(3):1134–1143. doi: 10.1128/jb.113.3.1134-1143.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey R. C., Brody S., Mikolajczyk S. D. Changes in the glutathione thiol-disulfide status of Neurospora crassa conidia during germination and aging. J Bacteriol. 1975 Jan;121(1):144–151. doi: 10.1128/jb.121.1.144-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J. A., Warner H. R. Isolation of an Escherichia coli mutant deficient in glutathione synthesis. J Bacteriol. 1975 Oct;124(1):140–148. doi: 10.1128/jb.124.1.140-148.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klubes P., Cerna I. Actions of 2-methyl-1,4-naphthoquinone in Bacillus cereus. Biochem Pharmacol. 1972 Jan 15;21(2):249–261. doi: 10.1016/0006-2952(72)90275-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandel H. G., Latimer R. G., Riis M. The actions of thioguanine in Bacillus cereus. Biochem Pharmacol. 1965 May;14(5):661–682. doi: 10.1016/0006-2952(65)90084-5. [DOI] [PubMed] [Google Scholar]
- Rotman Y., Fields M. L. A modified reagent for dipicolinic acid analysis. Anal Biochem. 1968 Jan;22(1):168–168. doi: 10.1016/0003-2697(68)90272-8. [DOI] [PubMed] [Google Scholar]
- SACKS L. E., BAILEY G. F. DRY RUPTURE OF BACTERIAL SPORES. J Bacteriol. 1963 Mar;85:720–721. doi: 10.1128/jb.85.3.720-721.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmit J. C., Brody S. Biochemical genetics of Neurospora crassa conidial germination. Bacteriol Rev. 1976 Mar;40(1):1–41. doi: 10.1128/br.40.1.1-41.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Levels of oxidized and reduced pyridine nucleotides in dormant spores and during growth, sporulation, and spore germination of Bacillus megaterium. J Bacteriol. 1977 Feb;129(2):857–865. doi: 10.1128/jb.129.2.857-865.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Most of the coenzyme A in dormant spores of Bacillus megaterium is in disulfide linkage to protein. Biochem Biophys Res Commun. 1977 Mar 7;75(1):45–50. doi: 10.1016/0006-291x(77)91286-4. [DOI] [PubMed] [Google Scholar]
- Setlow P. Identification and localization of the major proteins degraded during germination of Bacillus megaterium spores. J Biol Chem. 1975 Oct 25;250(20):8159–8167. [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem. 1970 Jul 25;245(14):3637–3644. [PubMed] [Google Scholar]
- Setlow P. Protease and peptidase activities in growing and sporulating cells and dormant spores of Bacillus megaterium. J Bacteriol. 1975 May;122(2):642–649. doi: 10.1128/jb.122.2.642-649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Setlow B., Setlow P. Levels of small molecules and enzymes in the mother cell compartment and the forespore of sporulating Bacillus megaterium. J Bacteriol. 1977 Jun;130(3):1130–1138. doi: 10.1128/jb.130.3.1130-1138.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]



