Abstract
Rationale: The development of lung cancer (LC) is accompanied by field changes in the airway mucosa that may have prognostic importance.
Objectives: To compare patients with prevalent LC to control subjects regarding their histologic dysplasia scores and chromosomal aneusomy as measured by fluorescence in situ hybridization (FISH).
Methods: The most advanced bronchial histology lesion was assessed from each of 44 LC cases and 90 cancer-free control subjects using a four-color FISH probe set encompassing the chromosome 6 centromere, 5p15.2, 7p12 (epidermal growth factor receptor), and 8q24 v-myc myelocytomatosis viral oncogene homolog (MYC) sequences. Histology grades were coded as dysplasia (moderate or severe) or carcinoma in situ (CIS).
Measurements and Main Results: CIS was the highest histologic grade for 32 subjects, and dysplasia was the highest grade for 102 subjects (54 moderate, 48 severe). Chromosomal aneusomy was seen in 64% of the LC cases, but in only 31% of the control subjects (odds ratio [OR], 4.68; 95% confidence interval [CI]. 1.97–11.04). Among those with any level of dysplasia, the OR for positive FISH and LC was 2.28 (95% CI, 0.75–6.86). Among those with CIS, the OR for positive FISH and LC was 5.84 (95% CI, 1.31–26.01).
Conclusions: Chromosomal aneusomy is associated with LC. Prospective examination of aneusomy as a precursor lesion that predicts LC is needed.
Keywords: chromosomal aneusomy, fluorescence in situ hybridization, carcinoma in situ, premalignancy
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Lung cancer (LC) develops through accumulation of multiple molecular and genetic changes, but the association between chromosomal aneusomy in bronchial lesions and LC risk is not well studied.
What This Study Adds to the Field
Carcinoma in situ and chromosomal aneusomy were identified as predictors of invasive LC in a case-control study of high-risk subjects. The interaction of these markers may improve the diagnostic capability in preneoplastic bronchial lesions.
Lung cancer (LC) is the most common fatal cancer in the United States and most parts of the world. About 160,000 deaths are expected to occur annually in the United States alone, and the incidence of LC in developing countries continues to rise (1, 2). Only about 15% of patients with LC survive 5 years with the best diagnostic and treatment options currently available. The main reason for the poor outcome is that more than two-thirds of cases are diagnosed at an advanced stage and, even when diagnosed in stage I, up to 40% of cancers recur after surgical resection. Smoking is the overwhelming risk factor, and most studies indicate that about 90% of cases are due to smoking (2). Familial and genetic factors also affect the susceptibility to LC development (3, 4).
To substantially improve the outcome for LC, new strategies of prevention and early detection are required (5). Screening with low-dose helical computed tomography scanning has been effective in finding small LC nodules that may be cured by surgery (6, 7), but the effect of this technique on survival is still uncertain (8, 9). Detecting radiographically occult intraepithelial bronchial lesions with sputum cytology and autofluorescence bronchoscopy (10, 11) could further contribute to improved survival in LC, as seen in other epithelial malignancies, such as cervical cancer and cancer of the colon (12, 13).
The development of centrally located bronchogenic carcinoma likely occurs through a stepwise process of carcinogenesis that is reflected by advancing histologic grade of dysplasia to carcinoma in situ (CIS) and invasive cancer (14). These changes are accompanied by the accumulation of chromosomal abnormalities throughout the airway mucosa, a process known as field cancerization (14, 15). The distinction between high-grade dysplastic lesions is subjective (16), and histologic grade was shown to be an uncertain predictor of progression to invasive cancer, reflecting the variability in the biological behavior of these lesions (17). There is therefore a need for biomarkers that can be used to more accurately predict the development of invasive cancer from a preneoplastic lesion than can histologic grade alone.
Chromosomal aneusomy has long been recognized as a hallmark for LC (18). The application of fluorescence in situ hybridization (FISH) technology to interphase cells has greatly facilitated the study of chromosomal changes in exfoliated cells and other airway epithelial samples (19). In previous studies, we have found that the detection of aneusomy by multitarget FISH assay increased the sensitivity of sputum cytology as a predictor of LC in high-risk subjects (20). In the present study, we investigated chromosomal aneusomy in situ in biopsies collected from heavy smokers by autofluorescence and white-light bronchoscopy, and correlated this biomarker with the histologic grade of the lesions and the presence of invasive LC. This study tested the hypotheses that chromosomal aneusomy is associated with histologic grade in bronchial epithelium, and that it is also associated with LC, accounting for histologic grade. Preliminary reports on the data contained in this article have been presented in abstract form at the Fourth International Chicago Symposium on Malignancies of the Chest and Head and Neck (21) and the 12th World Conference on Lung Cancer (22).
METHODS
Subjects
The study was performed using patient records and histologic sections that have been collected at the University of Colorado Cancer Center (UCCC) in Aurora, CO (106 individuals), the British Columbia Cancer Agency (BCCA) in Vancouver, Canada (19 individuals), and the University of Iceland Hospitals (UIH) in Reykjavik, Iceland (9 individuals). This study included all individuals in the UCCC database with CIS and severe dysplasia (SD) as the highest grade of mucosal abnormality, and 70% of those with moderate dysplasia (MD). The study population was specifically enriched with subjects with CIS lesions from the BCCA (Canada) and the UIH (Iceland).
The subjects studied were all considered to be at high risk for LC based on a history of at least 30 pack-years of smoking and spirometric evidence of airflow obstruction documented by an FEV1/FVC ratio of less than 75% and an FEV1 of less than 70% of predicted. Former smokers were defined as having quit at least 1 year before the time of enrollment. Pack-years was defined as the average number of packs smoked per day multiplied by the numbers of years smoked.
Subjects filled out a standard questionnaire, spirometry was performed, and results recorded. Flexible fiberoptic bronchoscopy was performed with both autofluorescence and white-light examination of the airways using either a Xillix LIFE II or OncoLIFE systems (Xillix, Richmond, BC, Canada), as previously described (10, 11), at the UCCC and BCCA sites; white-light examination alone was performed at the UIH. The BCCA cases had been diagnosed in a prospective study of early LC using autofluorescence and white-light bronchoscopy, or the subjects were enrolled as part of two National Cancer Institute–sponsored chemoprevention trials; in one case, the CIS biopsy was taken before chemoprevention, and in the other, after 6-month treatment with retinol. The Icelandic cases were identified from the computerized database of the Department of Pathology, UIH.
Protocols for tissue acquisition by bronchoscopy were approved by the Colorado Multiple Institutional Review Board, the Research and Development Committee of the Denver Veterans Affairs Medical Center, the HealthOne Institutional Review Board, the BCCA–University of British Columbia Clinical Research Ethics Board, the National Bioethics Committee of Iceland, and the Icelandic Data Processing Commission.
The determination of whether or not subjects had invasive LC at the time of bronchoscopy was made based on biopsy results, review of the clinical history, and matching to central cancer registries, vital statistics records, and national death indices at the respective institutions. The clinical information available on each individual included the following: (1) collection date of specimen studied; (2) collection site; (3) date of birth; (4) date of diagnosis of invasive LC (if present); (5) site of invasive LC; (6) histologic grade of preneoplastic lesion; (7) results of aneusomy detection by FISH analysis.
Histologic Analysis
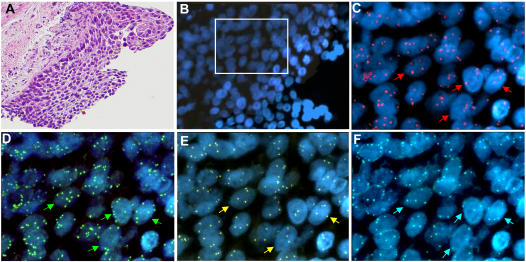
Archived formalin-fixed and paraffin-embedded biopsies were selected for having high-grade dysplasia at the time of the bronchoscopy. Blocks were retrieved and 4-μm sections prepared. One section was stained by hematoxylin and eosin (H&E) and reviewed by the study pathologist (W.A.F.), who determined the grade of premalignant change in the available specimen according to a modification of the World Health Organization criteria (23), in which bronchial dysplasias are assigned the following numerical scores: (1) normal; (2) reserve cell hyperplasia; (3) squamous metaplasia; (4) mild dysplasia; (5) MD; (6) SD; (7) CIS; and (8) invasive carcinoma. When multiple biopsies were available for a patient, the lesion with the highest dysplastic grade was used for analysis; only MDs or high-grade lesions (SD and CIS) were included in this study. Thus, each subject contributed only one biopsy to this analysis. A total of 134 subjects were included in the study, representing 32 with lesions determined as CIS, 48 with lesions classified as SD, and 54 with lesions classified as MD. At the time of the histologic evaluation, the most appropriate area for FISH analysis was selected and a computer image (Figure 1A) created and printed to guide selection of cells for FISH signal enumeration.
Figure 1.
Bronchial epithelium area selected for fluorescence fluorescence in situ hybridization (FISH) analysis. (A) Area with carcinoma in situ stained by hematoxylin and eosin. Magnification: ×20. (B) The same area in the sequential slide submitted to the LAVysion FISH assay stained by 4′,6-diamidino-2-phenylindole (DAPI). Magnification: ×40. The area included in the open rectangle is shown at 100× magnification for combination of fluors in (C) DAPI and SpectrumRed, (D) DAPI and SpectrumGreen, (E) DAPI and SpectrumGold, and (F) DAPI and SpectrumAqua. The specimen displayed aneusomy for (C) epidermal growth factor receptor, (D) 5p15.2, (E) v-myc myelocytomatosis viral oncogene homolog (MYC), and (F) Chromosome Enumeration Probe 6 (CEP6) regions. Arrows indicate single nuclei with extra copies of the tested target DNA.
FISH Analysis
The H&E-stained sections and one 4-μm-thick unstained section per subject were transferred to the FISH laboratory, with the accompanying images identifying the lesions. The unstained slide was subjected to a FISH assay using the LAVysion DNA probe set (Vysis/Abbott Molecular, Des Plaines, IL), including four DNA targets, 6p11.1-q11 (Chromosome Enumeration Probe CEP6, labeled with SpectrumAqua), 5p15.2 (D523, D5S721 labeled in SpectrumGreen, encompasses the SEMA5A gene), 7p12 (labeled in SpectrumRed, encompasses the epidermal growth factor receptor gene), and 8q24.12-q24.13 (labeled in SpectrumGold, encompasses the v-myc myelocytomatosis viral oncogene homolog [MYC] gene).
Initially, the slides were incubated for 2 hours at 56°C, deparaffinized in Citri-Solv (Fisher Scientific, Pittsburgh, PA), and washed in 100% ethanol for 5 minutes. The slides were then incubated in 2× sodium chloride, sodium citrate (SCC) buffer at 75°C for 15 minutes, digested in 0.25 mg/ml proteinase K/2× SSC at 45°C for 16 minutes, and washed in 2× SSC for 5 minutes before being dehydrated in an ethanol series. The probe set was applied according to the manufacturer's instructions to the selected hybridization areas, which were covered with 12-mm glass coverslips and sealed with rubber cement. Codenaturation and hybridization were performed in the Hybrite platform (Vysis), with temperature set for 1 minute at 85°C and 24 hours at 37°C. Posthybridization washes were performed with 2× SSC/0.3% NP40 at 72°C for 2 minutes, followed by wash in 2× SSC for 2 minutes at room temperature and dehydration in ethanol series. Chromatin was counterstained with 4′,6-diamidino-2-phenylindole (0.3 μg/ml in Vectashield Mounting Medium; Vector Laboratories, Burlingame, CA).
Analysis was performed on epifluorescence microscopes (Carl Zeiss MicroImaging, Thornwood, NY) equipped with single band pass interference filter sets for blue (4′,6-diamidino-2-phenylindole), aqua (SpectrumAqua), green (fluorescein isothiocyanate), yellow (SpectrumGold), and red (Texas red). For documentation, images were acquired with a cooled charge-coupled device camera (Photometrics, Tucson, AZ) in monochromatic layers and merged and processed using the CytoVision software (Applied Imaging, Inc., San Jose, CA). The areas selected by the pathologist in the H&E-stained sections were identified in the hybridized slide, and 30–50 nuclei were scored per area. A total of 30 areas with normal histology was also included in the experiments and similarly analyzed. A cell was considered abnormal (aneusomic) when showing three or more signals for two or more of the DNA targets. A premalignant lesion was considered abnormal (aneusomic) based on 100% specificity for the test (e.g., when the frequency of abnormal cells was higher than the highest frequency of aneusomic cells found in areas with normal histology [6.7%]).
Statistical Analysis
Categorical data (presence of LC, chromosomal aneusomy, and histologic grade) were summarized using frequencies and percents. Distribution of differences between cases and control subjects were examined using the Chi-square test. Logistic regression models were used to investigate the associations between cancer status and histologic dysplasia score and cancer status and FISH analysis outcome. Within the categories of histologic dysplasia (MD, SD, CIS), logistic regression models were developed to explore the associations between FISH analysis outcome and cancer status. Associations were expressed as odds ratios (ORs) with corresponding 95% confidence intervals (CI). MD and normal FISH results (no chromosomal aneusomy) were taken as reference. Univariate and multivariate models adjusting for covariates were implemented. Covariates included age (continuous), sex, smoking status (former vs. current), and pack-years. All analyses were performed using Microsoft Excel and SAS (version 9.1; SAS Institute, Inc., Cary, NC).
RESULTS
The analyzed population was comprised of 104 males and 30 females, with a mean age of 63.7 years. Subjects had a mean smoking history of 62.3 pack-years, and the majority of them (56.7%) were current smokers (Table 1). The distribution of age, gender, and pack-years of smoking were not different between LC cases and control subjects. The cell types of the 44 LC cases included 6 adenocarcinoma, 24 squamous cell, 1 small cell LC, and 13 unspecified non–small cell LC. Of the 44 cases with LC, 41 were known to have prevalent LC at the time of diagnosis, and 3 were diagnosed within 5 years after the bronchoscopy (12, 30, and 45 mo).
TABLE 1.
DEMOGRAPHICS IN THE CASE (INVASIVE LUNG CARCINOMA) AND CONTROL (FREE OF INVASIVE LUNG CARCINOMAS FOR AT LEAST 5 YR) COHORTS
|
P Values from χ2 Test
|
|||||
|---|---|---|---|---|---|
| Cases (%) | Controls (%) | Abnormal FISH (%) | Cancer Status* | FISH** | |
| Characteristic | (n = 44) | (n = 90) | (n = 56) | (44:90) | (56:78) |
| Age, yr | 0.22 | 0.10 | |||
| 30–59 | 12 (27.3) | 37 (41.1) | 19 (33.9) | ||
| 60–69 | 18 (40.9) | 34 (37.8) | 18 (32.1) | ||
| ⩾70 | 14 (31.8) | 19 (21.1) | 19 (33.9) | ||
| Sex | 0.41 | 0.29 | |||
| Male | 36 (81.8) | 68 (75.6) | 46 (82.1) | ||
| Female | 8 (18.2) | 22 (24.4) | 10 (17.9) | ||
| Pack-years | 0.31 | 0.07 | |||
| ⩽50 | 18 (40.9) | 48 (53.3) | 22 (39.3) | ||
| 50–76 | 11 (25.0) | 22 (24.4) | 14 (25.0) | ||
| ⩾75 | 14 (31.8) | 19 (21.1) | 19 (33.9) | ||
| Missing | 1 (2.3) | 1 (1.1) | 1 (1.8) | ||
| Smoking status | 0.07 | 0.03 | |||
| Current | 30 (68.2) | 46 (51.1) | 26 (46.4) | ||
| Former | 14 (31.8) | 43 (47.8) | 30 (53.6) | ||
| Missing | (0.0) | 1. (1.1) | (0.0) | ||
Definition of abbreviation: FISH = fluorescence in situ hybridization.
Cases:controls.
Abnormal FISH:normal FISH.
The relationship between cancer status and both biomarkers, histologic grade and chromosomal aneusomy, is shown in Table 2. Differences between LC cases and control subjects are shown as crude and adjusted OR with 95% CIs. The proportion of subjects with LC increased from carriers of MD (18.5%) and SD (22.9%) to carriers of CIS lesions (71.9%). When the OR for SD and CIS were calculated using MD as a reference, we found no association for SD (OR, 1.27; 95% CI, 0.47–3.45), whereas CIS on histologic analysis was strongly associated with invasive LC (OR, 16.4; 95% CI, 5.02–53.72).
TABLE 2.
ASSOCIATION BETWEEN LUNG CANCER STATUS AND THE BIOMARKERS HISTOLOGIC GRADE AND CHROMOSOMAL ANEUSOMY
| Case* | Control† | |||
|---|---|---|---|---|
| (n = 44) | (n = 90) | |||
| Markers | No. (%) | No. (%) | Crude OR (95% CI) | Adjusted OR‡ (95% CI) |
| Histologic grade | ||||
| MD | 10 (22.7) | 44 (48.9) | 1 | 1 |
| SD | 11 (25) | 37 (41.1) | 1.31 (0.5–3.42) | 1.27 (0.47–3.45) |
| CIS | 23 (52.3) | 9 (10) | 11.24 (4.01–31.56) | 16.41 (5.02–53.72) |
| Chromosomal aneusomy | ||||
| Negative | 16 (36.4) | 62 (68.9) | 1 | 1 |
| Positive | 28 (63.6) | 28 (31.1) | 3.88 (1.81–8.28) | 4.68 (1.97–11.04) |
Definition of abbreviations: CI = confidence interval; CIS = carcinoma in situ; MD = moderate dysplasia; OR = odds ratio; SD = severe dysplasia.
Invasive lung carcinoma.
Confirmed free of invasive lung cancer since bronchoscopy or to time of death from another cause.
Adjusted for age, sex, smoking status, and pack-years.
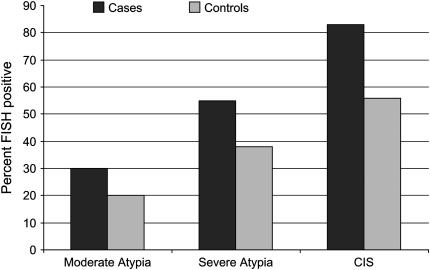
Chromosomal aneusomy (illustrated in Figure 1) was found in premalignant lesions of 56 subjects (41.8%), whereas the lesions in the remaining 78 subjects (58.2%) showed normal FISH results. Among the 44 LC cases, chromosomal aneusomy was found in 28 (63.6%), whereas, in control subjects, aneusomic lesions were seen in 28 of 90 cases (31.1%; OR, 4.68; 95% CI, 1.97–11.04). The proportion of subjects with chromosomal aneusomy increased from MD (22.2%) to SD (41.7%) and CIS lesions (75%), and showed a similar trend for cases and control subjects (Figure 2). The odds of the presence of aneusomy were over 4.5-times greater in LC cases than in those subjects who remained cancer free (Table 3). These odds were lower among those with MD (OR, 2.31; 95% CI, 0.27–20.04) and SD (OR, 3.07; 95% CI, 0.45–21.01), but increased among those with CIS (OR, 5.84; 95% CI, 1.31–26.01). Aneusomy was also significantly associated with smoking status in this study population (P = 0.04), but not with other demographic characteristics.
Figure 2.
Frequency of moderate dysplasia, severe dysplasia, and carcinoma in situ (CIS) among cases and control subjects.
TABLE 3.
ASSOCIATION BETWEEN CANCER STATUS AND CHROMOSOMAL ANEUSOMY AMONG THE HISTOLOGIC GRADES
| Chromosomal Aneusomy
|
||||||
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| Histologic Grade | (n = 56)
|
(n = 78)
|
Crude OR‡ (95% CI) | Adjusted OR‡ (95% CI) | ||
| Case* | Control† | Case* | Control† | |||
| All subjects | 28 | 28 | 16 | 62 | 3.88 (1.81–8.28) | 4.68 (1.97–11.04) |
| MD | 3 | 9 | 7 | 35 | 1.67 (0.36–7.76) | 2.31 (0.27–20.04) |
| SD | 6 | 14 | 5 | 23 | 1.97 (0.51–7.68) | 3.07 (0.45–21.01) |
| CIS | 19 | 5 | 4 | 4 | 3.80 (0.69–20.81) | 5.84 (1.31–26.01) |
For definition of abbreviations, see Table 2.
Invasive lung carcinoma.
Confirmed free of invasive lung cancer since bronchoscopy or to time of death from another cause.
Adjusted for age, sex, smoking status, and pack-years.
Of the 23 CIS lesions with a known LC site, 19 (83%) exhibited chromosomal aneusomy, compared with 5 out of 9 (55%) in CIS lesions occurring in patients without LC. It must be noted that 14 out of the 23 CIS lesions with a known cancer site were obtained at biopsy sites adjacent to an invasive LC diagnosed at the same bronchoscopy. Of these 14 CIS lesions, 11 (79%) were aneusomic. When the 14 cases of CIS with adjacent LC were excluded, 8 of the 9 cases (89%) with LC at a site distant (in a different lobe) from CIS exhibited chromosomal aneusomy.
DISCUSSION
In this study, we have investigated histologic preneoplasia grade and chromosome aneusomy assessed by FISH assay in bronchial biopsies as correlates of prevalent LC. Aneusomy was present in 63.6% of LC cases compared with 31.1% of control subjects (OR, 4.68; 95%, CI 1.97–11.04), implying a significant association with invasive LC. Chromosomal aneusomy was associated with LC in biopsies with any dysplasia, but was particularly strongly associated in biopsies with CIS. It is likely that different probe sets can be defined that will improve the test characteristics of the FISH assay. FISH analysis on tissue samples may add valuable information to conventional histology in the assessment of preneoplastic intraepithelial bronchial lesions, and, in the future, guide the need for monitoring of high-risk patients with aneusomic lesions.
There are several limitations to this study. A total of 32 patients with CIS were available from three institutions; 23 had either a prevalent (20) or incident (3) LC. As 14 of 23 patients with CIS and invasive LC had the CIS at a site adjacent to the LC, it is possible that these subjects may have biased our results toward an association between chromosomal aneusomy and LC. We do not believe this to be the case, as similar fractions (11/14 vs. 8/9) of the patients with CIS and invasive LC at either adjacent or distant sites exhibited aneusomy. A larger study population will be required to evaluate this definitively. Two theories prevail regarding CIS adjacent to an invasive LC: that the CIS represents lateral spread of tumor cells within the epithelium without demonstrated invasion, or, alternatively, these CIS lesions may represent a region of advanced dysplasia within which an invasive carcinoma arose. A second limitation is that this study was largely cross-sectional. The question of whether aneusomy predicts the subsequent development of invasive LC in various histologic grades of premalignancy will require a prospective study. The difficulties of following premalignant lesions for an adequate length of time are illustrated by the fact that the present study represents samples from three sites, two of which (UCCC and BCCA) have performed bronchoscopy in a research setting for over 15 years each. As additional high-risk patients are enrolled and followed, adequate numbers of incident cancers may be accrued to address this in a prospective manner.
A wide variety of chromosomal changes are thought to be important in the development of LC (15, 24). These involve tumor suppressor genes, oncogenes, cell regulatory genes, and DNA repair genes. Chromosomal changes have been known to affect almost every chromosome, and may be found in all regions of the bronchial mucosa. These changes have been identified in airway samples from healthy smokers, from individuals with premalignant dysplastic lesions and in nonmalignant bronchial mucosa from patients with LCs (25). Multifocal and unpredictable histologic and molecular changes in the airways of high-risk smokers have been described as “field cancerization,” a phenomenon particularly reported in epithelial malignancies, such as oral squamous cell carcinoma, carcinoma of the pharynx, and LC (14). Multicolor interphase FISH analysis, as well as spectral karyotyping of metaphases of cultured bronchial epithelial cells, has enhanced the capacity to study chromosomal field changes in bronchial epithelium of high-risk smokers. In a recent study, FISH analysis of exfoliated cells was found to significantly increase the sensitivity of sputum cytology as a predictor of LC (26). FISH has also been used successfully in the diagnosis of breast cancer (27) and carcinoma of the bladder (28).
The development of autofluorescence bronchoscopy has provided an opportunity to study the natural history of preneoplastic lesions in airway epithelium using visual assessment at bronchoscopy and histologic analysis. Up to the present, the histologic grade has been the only clinical biomarker to assess the propensity of preneoplastic change to progress to CIS or invasive cancer. In a recently published prospective follow-up study of 54 individuals with various grades of premalignant lesions, progression to CIS or invasive LC was observed in 30% (10/34) of individuals with low-grade lesions over a median follow-up time of 52 months (17), and in 39% of those with high-grade lesions over median follow-up of 39 months. The difference in progression rate between low- and high-grade lesions was not significant. Fluctuation of histologic grade on repeat, same-site biopsy was observed with 26% of low-grade lesions and 61% of high-grade lesions absent from follow-up biopsies. There are several possible explanations for this finding, including complete removal of small lesions by initial biopsy or regression of dysplasia. Direct observation of continuous, linear progression to malignancy from low- and even high-grade lesions at specific bronchial sites is not to be expected in most individuals, particularly during a short period of observation. The approach to the treatment of these premalignant early lesions will require better definition of the natural history of these lesions with longer follow-up and more comprehensive evaluation of correlative biomarkers. CIS lesions are rare in the clinical setting. Most longitudinal studies indicate that the risk of progression of such a lesion to invasive LC is substantial (29, 30). The development of biomarkers that more accurately predict the progression to invasive cancer is particularly important in this setting.
On the basis of our cross-sectional data, the presence of chromosomal aneusomy might be hypothesized to denote a higher risk of developing invasive LC. Additional prospective studies of FISH analysis for chromosomal aneusomy are required to establish its potential role in the assessment and clinical management of preneoplastic bronchial lesions. Future studies in a larger population are warranted to assess the role of histologic grade and FISH in the evaluation of these lesions. Chromosomal aneusomy may also be one way to identify subjects with the lesions most suitable for chemoprevention trials.
Acknowledgments
The authors thank Jerry Haney (University of Colorado Cancer Center [UCCC] Tissue Procurement Core) and John Reeves (UCCC Cytogenetics Core) for technical assistance.
Supported in part by National Cancer Institute grants U01-CA85070 (W.A.F.), P01-CA58187, P30-CA46934, and the Landspitali-University Hospital Science Fund, Iceland.
Originally Published in Press as DOI: 10.1164/rccm.200708-1142OC on November 7, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66. [DOI] [PubMed] [Google Scholar]
- 2.Peto R, Lopez AD, Boreham J, Thun M, Heath C, Doll R. Mortality from smoking worldwide. Br Med Bull 1996;52:12–21. [DOI] [PubMed] [Google Scholar]
- 3.Jonsson S, Thorsteinsdottir U, Gudbjartsson DF, Jonsson HH, Kristjansson K, Arnason S, Gudnason V, Isaksson HJ, Hallgrimsson J, Gulcher JR, et al. Familial risk of lung carcinoma in the Icelandic population. JAMA 2004;292:2977–2983. [DOI] [PubMed] [Google Scholar]
- 4.Bailey-Wilson JE, Amos CI, Pinney SM, Petersen GM, de Andrade M, Wiest JS, Fain P, Schwartz AG, You M, Franklin W, et al. A major lung cancer locus maps to chromosome 6q23-25. Am J Hum Genet 2004;75:460–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch FR, Franklin WA, Gazdar AF, Bunn PA. Early detection of lung cancer: clinical perspectives of recent advances in biology and radiology. Clin Cancer Res 2001;7:5–22. [PubMed] [Google Scholar]
- 6.Sobue T, Moriyama N, Kaneko M, Kusumoto M, Kobayashi T, Tsuchiya R, Kakinuma R, Ohmatsu H, Nagai K, Nishiyama H, et al. Screening for lung cancer with low-dose helical computerized tomography: anti-lung cancer association project. J Clin Oncol 2002;20:911–920. [DOI] [PubMed] [Google Scholar]
- 7.Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763–1771. [DOI] [PubMed] [Google Scholar]
- 8.Mulshine JL, Sullivan DC. Lung cancer screening. N Engl J Med 2005;352:2714–2720. [DOI] [PubMed] [Google Scholar]
- 9.Bach PB, Jett JR, Pastorino U, Tockman MS, Swensen SJ, Begg CB. Computed tomography screening and lung cancer outcomes. JAMA 2007;297:953–961. [DOI] [PubMed] [Google Scholar]
- 10.Lam S, Kennedy T, Unger M, Miller YE, Gelmont D, Rusch V, Gipe B, Howard D, LeRiche JC, Coldman A, et al. Localization of bronchial intraepithelial lesions by fluorescence bronchoscopy. Chest 1998;13:696–702. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch FR, Prindiville SA, Miller YE, Franklin WA, Dempsey EC, Murphy JR, Bunn PA, Kennedy TC. Fluorescence versus white-light bronchoscopy for detection of preneoplastic lesions: a randomized study. J Natl Cancer Inst 2001;93:1385–1391. [DOI] [PubMed] [Google Scholar]
- 12.Anderson GH, Boyes DA, Benedet JL, LeRiche JC, Matisic JP, Suen KC, Worth AJ, Millner A, Bennett OM. Organization and results of the cervical cytology screening program in British Columbia, 1955–85. Br Med J (Clin Res Ed) 1988;296:975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF, et al.; and The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med 1993;392:1977–1981. [DOI] [PubMed] [Google Scholar]
- 14.Braakhuis BJM, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter's concept of field cancerization: evidence and clinical implications. Cancer Res 2003;63:1727–1730. [PubMed] [Google Scholar]
- 15.Wistuba II, Behrens C, Milchgrub S, Bryant D, Hung J, Minna JD, Gazdar AF. Sequential molecular abnormalities are involved in the multistage development of squamous cell lung carcinoma. Oncogene 1999;18:643–650. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson AG, Perry LJ, Cury PM, Jackson P, McCormick CM, Corrin B, Wells AU. Reproducibility of the WHO/IASLC grading system for pre-invasive squamous lesions of the bronchus: a study of inter-observer and intra-observer variation. Histopathology 2001;38:202–208. [DOI] [PubMed] [Google Scholar]
- 17.Breuer RH, Pasic A, Smit EF, van Vliet E, Vonk Noordegraaf A, Risse EJ, Postmus PE, Sutedja TG. The natural course of preneoplastic lesions in bronchial epithelium. Clin Cancer Res 2005;11:537–543. [PubMed] [Google Scholar]
- 18.Taguchi T, Zhou JY, Feder M, Litwin S, Klein-Szanto AJ, Testa JR. Detection of aneuploidy in interphase nuclei from non–small cell lung carcinomas by fluorescence in situ hybridization using chromosome specific repetitive DNA probes. Cancer Genet Cytogenet 1996;89:120–125. [DOI] [PubMed] [Google Scholar]
- 19.Romeo MS, Sokolova IA, Morrison LE, Zeng C, Barón AE, Hirsch FR, Miller YE, Franklin WA, Varella-Garcia M. Chromosomal abnormalities in non–small cell lung carcinomas and in bronchial epithelia of high-risk smokers detected by multi-target interphase fluorescence in situ hybridization. J Mol Diagn 2003;5:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varella-Garcia M, Kittelson J, Schulte AP, Vu KO, Wolf HJ, Zeng C, Hirsch FR, Byers T, Kennedy T, Miller YE, et al. Multi-target fluorescence in situ hybridization assay increases sensitivity of sputum cytology as a predictor of lung cancer. Cancer Detect Prev 2004;28:244–251. [DOI] [PubMed] [Google Scholar]
- 21.Jonsson S, Varella-Garcia M, Miller YE, Wolf HJ, Lewis M, Kennedy TC, Keith RL, Hirsch F, Franklin WA. Aneusomy, angiogenesis and histology in bronchial biopsies from smokers at high risk for lung cancer. J Thorac Oncol 2006;1:889. [Google Scholar]
- 22.Jonsson S, Varella-Garcia M, Miller YE, Wolf HJ, Byers T, Kiatsimkul P, Bjornsson J, Lam SC, Hirsch FR, Franklin WA. Aneusomy by FISH analysis and histology as predictors of invasive lung cancer in bronchial biopsies from high risk subjects. J Thorac Oncol 2007;2(Suppl 4):S400. [Google Scholar]
- 23.Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J 2001;18:1059–1068. [DOI] [PubMed] [Google Scholar]
- 24.Balsara BR, Testa JR. Chromosomal imbalances in human lung cancer. Oncogene 2002;21:6877–6883. [DOI] [PubMed] [Google Scholar]
- 25.Varella-Garcia M, Chen L, Powell R, Hirsch FR, Kennedy TC, Keith R, Miller YE, Mitchell JD, Franklin WA. Spectral karyotyping detects chromosome damage in bronchial cells of smokers and patients with cancer. Am J Respir Crit Care Med 2007;176:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prindiville SA, Byers T, Hirsch FR, Franklin WA, Miller YE, Vu KO, Wolf HJ, Barón AE, Shroyer KR, Zeng C, et al. Sputum cytological atypia as a predictor of incident lung cancer in a cohort of heavy smokers with airflow obstruction. Cancer Epidemiol Biomarkers Prev 2003;12:987–993. [PubMed] [Google Scholar]
- 27.Heselmeyer-Haddad K, Chaudhri N, Stoltzfus P, Cheng JC, Wilber K, Morrison L, Auer G, Ried T. Detection of chromosomal aneuploidies and gene copy number changes in fine needle aspirates is a specific, sensitive, and objective genetic test for the diagnosis of breast cancer. Cancer Res 2002;62:2365–2369. [PubMed] [Google Scholar]
- 28.Halling KC, King W, Sokolova IA, Meyer RG, Burkhardt HM, Halling AC, Cheville JC, Sebo TJ, Ramakumar S, Stewart CS, et al. A comparison of cytology and fluorescence in situ hybridization for the detection of urothelial carcinoma. J Urol 2000;164:1768–1775. [PubMed] [Google Scholar]
- 29.Venmans BJ, van Boxem TJ, Smit EF, Postmus PE, Sutedja TG. Outcome of bronchial carcinoma in situ. Chest 2000;117:1572–1576. [DOI] [PubMed] [Google Scholar]
- 30.Bota S, Auliac JB, Paris C, Métayer J, Sesboüé R, Nouvet G, Thiberville L. Follow-up of bronchial precancerous lesions and carcinoma in situ using fluorescence endoscopy. Am J Respir Crit Care Med 2001;164:1688–1693. [DOI] [PubMed] [Google Scholar]