Abstract
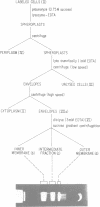
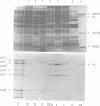
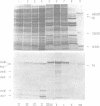
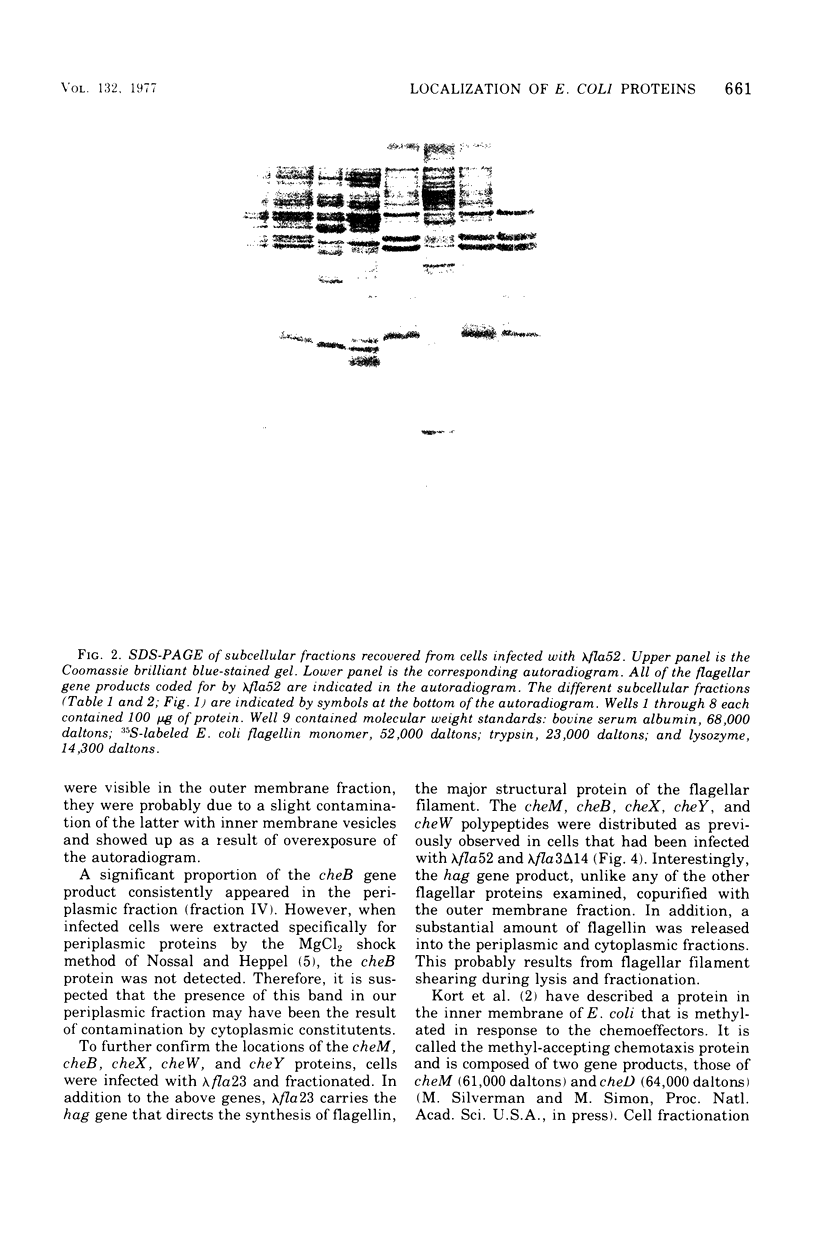
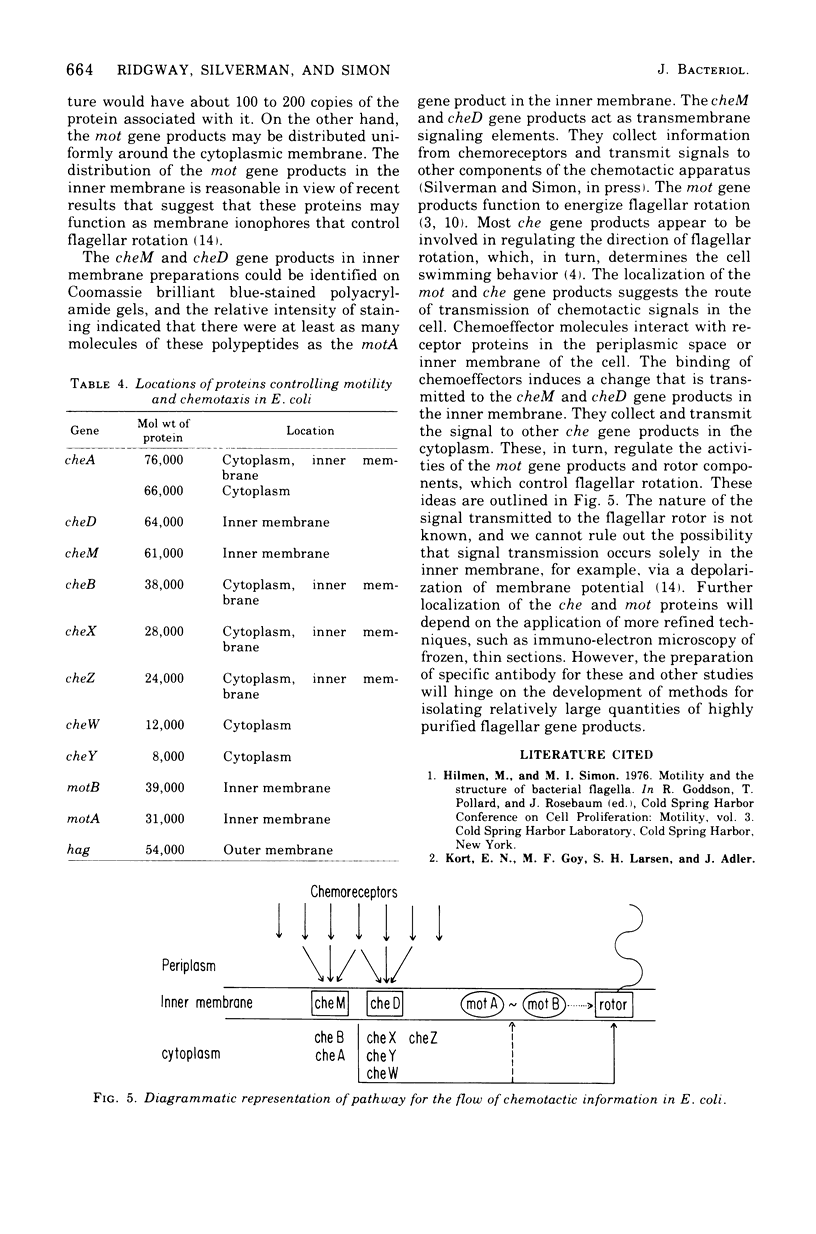
Flagellar proteins controlling motility and chemotaxis in Escherichia coli were selectively labeled in vivo with [35S]methionine. This distribution of these proteins in subcellular fractions was examined by sodium dodecyl sulfatepolyacrylamide gel electrophoresis and autoradiography. The motA, motB, cheM, and cheD gene products were found to be confined exclusively to the inner cytoplasmic membrane fraction, whereas the cheY, cheW, and cheA (66,000 daltons) polypeptides appeared only in the soluble cytoplasmic fraction. The cheB, cheX, cheZ, and cheA (76,000 daltons) proteins, however, were distributed in both the cytoplasm and the inner membrane fractions. The hag gene product (flagellin) was the only flagellar protein examined that copurified with the outer lipopolysaccharide membrane. Differences in the intracellular locations of the che and mot gene prodcuts presumably reflect the functional attributes of these components.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Larsen S. H., Adler J., Gargus J. J., Hogg R. W. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S. H., Reader R. W., Kort E. N., Tso W. W., Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974 May 3;249(452):74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Parkinson J. S. Genetics of chemotactic behavior in bacteria. Cell. 1975 Mar;4(3):183–188. doi: 10.1016/0092-8674(75)90166-x. [DOI] [PubMed] [Google Scholar]
- Silerman M., Matsumura P., Draper R., Edwards S., Simon M. I. Expression of flagellar genes carried by bacteriophage lambda. Nature. 1976 May 20;261(5557):248–250. doi: 10.1038/261248a0. [DOI] [PubMed] [Google Scholar]
- Silverman M., Matsumura P., Hilmen M., Simon M. Characterization of lambda Escherichia coli hybrids carrying chemotaxis genes. J Bacteriol. 1977 May;130(2):877–887. doi: 10.1128/jb.130.2.877-887.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Matsumura P., Simon M. The identification of the mot gene product with Escherichia coli-lambda hybrids. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3126–3130. doi: 10.1073/pnas.73.9.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Identification of polypeptides necessary for chemotaxis in Escherichia coli. J Bacteriol. 1977 Jun;130(3):1317–1325. doi: 10.1128/jb.130.3.1317-1325.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Operon controlling motility and chemotoxis in E. coli. Nature. 1976 Dec 9;264(5586):577–580. doi: 10.1038/264577a0. [DOI] [PubMed] [Google Scholar]
- Springer W. R., Koshland D. E., Jr Identification of a protein methyltransferase as the cheR gene product in the bacterial sensing system. Proc Natl Acad Sci U S A. 1977 Feb;74(2):533–537. doi: 10.1073/pnas.74.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmelcman S., Adler J. Change in membrane potential during bacterial chemotaxis. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4387–4391. doi: 10.1073/pnas.73.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]