Abstract
Rationale: Airways hyperresponsiveness (AHR) is a hallmark feature of asthma, and can be caused by various disparate mechanisms. Mouse models of AHR have been useful for studying these mechanisms in isolation, but such models still typically do not exhibit the same degree of AHR as seen in severe human asthma. We hypothesized that more severe AHR in mice could be achieved by imbuing them with more than one mechanism of AHR.
Objectives: We sought to determine if the airway wall thickening accompanying allergic inflammation and the exaggerated smooth muscle shortening induced by intratracheal cationic protein could act together to produce a severe form of AHR.
Methods: We used the forced oscillation technique to measure methacholine responsiveness in BALB/c mice that had been sensitized and challenged with ovalbumin followed by an intratracheal instillation of poly-l-lysine.
Measurements and Main Results: We found that both ovalbumin and poly-l-lysine treatment alone caused moderate levels of AHR. When the two treatments were combined, however, they synergized in terms of their effect on lung stiffness to an extent that could even be fatal, reflecting a significantly enhanced level of airway closure.
Conclusions: Our results suggest that mechanistic synergy between airway wall thickening and exaggerated smooth muscle shortening produces a more germane mouse model of asthma that may have particular relevance to the pathophysiology of the acute severe asthma exacerbation.
Keywords: airway hyperresponsiveness, methacholine, mouse model, asthma exacerbation
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
The causes of asthma attacks are unknown, despite the evidence that such attacks may simultaneously involve both the lung periphery and the central airways.
What This Study Adds to the Field
Exaggerated central airway constriction on a background of lung inflammation can cause extreme decrement in lung function, and even death, in mice. This is due to a synergy between smooth muscle shortening and airway wall thickening.
Asthma has been increasing in prevalence over the past 20 years (1), and in particular has been associated with increased hospital visits and death (1, 2). A cardinal feature of asthma is airway hyperresponsiveness (AHR), defined as an abnormally large decrement in lung mechanical function after challenge with a smooth muscle agonist. The genesis of AHR remains controversial, in part because it can be caused by very different factors (3–5), including abnormalities in the smooth muscle that surrounds the airways (6–8) and reductions in the various mechanical loads that normally oppose smooth muscle shortening (9, 10). These different factors almost certainly play varied roles in different individuals with asthma, contributing to the characterization of asthma as a complex disease.
Current asthma research focuses on mouse models of allergic airway inflammation because of the important role of immunology in asthma pathogenesis (11), and because the mouse presents a number of practical advantages compared with other species (12). Although the allergic mouse is hyperresponsive to methacholine challenge, the AHR it exhibits is not as extreme as that seen in the more severe forms of human asthma (11, 13). Furthermore, we have shown that the AHR in allergically inflamed BALB/c mice appears to be due almost entirely to enhanced closure of small airways caused by a physically thickened and more secretion-laden airway epithelium (14). These represent only a subset of the mechanisms potentially responsible for AHR in humans with asthma. Another candidate for the prime abnormality in asthma is exaggerated contractility of airway smooth muscle (15), which can also be modeled in animals. We have shown, for example, that mice become hypersensitive to methacholine after intratracheal administration of poly-l-lysine (PLL), which mimics the cationic protein of inflammatory cells (16).
The mouse has thus clearly been useful for studying individual mechanisms behind AHR (14, 16–18). Nevertheless, doubts have been expressed about the validity of using mice to model asthma itself (11). These doubts may have arisen because a focus on individual mechanisms of AHR does not address the fact that multiple mechanisms are likely operative in human patients with asthma who are typically both hyperresponsive and hypersensitive to methachline challenge (4). Our goal in the present study was therefore to investigate the notion that an effective mouse model of asthma should exhibit more than one mechanism of AHR. Accordingly, we sought to develop a more germane mouse model of asthma by combining the airway wall thickening that accompanies allergic lung inflammation with the exaggerated smooth muscle shortening induced by intratracheal cationic protein. Some of the results of this study have been published in abstract form (19, 20).
METHODS
Experiment Groups
Female mice were obtained from Jackson Laboratories (Bar Harbor, ME) at approximately 8 weeks of age. We studied four groups of mice (n = 5–9 per group) from each of the BALB/c, A/J, and C57BL/6 strains. The groups were as follows: (1) naive, (2) Ova (sensitized and challenged with ovalbumin [Ova] to induce an acute allergic inflammation), (3) PLL (treated with an intratracheal instillation of the cationic protein PLL), and (4) PLL+Ova (both allergically inflamed and treated with PLL). Our studies conformed to the National Research Council Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the University of Vermont.
Experimental Protocol
The anesthetized, tracheostomized mice were connected to a computer-controlled small-animal mechanical ventilator (flexiVent; SCIREQ, Montreal, PQ, Canada) for mechanical ventilation at 200 breaths/minute and a tidal volume of 0.2 ml against a positive end-expiratory pressure (PEEP) of 3 cm H2O. The animals were paralyzed with an intraperitoneal injection of pancuronium bromide (0.08 μg/kg). The experimental protocol began with the delivery of a deep breath to an airway pressure limit of 25 cm H2O. Approximately 1 minute later, the animals were exposed to methacholine aerosol at a concentration of 1.25 mg/ml for 40 seconds by directing the inspiratory line of the ventilator circuit through the aerosolization chamber of an ultrasonic nebulizer (Mystique; Airsep Corp., Buffalo, NY) while the animals were ventilated at 30 breaths/minute with a tidal volume of approximately 0.4 ml. At the end of the 40-second challenge, the nebulizer was immediately taken out of the inspiratory circuit and mechanical ventilation was resumed at 200 breaths/minute with a tidal volume of 0.2 ml. Every 10 seconds for the following 5 minutes, ventilation was interrupted to allow a 1-second passive expiration against the applied level of PEEP of 3 cm H2O. This was followed by the application of a 2-second volume perturbation to the lungs while the pressure required to generate the perturbations was measured (13, 21). The volume perturbation consisted of the superposition of 12 sinusoidal components having frequencies from 1 to 19.625 Hz with random phases and amplitudes that were scaled inversely with frequency. The phase of the perturbation was adjusted so that lung volume changed above the volume set by PEEP. The entire procedure, beginning with the two deep breaths, was then repeated using increasing methacholine concentrations of 3.125, 12.5, and 50.0 mg/ml.
At the end of the protocol, a lung lavage was performed by instilling 1 ml of phosphate-buffered saline containing 3.2% sodium citrate into the trachea with a syringe and then withdrawing it again (withdrawn volume, ∼0.8 ml). The lavage fluid was stored on ice for later analysis of cell counts, and the mice were killed with an overdose of sodium pentobarbital followed by opening of the thoracic cavity.
Measurement of Respiratory Mechanical Impedance
The pressure and flow data obtained during application of each volume perturbation were used to calculate a complex input impedance of the respiratory system (Zrs) (21), which was then fit to the equation of a lung model consisting of a single airway serving a constant-phase viscoelastic tissue unit. This provided values for the parameters R, G, and H of the constant-phase model of Zrs. R is a measure of the flow resistance of the conducting pulmonary airways (13), G reflects viscous dissipation of energy in the respiratory tissues (tissue resistance), H reflects elastic energy storage in the tissues (tissue stiffness) (22). We thus obtained a time course of the parameters R, G, and H for the 5 minutes after bronchial challenge in the mice.
Computational Model of the Mouse Lung
As an aid to data interpretation, we simulated our experiments using a computational model of the mouse lung. This enabled us to pursue what has come to be called in silico experimentation, allowing us to test hypotheses in a way that would not have been possible in vivo. To simulate the enhanced smooth muscle shortening occurring in the PLL group, the airways of the model were forced to follow a fractional narrowing profile that caused the simulated and experimental time courses of R to match. To simulate the effects of antigen treatment in the Ova group, an 18-μm-thick lining was added to all airways in the model to represent epithelial thickening, and the critical threshold radius at which airways close was increased from 38 to 45 μm to simulate the effect of increased airway secretions. PLL and Ova treatments together were simulated by combining the above features in a single simulation.
Statistics
Comparisons of cell counts (Table 1) and impedance parameter values between groups of mice at individual methacholine concentrations (Figure 1) were made by unpaired t test using the Origin software package (OriginLab Corp., Northampton, MA). Statistical significance was taken as P < 0.05.
TABLE 1.
TOTAL CELL COUNTS IN BRONCHOALVEOLAR LAVAGE FLUID
| Experimental Group
|
||||
|---|---|---|---|---|
| Mouse Strain | Naive | PLL | Ova | PLL+Ova |
| BALB/c | 69 ± 12 | 78 ± 11 | 449 ± 104* | 338 ± 78* |
| A/J | 50 ± 7 | 38 ± 5 | 386 ± 96* | 343 ± 129* |
| C57BL/6 | 53 ± 7 | 81 ± 26 | 134 ± 32* | 178 ± 39* |
Definition of abbreviations: Ova = ovalbumin; PLL = poly-l-lysine.
Values are mean ± SE. Cells counts are quoted in thousands of cells per milliliter of lavage fluid.
Statistically significantly different (unpaired t test, P < 0.05) from naive group of the same strain.
Figure 1.
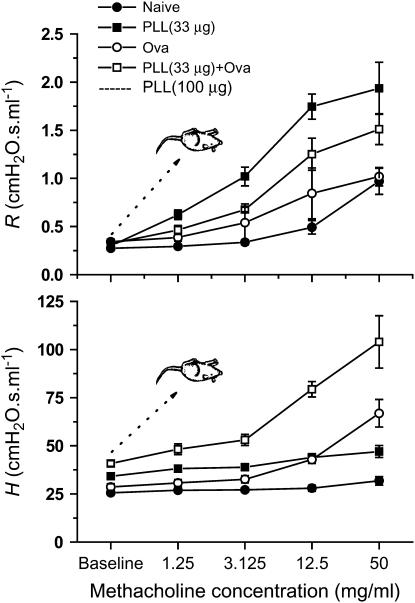
Methacholine dose–response relationships for airway resistance (R) and tissue stiffness (H) for the four groups of BALB/c mice. R is the peak value observed following each challenge. H is the final plateau value observed 5 minutes after each challenge. Allergically inflamed mice treated with 100 μg poly-l-lysine (PLL) died almost immediately after being challenged with the lowest dose of methacholine. Mice treated with the lower dose of 30 μg PLL, either alone or in combination with ovalbumin (Ova) sensitization and challenge, survived challenge with all doses of methacholine. The values of R in the PLL (30 μg)+Ova group were significantly greater (P < 0.05, unpaired t test) than their respective values in each of the naive, PLL, and Ova groups, with the exception of the Ova group at baseline and 3.125 mg/ml methacholine. The values of H in the PLL+Ova group were significantly greater than those in the other three groups at all methacholine concentrations.
Further details of methods used are provided in the online supplement.
RESULTS
Synergistic Effects of Cationic Protein Treatment and Allergic Inflammation
In our initial experiments, five BALB/c mice were subjected to sensitization and challenge with the foreign protein Ova using the protocol of our previous study (14). On the day of the experiment, the animals were anesthetized, tracheostomized, connected to the mechanical ventilator, and then given the same intratracheal dose of PLL as we used previously (16). Thirty minutes later, we began a standard methacholine challenge procedure. However, we were unable to measure airway responsiveness because all the mice died immediately after challenge with the first dose (3.125 mg/ml) of methacholine aerosol, presumably due to the severity of the bronchoconstriction that was induced (Figure 1).
To avoid having the mice die prematurely, we reduced the severity of the injury by lowering the dose of PLL to 33 μg from the 100 μg that we administered in our original experiments (16). The antigen sensitization and challenge protocol remained the same as before (see Methods), and inflammation was confirmed by increased cell counts in bronchoalveolar lavage fluid obtained from the mice at the end of the experiment (Table 1). Using this preparation, we were able to challenge the mice with aerosols of methacholine up to a concentration of 50 mg/ml, which allowed them to survive through the entire experiment. Figure 1 shows dose–response relationships for airway resistance (R) and tissue stiffness (H) obtained from measurements of input impedance (see Methods) after sequential challenges with increasing doses of methacholine aerosol in the following four groups of BALB/c mice: (1) naive, (2) PLL, (3) Ova, and (4) PLL+Ova. R was elevated in the mice that received PLL, relative to control, likely due to enhanced smooth muscle shortening (16). H was elevated in the Ova group at the highest dose, likely due to enhanced airway closure (14). The key novel observation of the present study was that H was substantially elevated in the PLL+Ova group compared with either the PLL or Ova groups. This indicates that cationic protein and antigen treatments act together to significantly increase the amount of peripheral airway closure that occurs compared with either treatment alone. Somewhat curiously, the response in R in the PLL+Ova group was slightly smaller than in the PLL group.
Computational Modeling of Mouse Lung Mechanics during Methacholine Challenge
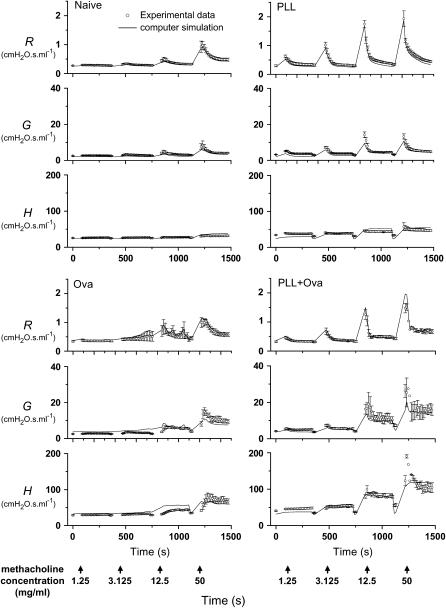
To understand this synergistic phenomenon on a quantitative basis, we used an anatomically based computational model of the mouse lung (see Methods) to simulate the time courses of R, H, and tissue damping (G) after each dose of methacholine. First, we set the parameters of the computational model to correspond to a normal BALB/c mouse. We made the airways in the model follow a time course of fractional narrowing that forced its prediction of R to follow the experimental measurements. Also, the radii of all airways in the model were monitored continuously throughout the simulation, and any airway that achieved a radius of 38 μm or less was immediately closed for the rest of the simulation. The resulting model predictions of time courses for G and H closely matched those found experimentally (Figure 2, top left), just as we found in previous studies (14, 16). Next, we forced the same model to follow the elevated R response of the PLL group, which required somewhat greater degrees of fractional airway narrowing at each dose of methacholine. Again, the corresponding time courses for G and H matched the experimental data (Figure 2, top right).
Figure 2.
Experimental measurements (open circles, mean ± SE) of the time courses of impedance parameters R, G, and H after increasing doses of methacholine aerosol in four groups of BALB/c mice. The solid lines show the corresponding predictions of the three parameters obtained using an anatomically based computational model of the mouse lung. The vertical arrows along the bottom indicate the start times of 40-second exposures to methacholine at the indicated concentrations. Deep inflations of the lung to a pressure limit of 25 cm H2O were given before each exposure. Ova = ovalbumin; PLL = poly-l-lysine.
To accurately simulate the behavior of the Ova group, we had to make two changes to the model as per our previous study (14). The first change was to add a lining to the inside of all the airways to represent a physically thickened epithelium, so that more airways reached the critical closure radius at some point during the simulation. The second change was to increase the critical radius itself from 38 to 45 μm, to represent an increased propensity for airway closure due to increased airway secretions. Forcing this modified model to follow the time course of R in the Ova group required less smooth muscle shortening than for the naive and PLL simulations (see Methods), but still gave accurate predictions of the corresponding time courses of G and H (Figure 2, bottom left).
Finally, we applied the modified model to the PLL+Ova data by again forcing it to match the measured R time course. This required a somewhat greater degree of airway narrowing because the peak responses in R were elevated compared with the naive group. Our most important result is that the predicted elevations in the G and H responses were sufficient to match those measured experimentally (Figure 2, bottom right). Assuming that PLL did not itself affect wall thickness in the Ova-treated mice, these simulations show that we can account for the methacholine responsiveness of the PLL+Ova group as being due to the combined effects of the individual mechanisms present in the PLL and Ova groups—namely, increased airway smooth muscle shortening, wall thickening, and secretions.
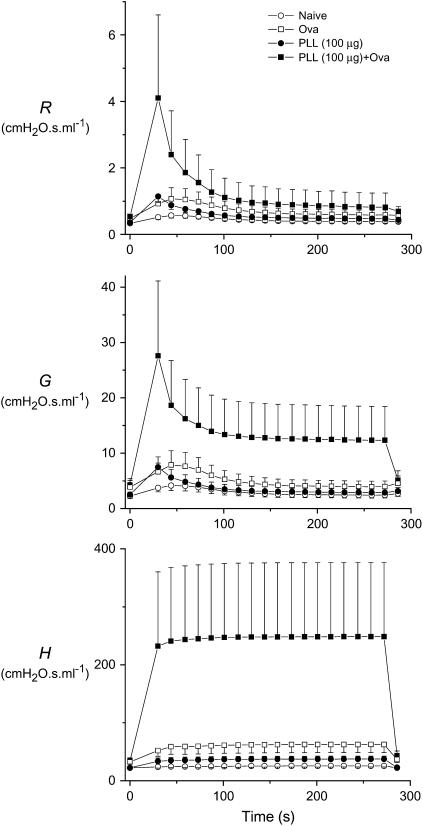
Our ability to predict lung responsiveness in the presence of allergic inflammation and low-dose cationic protein treatment, both individually and in combination, caused us to wonder if the computational model would also predict the kind of extreme bronchoconstriction we believe was responsible for the deaths of the mice that received 100 μg of PLL. We therefore forced the model of the allergically inflamed mouse (with thickened airway walls and increased closure radius) to follow the time course of R measured in our previous study (16) of normal BALB/c mice that had been treated with 100 μg of PLL and challenged with 12.5 mg/ml methacholine aerosol. Figure 3 shows the predicted time courses of R, G, and H, all of which are highly variable (note the large error bars) and greatly elevated compared with the simulations of control mice (14) or of mice that were either allergically inflamed (14) or received cationic protein treated alone (16). Furthermore, the elevation is most pronounced in H (Figure 3, bottom panel), which rises more than 10-fold above its baseline (premethacholine) value. Most of this rise in H is caused by closure of small airways, and corresponds to the derecruitment of more than 90% of the lung. If this were to actually happen in a real lung, it would presumably be functionally catastrophic and might explain the sudden deaths we observed in vivo. The computational model thus predicts that allergic inflammation and cationic protein treatment have a strongly synergistic effect on airway responsiveness to methacholine.
Figure 3.
Computational model prediction of the effect of simultaneous allergic inflammation and treatment with 100 μg intratracheal poly-l-lysine (PLL) on airway responsiveness in BALB/c mice, compared with either treatment separately and with untreated (naive) animals. R, G, and H are the three parameters characterizing the input impedance, Z. The data shown are mean ± SE obtained from a Monte-Carlo simulation in which the model was run 16 times, each time using a different random sampling of the baseline airway diameters for the mouse tabulated by Gomes and Bates (54) after modification so that the simulated Z matched that measured in the BALB/c mouse (14). Ova = ovalbumin.
Effects of Mouse Strain
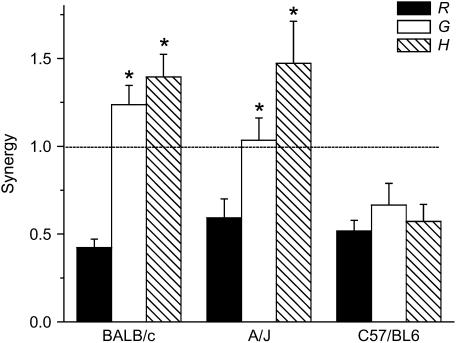
To establish the generality of our results, we repeated our experiments in two other strains of mice, the A/J because it is innately hyperresponsive to methacholine (18, 23) and the C57BL/6 because it is widely used in the generation of genetically manipulated mice. To quantitate the synergistic effects of cationic protein and antigen treatment, we calculated the mean elevations in R, G, and H, after each dose of methacholine as fractions of their respective baseline values measured before any of the challenges were given. We then calculated, for each parameter and each mouse strain, the ratio of the fractional elevation in the PLL+Ova group to the sum of the elevations in the PLL and Ova groups. A ratio greater than 1 indicates that combining cationic protein and antigen treatments causes a degree of hyperresponsiveness that is greater than the sum of the two conditions alone, meaning that they act together in a synergistic fashion. Figure 4 shows the mean ratios (termed “synergy”) obtained from the four methacholine challenges. The synergy for R is significantly less than 1 in all three strains, whereas synergy for both G and H is greater than 1 and significantly greater than synergy for R in the BALB/c and A/J mice (Figure 4). This means that if the effects of cationic protein and antigen treatment had been additive in R, they would have caused synergy in G and H to be substantially greater than unity. Interestingly, the ratios for R, G, and H are all similar and well below 1 in the C57/BL6 mice (Figure 4); although this strain did mount an inflammatory reaction against antigen (Table 1), the reaction did not manifest as an increased propensity of the airways to close during bronchoconstriction.
Figure 4.
Measures of synergy between cationic protein and antigen treatments. The mean elevations in R, G, and H after each dose of methacholine were calculated as fractions of their respective baseline values measured before any of the challenges were given. Synergy is the ratio of the elevation in a parameter from the PLL+Ova group to the sum of the elevations from the PLL and Ova groups. Ova = ovalbumin; PLL = poly-l-lysine. *Significant difference compared with R in the same strain (unpaired t test).
DISCUSSION
The major result of the present study is that allergic inflammation and intratracheal cationic protein together produce a response to methacholine in mice that is substantially greater than either treatment alone. In particular, in two of the mouse strains we studied, the plateau response in H after both Ova and PLL treatment was more than the sum of the responses after either treatment alone (Figures 1 and 4). We interpret the plateau response in H as largely reflecting derecruitment of lung units due to closure of small airways. This interpretation is based on several lines of evidence. First, we have shown previously using our computational model that, when airways narrow down to a critical radius, they must be immediately and irreversibly closed to simulate the simultaneous changes in R, G, and H seen after challenge with methacholine aerosol (14). Furthermore, it has been found by several groups of investigators that the ratio G/H, known as hysteresivity, always increases during bronchoconstriction, a situation in which the lung becomes mechanically heterogeneous. However, it has also been shown both numerically (24) and analytically (25) that an increase in hysteresivity is only expected when heterogeneities are not too extreme. If some airways are allowed to become arbitrarily narrow, leading to extreme differences in regional time constants throughout the lung, then hysteresivity should start to decrease again. The fact that this is not observed experimentally indicates that airways can only narrow to a certain extent, after which they snap shut altogether, presumably due to the sudden formation of liquid bridges across the lumen (26). We have also exploited the phenomenon of absorption atelectasis to obtain direct evidence that closure of small airways occurs during bronchoconstriction (18, 27).
Our present results thus indicate that PLL and Ova together have a synergistic effect on the amount of airway closure that persists after the induction of bronchoconstriction. Furthermore, the strength of this synergy depends on the dose of intratracheal PLL that is administered before methacholine challenge. That is, although synergy was clearly evident when 33 μg PLL was instilled, the H response was only just greater than the sum of the responses to Ova and PLL alone (Figure 4), and the mice easily recovered from the effects of an entire methacholine challenge protocol (Figure 1). By contrast, when we instilled three times the dose of PLL, the allergically inflamed mice died immediately after challenge with the lowest dose of methacholine.
We explain the above synergy as arising from the combined effects of the increased airway secretions and wall thickness accompanying allergic inflammation and the increased smooth muscle shortening caused by cationic protein. Support for this explanation is provided by our computational model, which is able to accurately reproduce the principal features of R, G, and H after methacholine challenge in the four groups of BALB/c mice we studied (Figure 2). The modeling of the data from the naive, PLL, and Ova groups is exactly as we have already established in previous studies (14, 16). The novel result shown in the present study is that, when the model is simultaneously imbued with the characteristics it needs to mimic the data from both the PLL (Figure 2, top right) and Ova (Figure 2, bottom left) groups, it then accurately captures the behavior of the PLL+Ova group (Figure 2, bottom right). Furthermore, when the model was made to constrict to a greater extent, as appropriate for the higher dose of 100 μg PLL, it predicted that, on average, about 90% of the lung would derecruit (Figure 3, bottom panel). The model also showed substantial variability from one individual simulation to the next (Figure 3), further emphasizing the mechanical instability of the allergically inflamed lung that has been treated with a large dose of intratracheal PLL.
The computational model is not, of course, a perfect representation of the mouse lung. Indeed, despite its anatomic detail, the model still contains what we know to be simplifying assumptions. For example, we assume that all airways narrow by the same fractional amount during bronchoconstriction, whereas we know that airway narrowing is heterogeneous (24, 28). We also ascribe the Ova-induced radial thickening of the airway wall solely to thickening of the epithelial layer, which we assume is the same for all airways; yet, if thickening also involves expansion of the other components of the wall, then it would be expected to increase with the size of the airway. Our justification for making assumptions such as these is based on the notion that, although they might change the details of the model simulations, the overall message will remain the same because the global structure of the model is anatomically realistic. Accordingly, we believe that the ability of the model to predict experimental results on the basis of putative mechanisms shows, at the very least, that the mechanisms are biologically plausible.
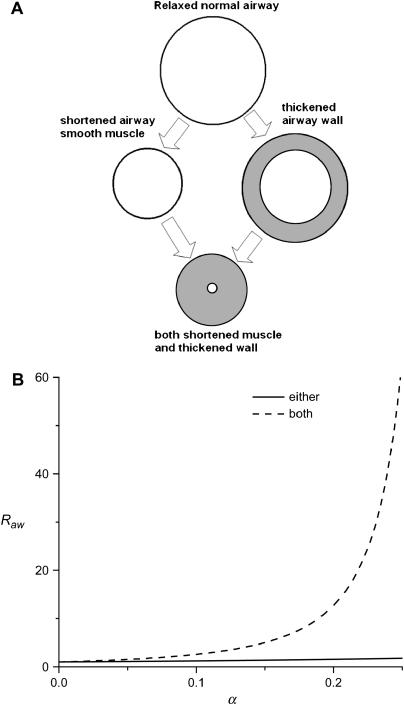
The considerable structural complexity of our computational model is obviously key to its ability to credibly test hypotheses about mechanisms of AHR in the mouse. By the same token, however, this very complexity can be an impediment to intuitive understanding of how the mechanisms actually operate, particularly when they occur in combination. It is therefore worth considering a simplified explanation of the synergy between airway wall thickening and smooth muscle shortening that, although highly idealized, nevertheless captures the essential mechanisms involved. Such a simplified interpretation is illustrated in Figure 5A, which shows a cylindrical airway surrounded by a uniform layer of smooth muscle. Assuming laminar flow through this airway, its resistance, Raw, is inversely related to the diameter of its lumen. We allow for the possibility that Raw can increase above its baseline value in two distinct ways: (1) by the circumferential shortening of the smooth muscle (i.e., a response to contractile stimulation) and (2) by the inward thickening of the airway wall (i.e., inflammation of the bronchial mucosa). We quantify these two mechanisms, respectively, in terms of (1) the fraction by which the smooth muscle shortens and (2) the fraction of the lumen radius occupied by expanded wall material. Both fractions are 0 under baseline conditions. Full occlusion of the airway lumen occurs when either fraction achieves a value of 1 while the other remains at 0. When both smooth muscle shortening and airway wall thickening increase together, however, full occlusion of the lumen is achieved when both fractions reach only a little over 0.3 (see the online supplement). This represents a synergy between the two mechanisms—that is, the combined effect of smooth muscle shortening and wall thickening on the elevation of Raw is greater than the sum of their individual effects. This is shown graphically in Figure 5B, from which it is apparent that the strength of the synergy increases rapidly as smooth muscle shortening and wall thickening themselves increase. The synergistic mechanism just described is identical to that operative in the airways of our anatomically based computational model of the mouse lung. The effects of this mechanism are obviously most evident in the smaller airways because a given amount of thickening causes the airway wall to impinge on the lumen to a proportionately greater degree in smaller compared with larger airways.
Figure 5.
(A) Simple model of a single constricting airway. We consider narrowing of this airway by two distinct mechanisms: (1) shortening of the airway smooth muscle by a factor α < 1 and (2) thickening of the bronchial mucosa such that the airway lumen is reduced by the same factor. (B) Airway resistance (relative to baseline) as a function of smooth muscle shortening and wall thickening when either occurs separately or both occur together (both effects are quantified by the fraction α).
The above explanation is based on the supposition that there is at least some overlap between those airways that become hypersensitive as a result of exposure to PLL and those that become inflamed from the Ova treatment. We expect such an overlap to exist in our PLL+Ova mice, even though the PLL, being instilled via the trachea, might be expected to affect the central airways predominately, because the Ova treatment leads to a generalized inflammation of the entire lung. Nevertheless, a positive synergy between the effects of cationic protein treatment and allergic inflammation is not always observed for all parameters of Zrs. In fact, synergy was negative for R in all three strains of mice (Figure 4), which we explain as follows. Cationic protein treatment is believed to compromise the barrier function of the airway epithelium, making the underlying smooth muscle more accessible to agonist deposited in the airway lumen and thus increasing methacholine sensitivity in R (16, 29). By contrast, we have recently argued that the epithelial thickening caused by allergic inflammation increases the physical barrier between the airway lumen and the underlying smooth muscle (14), manifest as a reduced and delayed peak response in R (Figure 2, lower left). When cationic protein and antigen treatment are combined, the magnitude of the resulting response in R represents a balance between these two opposing effects, and for the data shown in Figure 1 this balance tips in favor of an increased epithelial barrier. Even so, synergy values for G and H were significantly greater than synergy for R in both BALB/c and A/J strains, exceeding unity (Figure 4). If we had used a dose of PLL that caused synergy for R to reach a value of unity, the corresponding synergy values for G and H would have been substantially higher, possibly even to the point of compromising life support.
The synergistic amplification of AHR in the genetically hyperresponsive A/J strain was similar to that in the BALB/c strain (Figure 4). Although A/J mice mount a robust respond to aerosol methacholine challenge (23), the response is located more centrally than in BALB/c mice (18). When this constriction is superimposed on existing inflammation of the peripheral airways, the net result is a similar degree of elevation of R, G, and H. By contrast, the C57BL/6 strain showed no synergy at all (Figure 4) because the mice did not develop AHR after antigen treatment despite clearly developing an inflammatory reaction (Table 1). This implies that acute allergic inflammation in this strain did not cause an increase in the thickness of the airway epithelium, in agreement with the findings of others (30). Indeed, Takeda and colleagues (30) observed that peribronchial inflammation does not occur in C57BL/6 mice. In any case, the results in Figure 4 show that the potential for synergistic closure of airways in the lung during bronchoconstriction depends both on the susceptibility for developing an inflammatory thickening of the airway walls and on the intrinsic responsiveness of the airway smooth muscle.
The synergy we have identified may have important relevance to acute exacerbations of asthma, which significantly influence the morbidity, mortality, and health care costs associated with the disease and may contribute to long-term functional consequences (31, 32). The pathophysiology of asthma itself has been extensively studied for decades (33–35), and is often considered to be a dysfunction of the small peripheral airways (31, 36, 37). Even so, the pathophysiology of the asthma exacerbation is still not well understood, in part because it is difficult to study acutely ill patients. Nevertheless, several lines of evidence suggest that asthma exacerbations involve large airways. Helium–oxygen mixtures may acutely relieve the airflow limitation and dyspnea of an acute asthma attack (38), presumably by reducing airflow turbulence in large airways (38, 39). Also, patients with severe asthma appear to benefit more from treatment with ipratropium bromide, which acts on receptors localized to the large conducting airways (40), and a preferential increase in smooth muscle mass in the large airways distinguishes fatal from nonfatal asthma (41). Asthma exacerbations may thus represent the acute narrowing of conducting airways superimposed on a background of generalized lung inflammation.
The nature of the asthma exacerbation thus appears to be highly reminiscent of the situation we encountered in the mice of the present study in which airway closure was a significant feature of the response to bronchial challenge. Of course, the analogy to the asthma exacerbation may be limited by differences in structure between the mouse and human lungs. For example, the human lung is known to have a significant amount of collateral ventilation (42), which may reduce the functional importance of airway closure relative to its role in mice. Also, even though there is now compelling evidence from a variety of imaging studies (43–45) that substantial airway closure may occur in humans with asthma after bronchospasm, it remains unclear to what extent this closure persists as subjects breathe above functional residual capacity, so we clearly need to be cautious about extrapolating to the human situation. Nevertheless, our results suggest that synergy between different mechanisms of AHR may be an important factor in asthma exacerbations, even if the precise nature of that synergy is not identical to the situation we find in mice. This being the case, the PLL-treated allergically inflamed mouse may be useful as a model of the asthma exacerbation and as a platform for testing approaches to therapy. Such therapies might include treatments aimed at reducing surface tension in the lung. Indeed, β-agonists have some efficacy in this regard, because they have been shown to stimulate surfactant release (46) in addition to their well-known action as a smooth muscle relaxant, thus explaining their efficacy in the setting of an acute asthma attack. Other potential therapies might target specific components of inflammatory exudate, such as fibrin, which is known to interfere with surfactant function and increase the likelihood that small airways will close (47). Last, our results are consistent with the efficacy of a new asthma treatment, thermoplasty, which eliminates smooth muscle from the central airways (48, 49) and also possibly affects the epithelium (49).
Finally, our findings about the insidious potential for synergy to arise between two distinct mechanisms of AHR may also have implications for the study of complex diseases in general. It is becoming increasingly recognized that many common pathologies cannot be understood in terms of a single underlying mechanism. Substantial efforts are underway to try to understand complex diseases as derangements in the behavior of gene or protein networks (50, 51) (i.e., at the level of the genome or proteome), but less attention has been given to the possibility that interactions at the level of the physiome (52) may also be key to understanding complex disease. Indeed, there is no reason to suppose that pathophysiology at this level should be any less complex than at lower levels of scale, and the present study shows how interactions at the level of the physiome can lead to severe and even fatal consequences in the case of asthma.
In conclusion, we have shown that the combination of two distinct mechanisms of AHR, increased smooth muscle shortening and increase airway wall thickness, can act synergistically to produce an extreme decrement in lung mechanical function, even when either mechanism alone is well tolerated. We suggest that mechanistic synergy may be key to understanding the pathophysiology of the severe asthma exacerbation. Our results also highlight the need to think of causes of asthma as arising from interactions between multiple mechanisms, and seem to validate the current trend toward combining multiple therapeutic modalities (53).
Supplementary Material
Supported by NIH grants R01 HL67273 and HL75593 and the National Center for Research Resources (NCRR) Center for Biomedical Research Excellence (P20 RR15557).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200706-832OC on October 25, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Jarvis D, Burney P. Epidemiology of asthma. In: Busse W, Holgate S, editors. Asthma and rhinitis, 2nd ed. Oxford, UK: Blackwell Science; 2000. pp. 17–32.
- 2.Pearce N, Beasley R, Crane J, Burgess C. Epidemiology of asthma mortality. In: Busse W, Holgate S, editors. Asthma and rhinitis, 2nd ed. Oxford, UK: Blackwell Science; 2000. pp. 65–72.
- 3.Brusasco V, Pellegrino R. Complexity of factors modulating airway narrowing in vivo: relevance to assessment of airway hyperresponsiveness. J Appl Physiol 2003;95:1305–1313. [DOI] [PubMed] [Google Scholar]
- 4.Moreno RH, Hogg JC, Pare PD. Mechanics of airway narrowing. Am Rev Respir Dis 1986;133:1171–1180. [DOI] [PubMed] [Google Scholar]
- 5.Macklem PT. Bronchial hyporesponsiveness. Chest 1987;91(6, Suppl):189S–191S. [DOI] [PubMed] [Google Scholar]
- 6.Borger P, Tamm M, Black JL, Roth M. Asthma: is it due to an abnormal airway smooth muscle cell? Am J Respir Crit Care Med 2006;174:367–372. [DOI] [PubMed] [Google Scholar]
- 7.Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma: a 3-D morphometric study. Am Rev Respir Dis 1993;148:720–726. [DOI] [PubMed] [Google Scholar]
- 8.Tuck SA, Maghni K, Poirier A, Babu GJ, Periasamy M, Bates JHT, Leguillette R, Lauzon AM. Time course of airway mechanics of the (+)insert myosin isoform knockout mouse. Am J Respir Cell Mol Biol 2004;30:326–332. [DOI] [PubMed] [Google Scholar]
- 9.Ding DJ, Martin JG, Macklem PT. Effects of lung volume on maximal methacholine-induced bronchoconstriction in normal humans. J Appl Physiol 1987;62:1324–1330. [DOI] [PubMed] [Google Scholar]
- 10.Adler A, Bates JHT. A micromechanical model of airway-parenchymal interdependence. Ann Biomed Eng 2000;28:309–317. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel S, Holgate ST. The mouse trap: it still yields few answers in asthma. Am J Respir Crit Care Med 2006;174:1173–1176. [Discussion, 1176–1178.] [DOI] [PubMed] [Google Scholar]
- 12.Bates JHT, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol 2003;94:1297–1306. [DOI] [PubMed] [Google Scholar]
- 13.Tomioka S, Bates JH, Irvin CG. Airway and tissue mechanics in a murine model of asthma: alveolar capsule vs. forced oscillations. J Appl Physiol 2002;93:263–270. [DOI] [PubMed] [Google Scholar]
- 14.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JHT. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol 2004;96:2019–2027. [DOI] [PubMed] [Google Scholar]
- 15.Solway J, Fredberg JJ. Perhaps airway smooth muscle dysfunction contributes to asthmatic bronchial hyperresponsiveness after all. Am J Respir Cell Mol Biol 1997;17:144–146. [DOI] [PubMed] [Google Scholar]
- 16.Bates JHT, Wagers SS, Norton RJ, Rinaldi LM, Irvin CG. Exaggerated airway narrowing in mice treated with intra-tracheal cationic protein. J Appl Physiol 2006;100:500–506. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro SD. Animal models of asthma: pro: allergic avoidance of animal (model[s]) is not an option. Am J Respir Crit Care Med 2006;174:1171–1173. [DOI] [PubMed] [Google Scholar]
- 18.Wagers SS, Haverkamp HC, Bates JHT, Norton RJ, Thompson-Figueroa JA, Sullivan MJ, Irvin CG. Intrinsic and antigen-induced airway hyperresponsiveness are the result of diverse physiological mechanisms. J Appl Physiol 2007;102:221–230. [DOI] [PubMed] [Google Scholar]
- 19.Cojocaru A, Haverkamp H, Irvin CG, Bates JHT. Synergistic mechanisms of airway hyperresponsiveness: dependence on mouse strain. Am J Respir Crit Care Med 2007;175:A156. [Google Scholar]
- 20.Irvin CG, Rinaldi LM, Bates JHT. Peripheral lung inflammation and enhanced narrowing of central airway s combine synergistically to produce extreme hyperresponsiveness in mice [abstract]. Proc Am Thorac Soc 2005;2:A783. [Google Scholar]
- 21.Gomes RF, Shen X, Ramchandani R, Tepper RS, Bates JH. Comparative respiratory system mechanics in rodents. J Appl Physiol 2000;89:908–916. [DOI] [PubMed] [Google Scholar]
- 22.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 1992;72:168–178. [DOI] [PubMed] [Google Scholar]
- 23.Duguet A, Biyah K, Minshall E, Gomes R, Wang CG, Taoudi-Benchekroun M, Bates JHT, Eidelman DH. Bronchial responsiveness among inbred mouse strains: role of airway smooth-muscle shortening velocity. Am J Respir Crit Care Med 2000;161:839–848. [DOI] [PubMed] [Google Scholar]
- 24.Thorpe CW, Bates JHT. Effect of stochastic heterogeneity on lung impedance during acute bronchoconstriction: a model analysis. J Appl Physiol 1997;82:1616–1625. [DOI] [PubMed] [Google Scholar]
- 25.Bates JH, Allen GB. The estimation of lung mechanics parameters in the presence of pathology: a theoretical analysis. Ann Biomed Eng 2006;34:384–392. [DOI] [PubMed] [Google Scholar]
- 26.Otis DR Jr, Johnson M, Pedley TJ, Kamm RD. Role of pulmonary surfactant in airway closure: a computational study. J Appl Physiol 1993;75:1323–1333. [DOI] [PubMed] [Google Scholar]
- 27.Lundblad LK, Thompson-Figueroa J, Allen GB, Rinaldi L, Norton RJ, Irvin CG, Bates JHT. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med 2007;175:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutchen KR, Hantos Z, Petak F, Adamicza A, Suki B. Airway inhomogeneities contribute to apparent lung tissue mechanics during constriction. J Appl Physiol 1996;80:1841–1849. [DOI] [PubMed] [Google Scholar]
- 29.Homma T, Bates JH, Irvin CG. Airways hyperresponsiveness induced by cationic proteins in vivo: site of action. Am J Physiol Lung Cell Mol Physiol 2005;289:L413–L418. [DOI] [PubMed] [Google Scholar]
- 30.Takeda K, Haczku A, Lee JJ, Irvin CG, Gelfand EW. Strain dependence of airway hyperresponsiveness reflects differences in eosinophil localization in the lung. Am J Physiol Lung Cell Mol Physiol 2001;281:L394–L402. [DOI] [PubMed] [Google Scholar]
- 31.Wenzel S, Fahy JW, Irvin CG, Peters SP, Spector S, Szelfer SJ. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations and unanswered questions. Am J Respir Crit Care Med 2000;162:2341–2351. [DOI] [PubMed] [Google Scholar]
- 32.Irvin CG. Interaction between the growing lung and asthma: role of early intervention. J Allergy Clin Immunol 2000;105:S540–S546. [DOI] [PubMed] [Google Scholar]
- 33.Woolcock AJ, Read J. The static elastic properties of the lungs in asthma. Am Rev Respir Dis 1968;98:788–794. [DOI] [PubMed] [Google Scholar]
- 34.Gold WM, Kaufman HS, Nadel JA. Elastic recoil of the lungs in chronic asthmatic patients before and after therapy. J Appl Physiol 1967;23:433–438. [DOI] [PubMed] [Google Scholar]
- 35.Finucane KE, Colebatch HJ. Elastic behavior of the lung in patients with airway obstruction. J Appl Physiol 1969;26:330–338. [DOI] [PubMed] [Google Scholar]
- 36.Kaminsky DA, Irvin CG. New insights from lung function. Curr Opin Allergy Clin Immunol 2001;1:205–209. [DOI] [PubMed] [Google Scholar]
- 37.Pare PD, Lawson LM, Brooks LA. Patterns of response to inhaled bronchodilators in asthmatics. Am Rev Respir Dis 1983;127:680–685. [DOI] [PubMed] [Google Scholar]
- 38.Ho AM, Lee A, Karmakar MK, Dion PW, Chung DC, Contardi LH. Heliox vs air-oxygen mixtures for the treatment of patients with acute asthma: a systematic overview. Chest 2003;123:882–890. [DOI] [PubMed] [Google Scholar]
- 39.Bhansali PV, Irvin CG, Dempsey JA, Bush R, Webster JG. Human pulmonary resistance: effect of frequency and gas physical properties. J Appl Physiol 1979;47:161–168. [DOI] [PubMed] [Google Scholar]
- 40.Bethel RD, Irvin CG. Anticholinergic drugs and asthma. Sem Respir Med 1987;8:366–371. [Google Scholar]
- 41.Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis 1993;147:405–410. [DOI] [PubMed] [Google Scholar]
- 42.Macklem PT. Airway obstruction and collateral ventilation. Physiol Rev 1971;51:368–436. [DOI] [PubMed] [Google Scholar]
- 43.Samee S, Altes T, Powers P, de Lange EE, Knight-Scott J, Rakes G, Mugler JP III, Ciambotti JM, Alford BA, Brookeman JR, et al. Imaging the lungs in asthmatic patients by using hyperpolarized helium-3 magnetic resonance: assessment of response to methacholine and exercise challenge. J Allergy Clin Immunol 2003;111:1205–1211. [DOI] [PubMed] [Google Scholar]
- 44.Venegas JG, Winkler T, Musch G, Vidal Melo MF, Layfield D, Tgavalekos N, Fischman AJ, Callahan RJ, Bellani G, Harris RS. Self-organized patchiness in asthma as a prelude to catastrophic shifts. Nature 2005;434:777–782. [DOI] [PubMed] [Google Scholar]
- 45.King GG, Eberl S, Salome CM, Meikle SR, Woolcock AJ. Airway closure measured by a Technegas bolus and SPECT. Am J Respir Crit Care Med 1997;155:682–688. [DOI] [PubMed] [Google Scholar]
- 46.Ewing CK, Duffy DM, Roberts JM. Characterization of the beta-adrenergic receptor in isolated human fetal lung type II cells. Pediatr Res 1992;32:350–355. [DOI] [PubMed] [Google Scholar]
- 47.Wagers SS, Norton RJ, Rinaldi LM, Bates JHT, Sobel BE, Irvin CG. Extravascular fibrin, plasminogen activator, plasminogen activator inhibitors, and airway hyperresponsiveness. J Clin Invest 2004;114:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cox G, Thomson NC, Rubin AS, Niven RM, Corris PA, Siersted HC, Olivenstein R, Pavord ID, McCormack D, Chaudhuri R, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med 2007;356:1327–1337. [DOI] [PubMed] [Google Scholar]
- 49.Solway J, Irvin CG. Airway smooth muscle as a target for asthma therapy. N Engl J Med 2007;356:1367–1369. [DOI] [PubMed] [Google Scholar]
- 50.Bray D. Molecular networks: the top-down view. Science 2003;301:1864–1865. [DOI] [PubMed] [Google Scholar]
- 51.Kitano H. Systems biology: a brief overview. Science 2002;295:1662–1664. [DOI] [PubMed] [Google Scholar]
- 52.Crampin EJ, Halstead M, Hunter P, Nielsen P, Noble D, Smith N, Tawhai M. Computational physiology and the Physiome Project. Exp Physiol 2004;89:1–26. [DOI] [PubMed] [Google Scholar]
- 53.Moore WC, Peters SP. Update in asthma 2006. Am J Respir Crit Care Med 2007;175:649–654. [DOI] [PubMed] [Google Scholar]
- 54.Gomes RF, Bates JH. Geometric determinants of airway resistance in two isomorphic rodent species. Respir Physiolo Neurobiol 2002;130:317–325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.