Abstract
Rationale: Costimulatory molecules, including the CD40–CD154 and CD80/86–CD28 dyads, play a prominent role in regulating inflammation in the adaptive immune response. Studies from our group and others suggest a potentially important role for these costimulatory cascades in innate immunity as well.
Objectives: To determine the role of CD80/86 alone and in combination with CD40 in lethal polymicrobial sepsis in mice and humans.
Methods: The murine cecal ligation and puncture (CLP) model was used to determine the role of CD80/86 alone and in combination with CD40 using wild-type mice, CD80/86−/− mice, and novel CD40/80/86−/− mice. Expression of cell-bound and soluble costimulatory molecules was assessed in humans via ELISA and flow cytometry.
Measurements and Main Results: Lethal CLP was associated with up-regulation of CD40 and CD80/86 and their respective ligands CD28 and CD154 on innate effector cells. Blockade or deletion of CD80/86 attenuated mortality and inflammatory cytokine production during CLP. CD40/80/86−/− mice exhibited further reductions in mortality, lung injury, and inflammatory cytokine production compared with CD80/86−/− mice. Finally, humans with sepsis had increased monocyte expression of CD40 and CD80 compared with healthy control subjects; with higher levels in subjects requiring vasopressor support. Levels of soluble CD28 and CD154 were significantly higher in patients who died compared with those who lived.
Conclusions: These data demonstrate a central role for CD40 and CD80/86 in the innate immune response and suggest that combined inhibition of CD40 and CD80/86 may improve mortality in sepsis. Expression of costimulatory molecules may serve as biomarkers for outcome in septic patients.
Keywords: costimulation, innate immunity, sepsis
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
At present, there is little known about the role of costimulatory molecules in regulating inflammation in severe sepsis.
What This Study Adds to This Field
This study suggests that costimulatory molecules have an important role in regulating inflammation and mortality in lethal sepsis, and provides support for a paradigm in which costimulatory molecules act synergistically to regulate severe inflammatory responses to bacterial infection.
Sepsis, defined as the systemic inflammatory response to infection, affects more than 700,000 people per year in the United States, resulting in 250,000 deaths and a net cost of $17 billion per year (1, 2). Sepsis is the leading cause of death in the intensive care unit and the tenth leading cause of death overall. Despite appropriate antibiotics and advances in therapy, mortality exceeds 25% (1, 2).
The host inflammatory response in sepsis is complex, involving an interplay between pro- and antiinflammatory mediators, both of which have divergent effects on pathogen control, systemic inflammation, and susceptibility to recurrent infection. Numerous attempts to alter this balance in sepsis through modulation of individual cytokines have been unsuccessful (3–6). This suggests that once the inflammatory cascade has been initiated, individual cytokines are part of a redundant circuit that proceeds independently of any single component. As a consequence, investigators have focused on early receptors/mediators that may be a first step in innate inflammation. As macrophages play a critical role for production of inflammatory cytokines/chemokines in sepsis, a number of studies have focused on pathways of macrophage activation (7–11).
One potential mechanism for macrophage activation is through engagement of costimulatory receptors. These include CD40 and CD80/86 (B7-1/B7-2) with their respective ligands CD154 and CD28. These interactions are critical for T-cell activation and inflammation in models of adaptive immunity in vitro and in vivo (12–14). Data from our laboratory suggest that neutrophils (PMNs) can activate macrophages in vitro via engagement of the costimulatory receptors CD40 and CD80/86, establishing a potential role for these cascades in regulating inflammation in the innate immune response in vivo (11).
Prior investigations have already begun to define a potential role for these costimulatory receptors in the innate immune response in vivo. CD40−/− mice are resistant to hyperoxic and LPS-induced lung injury and CD40 is up-regulated on peripheral blood mononuclear cells in septic patients (15–17). Our laboratory documented improved survival and attenuations in remote organ injury and cytokine production, specifically IL-12, in CD40−/− mice subjected to polymicrobial sepsis (18). Evidence also suggests an important role for the CD80/86–CD28 system in innate immunity in vivo. CD28 is integral to abdominal abscess formation and control of inflammation in superantigen shock in mice (19–21). Polymicrobial sepsis is associated with altered CD86 expression on Kupffer cells and peritoneal macrophages, with CD86−/− mice having attenuated IL-10 production after cecal ligation and puncture (CLP) (22, 23). Finally, one report documents overwhelming inflammation and death in humans treated with an agonistic CD28 monoclonal antibody (mAb) (24).
Inhibition of individual costimulatory cascades has been used successfully in animals and more recently in humans to partially control inflammation in autoimmune disease and graft rejection (23, 25, 26). However, numerous studies show that simultaneous inhibition of multiple costimulatory cascades has an additive/synergistic effect on immunomodulation. Specifically, simultaneous blockade of CD154 and CD28 results in additional xenograft tolerance in mice compared with either alone, with similar results in models of murine lupus and experimental allergic encephalitis (12–14, 27). However, to date, no data exist about the effect of this strategy on regulating inflammation in models of innate immunity, such as lethal polymicrobial sepsis.
Because of our observation that simultaneous blockade of CD40 and CD80/86 can attenuate PMN-mediated activation of macrophages in vitro (11), we tested whether CD40 and CD80/86 are induced in murine polymicrobial sepsis after CLP and whether theses receptors act synergistically to regulate mortality and inflammation in this model. Finally, we investigated the relevance of these findings for humans by assessing whether the expression of these costimulatory molecules could serve as biomarkers for disease severity and/or outcome in patients with sepsis. Some of the results of these studies have been reported in abstract form (28–30).
METHODS
Mice
All studies were approved by the Institutional Animal Care and Use Committee of Oregon Health and Science University (Portland, OR). Six- to 10-week-old female C57BL/6 mice (stock no. 00664) and CD80/86−/− mice (stock no. 003610) were obtained from Jackson Laboratory (Bar Harbor, ME). CD40−/− mice (stock no. 002928; Jackson Laboratory) and CD80/86−/− mice were crossbred to create CD40/80/86−/− mice. CD40/80/86−/− deletion was confirmed by polymerase chain reaction and fluorescence-activated flow cytometry. CD40/80/86−/− mice have a normal lifespan and breeding pattern. Mice were housed under specific pathogen–free conditions. All experiments were approved by the Oregon Health and Science University Animal Care and Use Committee. CLP was performed as previously described (18). Briefly, mice were anesthetized with 2.5% isoflurane and underwent CLP with a 19-gauge needle. Mice received 1 ml of 0.9% saline subcutaneously for resuscitation. At the specified times, plasma, bronchoalveolar lavage (BAL) fluid, and peritoneal lavage (PL) fluid samples (3 ml) were collected from the mice as previously described (18, 31). For survival experiments, mice were monitored for a total of 14 days. For antibody inhibition, 200 μg of anti-CD86 (GL-1; eBioscience, San Diego, CA) and anti-CD80 (16-10A1; eBioscience) were injected intraperitoneally 4 hours before surgery.
Cytokines and tissue myeloperoxidase (MPO) were determined with commercially available immunoassays (R&D Systems [Minneapolis, MN] and Bender MedSystems [Vienna, Austria] [soluble CD28 only]; Hycult Biotechnology [Uden, The Netherlands] [MPO]) and performed according to the manufacturer's specifications. NF-κB DNA binding was determined by DNA-binding ELISA (Active Motif, Carlsbad, CA). Pulmonary capillary leakage was determined with Evan's blue dye as previously described (18). Quantitative cultures of plasma and peritoneal lavage samples were performed, using serial dilutions plated onto LB agar plates. Murine peritoneal PMNs were obtained from wild-type mice treated intraperitoneally with 1 cm3 of 3% thioglycollate (Sigma-Aldrich, St. Louis, MO) for 4 hours. Mice were lavaged and PMNs were washed twice in sterile phosphate-buffered saline before use. PMNs (5 × 105) were injected into the peritoneal cavity of wild-type or knockout mice for 4 hours, and then mice were killed and PL fluid and plasma were collected for cytokine analysis.
Flow Cytometry
Flow cytometry was performed as previously described (18, 32). Splenocytes, whole blood, and peritoneal lavage fluid were collected and 1 × 106 cells were incubated with 100 μl of FcBlock (murine cells only; BD Biosciences, San Jose, CA) for 15 minutes and then labeled with the following antibodies: CD40, CD154, CD80, CD86, CD4, CD8, LY6g (murine), CD11b, F4/80 (murine), CD28, and CD14 (human) at optimal concentration for 45 minutes in the dark. Red blood cells were lysed with RBC lysis buffer and then the cells were fixed with 0.1% paraformaldehyde and analyzed on a BD LSR II eight-color analyzer (BD Biosciences) with FloJo software (Tree Star, Ashland, OR). All reagents were purchased from BD Biosciences. BD CompBeads (BD Biosciences) were used to calibrate the instrument before each use. PMNs were identified by forward scatter/side scatter (FSC/SSC) characteristics as Ly6G+ (mice only); mononuclear cells were identified by FSC/SSC as CD11b+ and F4/80+ (mice) or as CD11b+ and CD14+ (human); T cells were identified by FSC/SSC and further subgrouped by CD4+ and CD8+ labeling. Isotype antibody–labeled cells were used to control for nonspecific staining.
Human Studies
All studies were approved by the institutional review boards of New York University (New York, NY) and Oregon Health and Science University. All patients meeting American College of Chest Physicians/Society of Critical Care Medicine criteria for sepsis in the first 24 hours of intensive care unit stay were eligible for inclusion (33). Septic shock was defined as sepsis with a need for treatment with vasopressors. Patients were excluded for the following reasons: the presence of a Do Not Resuscitate order or decision to institute comfort care measures, hemoglobin less than 7 g/dl, or the presence of active bleeding requiring more than 2 units of packed red blood cells. After obtaining informed consent, 25 cm3 of blood was collected into glass (serum) or ethylenediaminetetraacetic acid–coated tubes (platelet-poor plasma) on Days 1, 3–5, 7, and 14 or until death or hospital discharge. After obtaining preliminary data on soluble mediators, and optimization of labeling protocols, all subsequent subjects had additional blood collected for flow cytometry. Clinical data including SOFA (Sequential Organ Failure Assessment) and APACHE (Acute Physiology and Chronic Health Evaluation) scores were recorded at the time of each blood draw. Patients were monitored for 28 days or until death or hospital discharge.
Statistics
Survival was analyzed by Kaplan-Meier analysis. All comparisons between groups were performed by Mann-Whitney or Kruskal-Wallis analysis of variance, depending on the number of groups analyzed. For multigroup analysis, intergroup comparisons were performed by Dunn's test. Correlations were made by Spearman's test. All statistics were done with Prism 4.0 (GraphPad Software, San Diego, CA).
RESULTS
Expression of Costimulatory Molecules in Murine Sepsis
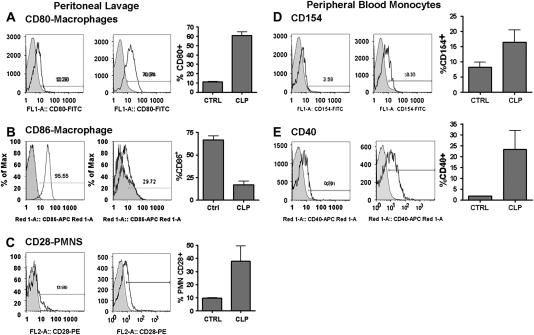
We first determined whether expression of the CD40 and CD80/86 systems was altered in polymicrobial sepsis induced by CLP. CD80 was up-regulated on mononuclear cells in PL fluid (Figure 1A) after CLP with similar results in peripheral blood and spleen (data not shown). In contrast, CD86 was differentially regulated in the different tissue compartments. Similar to CD80, CD86 was up-regulated in spleen (47.8 vs. 16.3%; P < 0.01) and peripheral blood (data not shown). However, as in previous reports (34), CD86 was decreased in PL fluid 18 hours after CLP (Figure 1B) compared with control subjects. Of note, there was little alteration in expression of either molecule after sublethal CLP (30-gauge needle) (data not shown). There was no difference in CD4+, CD8+ lymphocyte or PMN expression of CD80 or CD86 in any tissue compartment tested (data not shown). We next investigated the potential cellular sources of CD28. PMNs (Ly6G+) from control mice showed little expression of CD28 in any tissue compartment tested, with no PMNs observed in PL fluid. Sepsis was associated with marked upregulation of CD28 on PMNs in PL fluid (Figure 1C), with similar results in peripheral blood and spleen (data not shown). As expected, CD4+ and CD8+ cells constitutively expressed CD28. However, there was no change in either mean fluorescence intensity or percent CD28+CD4+ or CD28+CD8+ cells in the peripheral blood in mice after CLP compared with control mice (data not shown).
Figure 1.
Expression of CD40 and CD86 system after cecal ligation and puncture (CLP). C57BL/6 mice underwent CLP with a 19-gauge needle and were killed 18 hours later for flow cytometric analysis. Representative histograms for control and septic mice and a composite graph are presented for each molecule. Gray-shaded areas represent isotype control. (A) CD80 expression on peritoneal lavage mononuclear (CD11b+) cells; P < 0.001. (B) CD86 expression on splenic mononuclear cells; P < 0.001. (C) CD28 expression on peripheral blood neutrophils (PMNs) (Ly6G+); P = 0.05. (D) CD154 expression on CD11b+Ly6G+ peripheral blood mononuclear cells; P = 0.04. (E) CD40 expression on CD11b+Ly6G+ peripheral blood mononuclear cells; P = 0.04. Data represent three to five mice per group and were analyzed by Mann-Whitney t test.
Expression of CD40 and CD154 was also increased on CD11b+Ly6G+ monocytes in peripheral blood (Figures 1D and 1E) and spleen (data not shown) after CLP. There was no change in CD4+, CD8+, or PMN expression of CD154. Finally, CLP was associated with increased expression of soluble CD154 in plasma (7,958 vs. 4,658 pg/ml) as compared with control subjects.
Inhibition of CD40 and CD80/86 Improves Survival in Polymicrobial Sepsis
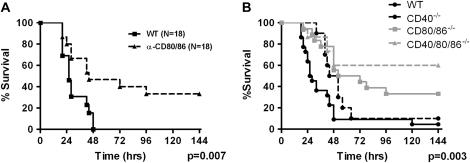
We have reported that CD40−/− mice have delayed mortality and attenuation of IL-6, IL-10, and IL-12 production after CLP (18). We next tested the role of CD80/86 in controlling lethality and inflammation in CLP. Because of the significant overlap in CD80 and CD86 signaling, initial experiments were carried out in mice with a targeted deletion of both molecules (CD80/86−/−). CD80/86−/− mice had a 33% reduction in mortality (93 vs. 60%) and a 2.5-fold improvement in median survival (30 vs. 78 h; P = 0.007) compared with wild-type mice (Figure 2B). Similar results were obtained with administration of an α-CD80/86 mAb 4 hours before CLP (Figure 2A). CD80/86−/− mice had no change in bacterial clearance compared with wild-type mice (see Figure 4D). Improvement in survival was associated with attenuation in plasma and BAL fluid IL-6 and IL-10 in the CD80/86−/− mice, with no change in IL-12 as compared with wild-type mice (Figure 3).
Figure 2.
Inhibition of CD80/86 alone or in combination with CD40 improves survival after cecal ligation and puncture (CLP). (A) Wild-type (WT) mice were injected intraperitoneally with either control IgG or 200 μg each of α-CD80 and α-CD86 4 hours before CLP and monitored for survival. n = 15 mice per group. (B) WT (n = 18), CD40−/− (n = 10), CD80/86−/− (n = 18), and CD40/80/86−/− (n = 15) mice underwent 19-gauge CLP and were monitored for survival. Data from CD40−/− mice have been previously published (31). For all survival experiments mice were monitored for a total of 14 days. Data are presented for the first 7 days, as there was no additional attrition between Days 7 and 14.
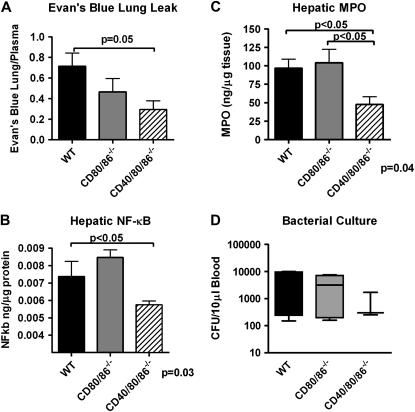
Figure 4.
CD40/80/86−/− mice have additional attenuation in remote organ injury after cecal ligation and puncture (CLP). CD40/80/86−/− mice have attenuated cytokine production after CLP. Wild-type (WT), CD80/86−/−, and CD40/80/86−/− mice underwent 19-gauge CLP and were killed 18 hours after CLP. (A) Pulmonary capillary leakage was determined with Evans blue dye as previously described (18, 31). (B) nuclear factor (NF)-κB was determined by p65 DNA–binding ELISA from liver homogenates. Data are normalized for total protein content. (C) Liver myeloperoxidase (MPO) was determined by ELISA. Data are presented as nanograms of MPO per microgram of hepatic protein. (D) Peripheral blood bacterial cultures 18 hours after CLP, as determined by serial dilutions. There were four to six mice per group. Data were analyzed by nonparametric analysis of variance, with P values shown at the bottom of each graph. Statistically significant group comparisons are bracketed.
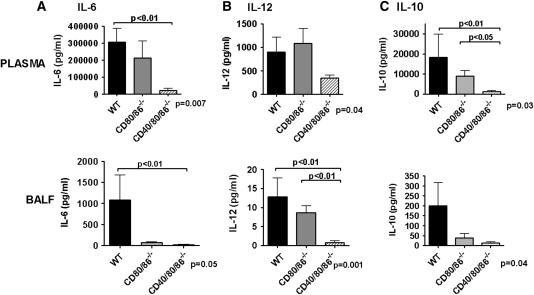
Figure 3.
CD40/80/86−/− mice have attenuated cytokine production after cecal ligation and puncture (CLP). Wild-type (WT), CD80/86−/−, and CD40/80/86−/− mice underwent 19-gauge CLP and were killed 18 hours after CLP. Plasma and bronchoalveolar lavage fluid (BALF) were collected and analyzed for IL-6 (A) IL-12 (B), and IL-10 (C) by commercially available ELISA. There were six to eight mice in each group. Data were analyzed by nonparametric analysis of variance, with P values shown at the bottom of each graph. Statistically significant group comparisons are bracketed.
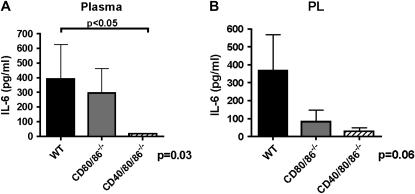
Because of reports of additive or synergistic effects of combined costimulatory molecule inhibition, we generated a novel CD40/80/86−/− mouse to test the hypothesis that CD40 and CD80/86 act synergistically to regulate inflammation in sepsis. CD40/80/86−/− mice had a further improvement in overall and median survival after CLP (60%) compared with wild-type (8%) and CD80/86−/− (33%) mice (P = 0.001), and prior data using CD40−/− mice (10%) (18, 31) (Figure 2B). Improvement in survival of CD40/80/86−/− mice was associated with greater attenuations in plasma and BAL fluid IL-6 at 6 hours after CLP (see Figures E1A and E1B in the online supplement) compared with wild-type and CD80/86−/− mice. At 18 hours after CLP, CD40/80/86−/− mice had attenuations in IL-6 and IL-10 in BAL fluid (Figure 3), PL fluid (see Figures E1E and E1F), and plasma (Figure 3) compared with wild-type and CD80/86−/− mice. However, in contrast to CD80/86−/− mice, CD40/80/86−/− mice showed a reduction in plasma and BAL fluid IL-12 at 6 hours (see Figure E1D) and 18 hours (Figure 3) after CLP.
We used pulmonary capillary leakage as determined with Evan's blue dye as a measure of distant organ injury. CD40/86/86−/− mice had a reduction in pulmonary capillary leakage compared with CD80/86−/− or wild-type mice after CLP (Figure 4A). CD40/80/86−/− mice also had further attenuation in hepatic PMN accumulation (determined by MPO) and NF-κB induction compared with wild-type and CD80/86−/− mice (Figures 4B and 4C). Finally, CD40/80/86−/− mice demonstrated no significant difference in circulating bacterial burden after CLP (Figure 4D).
The finding that the regulation of costimulatory molecules and their receptors is altered on PMNs and monocytes after CLP suggests that PMN–mononuclear cell interactions may contribute to the inflammatory response in vivo via CD40/80/86. To determine whether deletion of costimulatory molecules affected the ability of resident peritoneal cells to produce inflammatory cytokines, we injected wild-type PMNs intraperitoneally into wild-type, CD80/86−/−, or CD40/80/86−/− mice. Administration of wild-type PMNs resulted in significant IL-6 production in plasma and PL fluid from wild-type mice 4 hours postinjection compared with saline-injected control mice (data not shown). CD80/86−/− mice injected with wild-type PMNs had less PL fluid IL-6 compared with similarly treated wild-type mice, with no difference in plasma IL-6 (Figure 5). CD40/80/86−/− mice injected with wild-type PMNs had a further attenuation in plasma and PL fluid IL-6 compared with the CD80/86−/− mice (Figure 5).
Figure 5.
Neutrophils (PMNs) induce IL-6 production in vivo via CD40 and CD80/86. PMNs (5 × 105) from wild-type (WT) mice were injected intraperitoneally into WT, CD80/86−/−, or CD40/80/86−/− mice for 4 hours, at which point (A) plasma and (B) peritoneal lavage (PL) fluid were collected and analyzed for IL-6 via ELISA. There were five to eight mice per group. Data were analyzed by nonparametric analysis of variance, with P values shown at the bottom of each graph. Statistically significant group comparisons are bracketed.
Expression of Costimulatory Molecules Serves as a Biomarker for Outcome in Human Sepsis
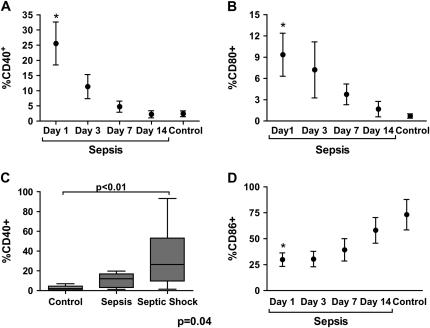
Finally, we investigated whether the mouse model accurately reflects alteration of costimulatory molecule expression in human sepsis. Thirty-five subjects who met the American College of Chest Physicians/Society of Critical Care Medicine consensus definition of sepsis and 11 healthy control subjects provided blood for analysis of soluble mediator analysis, and 14 consecutive septic subjects and 6 healthy control subjects provided whole blood for flow cytometry (33). The clinical characteristics of the septic subjects are presented in Table 1. Overall, the healthy control subjects were younger (37 vs. 57 yr) than septic patients, with no difference in sex distribution (data not shown). Thirteen of 14 subjects in whom flow cytometry was performed survived and had blood drawn on Days 1, 3, 7, and 14 or until death or hospital discharge. As in prior reports, sepsis was associated with marked upregulation of plasma IL-6 (1,056 ± 563 vs. 2.1 ± 1.3 pg/ml; P < 0.001), and IL-12 (7.9 ± 3.5 vs. 0.42 ± 0.17 pg/ml; P = 0.01) (35). CD40 and CD80 expression was up-regulated on the circulating monocytes (CD14+) of septic subjects on Day 1 compared with healthy control subjects (Figures 6A and 6B; and see the representative dot plot in Figure E2). At the time of final blood collection, expression levels of CD40 were significantly lower than those at admission (6.1 vs. 25.2%; P = 0.004) and were not different from those of healthy control subjects (6.1 vs. 2.5%; P = not significant). A similar trend was seen for CD80 expression (9.3 vs. 3.2%; P = 0.1). The decrease in CD40 and CD80 expression correlated tightly with reduced APACHE II (see Figure E2) and Simplified Acute Physiology Score II (SAPS II) scores (data not shown). In addition, expression levels of CD40 (Figure 6C) and CD80 (13.64% [septic shock] vs. 1.64% [sepsis] vs. 0.74% [healthy control subjects]; P = 0.002 by analysis of variance) on Day 1 were significantly higher in subjects with septic shock (defined as the need for vasopressors), compared with sepsis. There was no association between the monocyte expression levels of CD40 or CD80 and the need for mechanical ventilation, ventilator-free days, or the presence of bacteremia. CD86 was constitutively expressed on circulating monocytes in healthy individuals and levels were decreased in septic subjects on Day 1 compared with healthy control subjects, with levels returning to those of healthy control subjects at the time of the last blood collection (Figure 6D). As expected, CD28 was expressed at high levels on CD4+ and CD8+ cells, with no significant difference between septic and healthy subjects in either mean fluorescence intensity or percent positive cells (data not shown). However, in contrast to our observations in mice, there were only low levels of PMN surface CD28 expression in septic patients. Finally, similar to our observations in mice, CD154 was present on circulating monocytes with a trend toward higher levels in septic patients compared with healthy control subjects (3.9 vs. 1.2%; P = 0.08).
TABLE 1.
CLINICAL CHARACTERISTICS OF SUBJECTS ON ENROLLMENT
| All Septic Szubjects | Septic Subjects Selected for Flow Cytometry | |
|---|---|---|
| (n = 35) | (n = 14) | |
| Age, yr | 54.5 | 57.8 |
| Sex, % female | 45.7 | 50 |
| APACHE II score | 16.8 | 18.6 |
| Mechanical ventilation, % | 43 | 47 |
| Vasopressors, % | 54 | 64 |
| Bacteremia, % | 34 | 35.7 |
| 28-Day mortality, % | 17.1 | 7.1 |
Definition of abbreviation: APACHE = Acute Physiology and Chronic Health Evaluation.
Figure 6.
Humans with sepsis present alterations in costimulatory molecules. Healthy control subjects and 14 septic subjects underwent flow cytometric analysis for CD40 (A), CD80 (B), and CD86 (D) on circulating monocytes. Monocytes were identified on the basis of forward scatter/side scatter (FSC/SSC) and CD14+. Septic subjects had blood collected on Days 1, 3, 7, and 14 or until hospital discharge. Thirteen of 14 septic subjects survived until discharge. *P < 0.01 compared with control subjects. (C) Expression levels of CD40 on CD14+ monocytes in healthy control subjects (n = 6), septic subjects (n = 4), and septic shock patients (n = 10) on Day 1. Data were analyzed by nonparametric analysis of variance, with the P value shown at the bottom of the graph. Statistically significant group comparisons are bracketed.
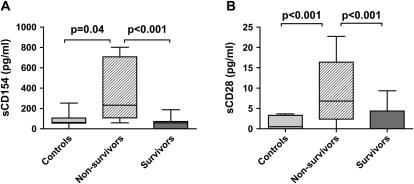
Finally, we determined the concentrations of the soluble isoforms of CD154 and CD28 in sepsis. Soluble CD154 (sCD154) is a well-established biomarker for diseases regulated by adaptive immunity and is a potent CD40 agonist, resulting in macrophage activation and systemic inflammation (32, 36–39). In a similar fashion, soluble CD28 (sCD28) acts as an activator of dendritic cells via CD80/86 (40). We demonstrate that sCD28:Fc results in similar upregulation of IL-6 in murine peritoneal macrophages compared with Fc-treated control subjects (340 vs. 5 pg/ml). Whereas the levels of sCD28 and sCD154 were no different in septic subjects compared with healthy control subjects, the plasma concentrations of both were higher in nonsurvivors compared with either survivors or healthy control subjects (Figure 7). In contrast, there was no difference in the plasma concentration of IL-6 between survivors and nonsurvivors (1,200 ± 916 vs. 861 ± 507 pg/ml; P = 0.83). There was no correlation between sCD28 or sCD154 and the concentrations of IL-6 and IL-12, the APACHE II score on admission, the need for mechanical ventilation, the presence of septic shock, or the presence of bacteremia.
Figure 7.
Expression of soluble CD28 and CD154 correlates with lethality in human sepsis. Levels of soluble CD154 (sCD154; A) and soluble CD28 (sCD28; B) were measured by ELISA in healthy control subjects (n = 11) and septic subjects (n = 35) on Day 1. Levels for both were higher in nonsurvivors (n = 6) compared with survivors (n = 29). Data were analyzed by nonparametric analysis of variance, P < 0.001. Statistically significant group comparisons are bracketed.
DISCUSSION
One of the major findings of this study is that deletion of the costimulatory molecules, CD40 and CD80/86, alone and in combination, markedly improves survival in the murine CLP model of lethal polymicrobial sepsis. Improved survival in CD80/86−/− mice firmly establishes a role for this receptor complex in regulating inflammation in overwhelming sepsis. The similar result in mice treated with an anti-CD80/86 mAb confirms the observation in the knockout mice, although further experiments with post–CLP treatment would be required to establish the potential of inhibiting CD80/86 as a therapeutic strategy. These data are consistent with the observation that stimulation of CD28 with an agonistic mAb in humans can result in a fulminant shocklike state with high lethality (24). The attenuation of circulating IL-6 and IL-10 in CD80/86−/− mice is consistent with one study suggesting that CD86 is a major regulator of IL-10 during CLP, and with other studies documenting CD28-mediated IL-6 production from dendritic cells in a CD80/86-dependent manner (34, 40). In contrast, CD80/86−/− mice had no change in circulating IL-12 after CLP. We have previously reported a critical role for CD40 in regulating IL-12 in sepsis, which suggested persistent activity of the CD40 system in CD80/86−/− mice and a potential role for combined inhibition of these cascades (18, 31).
The beneficial effect of combined blockade of multiple costimulatory pathways in inhibiting inflammation has been observed in many disease states governed by cellular immunity, including allograft rejection and autoimmunity (12–14, 27). The twofold improvement in survival of CD40/80/86−/− mice compared with wild-type, CD80/86−/−, and CD40−/− mice, suggests a similar synergistic interaction for these costimulatory molecules in sepsis, which is regulated by innate immunity. This improvement in survival was associated with attenuations in IL-6, IL-10, and IL-12 in multiple tissue compartments. Specifically, the reduction in IL-12 further implicates a specific role for CD40 in the regulation of IL-12 and helper T-cell type 1 cytokine production in sepsis (18). Reductions in inflammatory mediators were associated with a reduction in hepatic NF-κB activation, pulmonary capillary leakage, and hepatic PMN accumulation. These findings, combined with the lack of alteration in bacterial clearance, suggest that inhibiting multiple costimulatory receptors can prevent remote organ damage without compromising innate host defenses against bacterial pathogens. The marked effect of deleting both CD40 and CD80/86 suggests these molecules are an important early step in the inflammatory response to overwhelming infection.
Our results also suggest that monocytes/macrophages and PMNs play an important role in the inflammatory responses mediated by costimulatory molecules in sepsis. This is supported by the increase in CD40, CD154, and CD80 expression on peripheral blood monocytes and peritoneal macrophages after CLP and is in agreement with one report showing upregulation of CD80 on peritoneal dendritic cells after CLP (34). CD86, however, was differentially regulated after CLP in our study, with increased expression in spleen and reduced expression on peritoneal macrophages. The latter is consistent with other reports and our human observations (34). The significance of the compartment-specific changes in CD86 expression remains unclear and further studies are required to determine whether this represents tissue-specific differentiation, or selective transmigration of peripheral monocytes. The finding of increased CD28 expression on PMNs in peripheral blood and PL fluid after CLP, with little alteration in T-cell expression, suggests an important role for PMNs in costimulatory molecule–mediated inflammation as well. The ability of PMNs to induce IL-6 production in a CD40/80/86-dependent manner in vivo provides direct evidence of the ability of costimulatory cascades to regulate inflammation via innate immune effector cells, and supports in vitro data from our laboratory suggesting that PMNs activate macrophages via engagement of CD40, CD80/86, and intercellular adhesion molecules on the macrophage surface (11).
However, we cannot exclude other cellular sources for costimulatory molecule–mediated inflammation in our model. Costimulatory molecules are widely expressed and other potential sources include platelets (CD154), endothelial cells, natural killer cells, and T cells, all of which have been described to have important roles in the innate immune response (41–43). T-cell–expressed costimulatory ligands have been implicated in the immunoparalysis observed in the recovery phase of sepsis (44). This may in part explain the differences between our results and those in one study by Schwulst and coworkers (44). In that study, CD40 activation, via an agonistic monoclonal antibody, improved survival in mice subjected to a dual-hit model of nonlethal CLP followed by a secondary lethal bacterial pneumonia. This raises the possibility that there is a biphasic response to costimulatory receptor expression and cell type involved, depending on the severity and timing of the initial insult. Furthermore, unlike our study, in which peritoneal sepsis was the cause of death, death in their study was induced by secondary pneumonia, raising the possibility for differential, compartment-specific effects for CD40. Finally, their study assessed the role of CD40 via an agonist antibody, thus eliminating any effect of CD40 activation of CD154-expressing cells. This may be an important factor given the ability of CD154 ligation to activate platelets and T cells (43, 45, 46).
An eventual goal is to translate these findings to human sepsis. The up-regulation of costimulatory receptors on circulating monocytes and expressed costimulatory ligands on PMNs in septic patients lends further support to a pathophysiologic role for costimulatory molecules in the acute phases of sepsis. The increased expression of CD80 and CD40 on Day 1 on circulating monocytes in septic subjects, with the highest levels found in subjects with circulatory failure, suggests an important role for these cascades in early septic shock. The subsequent decrease in CD40 and CD80 expression levels as the severity of illness decreased lends further support for a causal role of these molecules in regulating inflammation in human sepsis.
We found that increased levels of soluble CD28 and CD154 identify a subset of patients with lethal sepsis. Prior studies showed that sCD28 and sCD154 can act as stimulatory ligands for their respective receptors and serve as biomarkers for disease presence and/or activity in acute coronary syndromes, tuberculosis, and autoimmune disease (32, 36–40). We have found a similar role for these molecules as biomarkers in sepsis. The lack of correlation between sCD28 or sCD154 and APACHE II or SOFA scores suggests that these costimulatory ligands may be more specific markers for mortality than traditional biomarkers such as IL-6 (47). Although suggestive, because of the relatively small number of nonsurvivors, and the marked heterogeneity known to exist in human sepsis, larger numbers of patients will need to be studied in order to draw definitive conclusions. Furthermore, the source of these soluble costimulatory ligands and the mechanism of solubilization are not clear. There are many potential sources for both ligands as activated platelets are a large reservoir of sCD154, and sCD28 is produced by alternative splicing of CD28 mRNA in T cells (37, 48)
In conclusion, these data support an important and novel role for costimulatory molecules alone and in combination in regulating inflammation in the innate immune response in overwhelming polymicrobial sepsis. These data support the rationale for blockade of these pathways in humans and suggest a new potential therapeutic modality for septic shock.
Supplementary Material
Supported by NIH K08 HL070710 (J.A.G.), R01 AI067522 (J.A.G.), and RO1 HL57879 (M.W.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200703-515OC on November 11, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–1310. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–150. [DOI] [PubMed] [Google Scholar]
- 3.Opal SM, Fisher CJ Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, et al.; Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. Crit Care Med 1997;25:1115–1124. [DOI] [PubMed] [Google Scholar]
- 4.Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, Dal Nogare A, Nasraway S, Berman S, Cooney R, et al.; NORASEPT II Study Group. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. Lancet 1998;351:929–933. [PubMed] [Google Scholar]
- 5.Cohen J, Carlet J; International Sepsis Trial Study Group. INTERSEPT: an international, multicenter, placebo-controlled trial of monoclonal antibody to human tumor necrosis factor-α in patients with sepsis. Crit Care Med 1996;24:1431–1440. [DOI] [PubMed] [Google Scholar]
- 6.Remick DG, Garg SJ, Newcomb DE, Wollenberg G, Huie TK, Bolgos GL. Exogenous interleukin-10 fails to decrease the mortality or morbidity of sepsis. Crit Care Med 1998;26:895–904. [DOI] [PubMed] [Google Scholar]
- 7.Matsukawa A, Takeda K, Kudo S, Maeda T, Kagayama M, Akira S. Aberrant inflammation and lethality to septic peritonitis in mice lacking STAT3 in macrophages and neutrophils. J Immunol 2003;171:6198–6205. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto J, Wiener-Kronish JP, Hashimoto S, Sawa T. Effects of Cl2MDP-encapsulating liposomes in a murine model of Pseudomonas aeruginosa–induced sepsis. J Liposome Res 2002;12:239–257. [DOI] [PubMed] [Google Scholar]
- 9.Sturm E, Havinga R, Baller JF, Wolters H, van Rooijen N, Kamps JA, Verkade HJ, Karpen SJ, Kuipers F. Kupffer cell depletion with liposomal clodronate prevents suppression of Ntcp expression in endotoxin-treated rats. J Hepatol 2005;42:102–109. [DOI] [PubMed] [Google Scholar]
- 10.Grivennikov SI, Tumanov AV, Liepinsh DJ, Kruglov AA, Marakusha BI, Shakhov AN, Murakami T, Drutskaya LN, Forster I, Clausen BE, et al. Distinct and nonredundant in vivo functions of TNF produced by T cells and macrophages/neutrophils: protective and deleterious effects. Immunity 2005;22:93–104. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino Y, Hoshino S, Gold JA, Raju B, Prabhakar S, Pine R, Rom WN, Nakata K, Weiden M. Mechanisms of PMN-mediated induction of HIV-1 replication in macrophages during pulmonary tuberculosis. J Infect Dis 2007;195:1303–1310. [DOI] [PubMed] [Google Scholar]
- 12.Daikh DI, Finck BK, Linsley PS, Hollenbaugh D, Wofsy D. Long-term inhibition of murine lupus by brief simultaneous blockade of the B7/CD28 and CD40/gp39 costimulation pathways. J Immunol 1997;159:3104–3108. [PubMed] [Google Scholar]
- 13.Schaub M, Issazadeh S, Stadlbauer TH, Peach R, Sayegh MH, Khoury SJ. Costimulatory signal blockade in murine relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol 1999;96:158–166. [DOI] [PubMed] [Google Scholar]
- 14.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 1996;381:434–438. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto N, Kawabe T, Imaizumi K, Hara T, Okamoto M, Kojima K, Shimokata K, Hasegawa Y. CD40 plays a crucial role in lipopolysaccharide-induced acute lung injury. Am J Respir Cell Mol Biol 2004;30:808–815. [DOI] [PubMed] [Google Scholar]
- 16.Adawi A, Zhang Y, Baggs R, Finkelstein J, Phipps RP. Disruption of the CD40–CD40 ligand system prevents an oxygen-induced respiratory distress syndrome. Am J Pathol 1998;152:651–657. [PMC free article] [PubMed] [Google Scholar]
- 17.Sugimoto K, Galle C, Preiser JC, Creteur J, Vincent JL, Pradier O. Monocyte CD40 expression in severe sepsis. Shock 2003;19:24–27. [DOI] [PubMed] [Google Scholar]
- 18.Gold JA, Parsey M, Hoshino Y, Hoshino S, Nolan A, Yee H, Tse DB, Weiden MD. CD40 contributes to lethality in acute sepsis: in vivo role for CD40 in innate immunity. Infect Immun 2003;71:3521–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzianabos AO, Chandraker A, Kalka-Moll W, Stingele F, Dong VM, Finberg RW, Peach R, Sayegh MH. Bacterial pathogens induce abscess formation by CD4+ T-cell activation via the CD28–B7-2 costimulatory pathway. Infect Immun 2000;68:6650–6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saha B, Jaklic B, Harlan DM, Gray GS, June CH, Abe R. Toxic shock syndrome toxin-1–induced death is prevented by CTLA4Ig. J Immunol 1996;157:3869–3875. [PubMed] [Google Scholar]
- 21.Saha B, Harlan DM, Lee KP, June CH, Abe R. Protection against lethal toxic shock by targeted disruption of the CD28 gene. J Exp Med 1996;183:2675–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding Y, Chung CS, Newton S, Chen Y, Carlton S, Albina JE, Ayala A. Polymicrobial sepsis induces divergent effects on splenic and peritoneal dendritic cell function in mice. Shock 2004;22:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med 2005;353:770–781. [DOI] [PubMed] [Google Scholar]
- 24.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 2006;355:1018–1028. [DOI] [PubMed] [Google Scholar]
- 25.Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor α inhibition. N Engl J Med 2005;353:1114–1123. [DOI] [PubMed] [Google Scholar]
- 26.Homann D, Jahreis A, Wolfe T, Hughes A, Coon B, van Stipdonk MJ, Prilliman KR, Schoenberger SP, von Herrath MG. CD40L blockade prevents autoimmune diabetes by induction of bitypic NK/DC regulatory cells. Immunity 2002;16:403–415. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Gao D, Lunsford KE, Frankel WL, Bumgardner GL. Targeting LFA-1 synergizes with CD40/CD40L blockade for suppression of both CD4-dependent and CD8-dependent rejection. Am J Transplant 2003;3:1251–1258. [DOI] [PubMed] [Google Scholar]
- 28.Mehta N, Nolan A, McCormack DG, Weiden JA. Role of macrophage costimulatory molecules in human sepsis [abstract]. Am J Respir Crit Care Med 2005;171:A522. [Google Scholar]
- 29.Gold JA, Nolan A, Kelly A, Weiden M. Role of combined costimulatory molecule inhibition in sepsis [abstract]. Am J Respir Crit Care Med 2007;175:A900. [Google Scholar]
- 30.Gold JA, Nolan A, Mehta N, Weiden M. Blockade of CD80/CD86 improves survival in murine polymicrobial sepsis [abstract]. Am J Respir Crit Care Med 2006;173:A644. [Google Scholar]
- 31.Nolan A, Weiden M, Hoshino Y, Gold JA. Cd40 but not CD154 knockout mice have reduced inflammatory response in polymicrobial sepsis: a potential role for Escherichia coli heat shock protein 70 in CD40-mediated inflammation in vivo. Shock 2004;22:538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoshino Y, Tse DB, Rochford G, Prabhakar S, Hoshino S, Chitkara N, Kuwabara K, Ching E, Raju B, Gold JA, et al. Mycobacterium tuberculosis–induced CXCR4 and chemokine expression leads to preferential X4 HIV-1 replication in human macrophages. J Immunol 2004;172:6251–6258. [DOI] [PubMed] [Google Scholar]
- 33.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ; ACCP/SCCM Consensus Conference Committee/American College of Chest Physicians/Society of Critical Care Medicine. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 1992;101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 34.Newton S, Ding Y, Chung CS, Chen Y, Lomas-Neira JL, Ayala A. Sepsis-induced changes in macrophage co-stimulatory molecule expression: CD86 as a regulator of anti-inflammatory IL-10 response. Surg Infect (Larchmt) 2004;5:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Presterl E, Staudinger T, Pettermann M, Lassnigg A, Burgmann H, Winkler S, Frass M, Graninger W. Cytokine profile and correlation to the APACHE III and MPM II scores in patients with sepsis. Am J Respir Crit Care Med 1997;156:825–832. [DOI] [PubMed] [Google Scholar]
- 36.Ludwiczek O, Kaser A, Tilg H. Plasma levels of soluble CD40 ligand are elevated in inflammatory bowel diseases. Int J Colorectal Dis 2003;18:142–147. [DOI] [PubMed] [Google Scholar]
- 37.Heeschen C, Dimmeler S, Hamm CW, van den Brand MJ, Boersma E, Zeiher AM, Simoons ML. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med 2003;348:1104–1111. [DOI] [PubMed] [Google Scholar]
- 38.Wiley JA, Geha R, Harmsen AG. Exogenous CD40 ligand induces a pulmonary inflammation response. J Immunol 1997;158:2932–2938. [PubMed] [Google Scholar]
- 39.Kiener PA, Moran-Davis P, Rankin BM, Wahl AF, Aruffo A, Hollenbaugh D. Stimulation of CD40 with purified soluble gp39 induces proinflammatory responses in human monocytes. J Immunol 1995;155:4917–4925. [PubMed] [Google Scholar]
- 40.Orabona C, Grohmann U, Belladonna ML, Fallarino F, Vacca C, Bianchi R, Bozza S, Volpi C, Salomon BL, Fioretti MC, et al. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol 2004;5:1134–1142. [DOI] [PubMed] [Google Scholar]
- 41.Schonbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci 2001;58:4–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leifeld L, Trautwein C, Dumoulin FL, Manns MP, Sauerbruch T, Spengler U. Enhanced expression of CD80 (B7-1), CD86 (B7-2), and CD40 and their ligands CD28 and CD154 in fulminant hepatic failure. Am J Pathol 1999;154:1711–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998;391:591–594. [DOI] [PubMed] [Google Scholar]
- 44.Schwulst SJ, Grayson MH, DiPasco PJ, Davis CG, Brahmbhatt TS, Ferguson TA, Hotchkiss RS. Agonistic monoclonal antibody against CD40 receptor decreases lymphocyte apoptosis and improves survival in sepsis. J Immunol 2006;177:557–565. [DOI] [PubMed] [Google Scholar]
- 45.van Essen D, Kikutani H, Gray D. CD40 ligand-transduced co-stimulation of T cells in the development of helper function. Nature 1995;378:620–623. [DOI] [PubMed] [Google Scholar]
- 46.Poudrier J, van Essen D, Morales-Alcelay S, Leanderson T, Bergthorsdottir S, Gray D. CD40 ligand signals optimize T helper cell cytokine production: role in Th2 development and induction of germinal centers. Eur J Immunol 1998;28:3371–3383. [DOI] [PubMed] [Google Scholar]
- 47.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med 1993;119:771–778. [DOI] [PubMed] [Google Scholar]
- 48.Magistrelli G, Jeannin P, Elson G, Gauchat JF, Nguyen TN, Bonnefoy JY, Delneste Y. Identification of three alternatively spliced variants of human CD28 mRNA. Biochem Biophys Res Commun 1999;259:34–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.