Abstract
Tissue mast cells (MC) are recognized as key effector cells of immediate-type allergic reactions releasing inflammatory mediators and cytokines on stimulation with antigen, but they also might be involved in IgE-independent inflammatory and tissue repair processes. The mechanism of human MC regulation in tissue is not fully understood. Here, we show that IL-4, in synergy with stem cell factor (SCF), regulates the function of purified human MC isolated from intestinal tissue. Whereas SCF induced only marginal proliferation of MC cultured in vitro up to 4 weeks, addition of IL-4 and SCF strongly increased the proliferation rate. Moreover, IL-4, which by itself had no visible effect on human MC, enhanced the release of histamine, leukotriene C4, and IL-5 in MC triggered by IgE receptor crosslinking. The IL-4 effects occurred in a dose-dependent fashion (ED50 = 100 pg/ml) and could be totally blocked by a competitive IL-4 receptor antagonist. Our data indicate that IL-4 is an important regulator of human MC function and suggest that mature MC retain the capacity to proliferate in a particular tissue environment.
Mast cells (MC) exert their effector functions by releasing proinflammatory and immuno-regulatory mediators on stimulation with IgE-dependent and IgE-independent agonists. We and others showed that stem cell factor (SCF) is a unique growth factor enhancing the IgE-dependent mediator release in mature human MC (1–4) and promoting the growth and maturation of human MC derived from bone marrow and other progenitors (5–10). In contrast, murine MC are regulated, apart from SCF, by multiple growth factors such as IL-3, IL-4, IL-6, IL-9, IL-10, and nerve growth factor (1, 2). Most of these findings could not be confirmed for human MC, but it was suggested that, apart from SCF, lymphocyte-derived factors such as IL-4 also may be involved in the control of human MC growth and function (8, 11). IL-4 produced by lymphocytes of the Th2 type and basophils regulates B cell maturation to IgE-producing plasma cells and T cell maturation toward the “Th2 phenotype” and plays a key role in the pathogenesis of allergic inflammation (12–14). However, it is unclear so far whether IL-4 also regulates human MC functions. In rodents, IL-4 enhances the growth of MC, particularly in combination with SCF or IL-3 (1, 2). In humans, IL-4 was studied for its effects on the development of MC from progenitor cells (5–10). These in vitro studies yielded conflicting results, possibly because of the heterogeneity of MC deriving from different origins (1). IL-4 either decreased the SCF-dependent development of MC from peripheral blood cells and fetal liver cells (5–7), possibly by down-regulating c-kit expression on MC progenitors (5, 15) or had no effect on the number of MC that developed from cord blood cells cultured with SCF and IL-6 (10). In contrast, IL-4 was found to induce Fcɛ receptor expression (7, 9), to promote the development of chymase-positive MC from immature progenitors (7, 10), and to prolong MC survival after withdrawal of SCF and IL-6 (8). The effect of IL-4 on mature human MC is largely unknown. Here, we studied IL-4 for its capacity to regulate mature human MC functions by using a recently developed in vitro culture system for MC isolated from human intestinal surgery tissue specimens (4, 16).
MATERIALS AND METHODS
MC Isolation, Purification, and Culture.
Human intestinal MC were isolated from surgery tissue specimens derived from individuals who underwent bowel resection because of cancer. The methods of mechanical and enzymatic tissue dispersion yielding single cell preparations containing 2–8% MC (mean 4 ± 2%, n = 47) has been described (3, 4, 16). After overnight culture in medium (RPMI 1640 supplemented with 10% heat-inactivated FCS, 25 mM Hepes, 2 mM glutamine and antibiotics) MC were enriched by positive selection after incubation with an anti-human-c-kit-antibody (mAb YB5.B8, 5 ng/ml; PharMingen) and immunomagnetic beads by using the MACS system (Miltenyi Biotec, Bergisch-Gladbach, Germany) as described (4, 16–18). The fraction containing the c-kit-positive cells (MC purity 60 ± 22%, n = 47) was washed, resuspended in culture medium at a density of 1–4 × 105 MC per ml, and cultured for up to 28 days in medium alone or in medium supplemented with recombinant human SCF (25 ng/ml, Prepro Tech, Rocky Hill, NJ), IL-4 (10 ng/ml, Novartis, Vienna, Austria), or both. Weekly, half of the culture medium was carefully exchanged, and cytokines were supplemented again at the same concentrations. MC purity and recovery (expressed as percent of MC numbers at start of culture) was determined by cell counting by using trypan blue staining and differentiation of the cells on cytospins stained with May-Grünwald/Giemsa (3, 4, 16–18). To block IL-4 activity, the competitive IL-4 inhibitor RY, an IL-4 mutant that binds to IL-4 receptor (IL-4R) with high affinity (Km = 500 pM), but has no agonistic activity and blocks the effect of wild-type IL-4, was used (19).
Mediator and Cytokine Release Assays.
MC were stimulated by IgE-receptor crosslinking using the purified mAb 29C6 (100 ng/ml; Hoffmann–La Roche) directed against a non-IgE binding epitope of the high-affinity IgE-receptor α-chain (20). Histamine release into supernatants was measured by commercial RIA (Coulter) and expressed in percent of total cellular histamine content determined after cell lysis. Leukotriene C4 (LTC4) release was measured by RIA as described (21). Tumor necrosis factor (TNF) α and IL-5 were measured in supernatants by ELISA (R & D Systems). Mediator release in response to mAb 29C6 was expressed as specific mediator release obtained by subtraction of spontaneous mediator release in response to buffer control.
MC RNA Preparation and Reverse Transcription–PCR (RT-PCR).
Total RNA was prepared from highly purified human MC preparations (purity >98%) by using the RNeasy Mini Kit (Quiagen, Hilden, Germany). RT-PCR was performed with primer pairs for TNF-α (17), IL-5 (18), and IL-4R α-chain (sense: 5′-CATGAAGGTCTTGCAGGAGC-3′; antisense: 5′-CAGGTGGTGTTATAGCACTG-3′). Positive controls were performed with primer pairs for glycerinalde-hydephosphate-dehydrogenase (GAPDH) (17, 18). The PCR products contained DNA fragments of 237 bp (TNF-α), 268 bp (IL-5), 561 bp (IL-4R α-chain), and 452 bp (GAPDH), respectively. To observe relative changes in mRNA expression, duplex PCR according to the primer-dropping method was performed as described (17). Duplex PCR was started with the primer pair for the cytokine of interest, and after some cycles later (three cycles for IL-5, seven cycles for TNF-α), the primer set of GAPDH was added. Samples from each reaction were collected after three different time points to assess the exponential DNA increase (after 25, 27, and 29 cycles for IL-5 mRNA detection, after 29, 31, and 33 cycles for TNF-α mRNA detection). To be certain to obtain specific cDNA, PCR fragments were sequenced by the dideoxy method using the T7 Sequencing kit (Amersham Pharmacia).
Immunocytochemistry and Proliferation Assays.
Immunocytochemistry was performed by using antibodies against human tryptase (mAb, 230 ng/ml; Chemicon), chymase (mAb, 100 ng/ml, Chemicon), IL-4R α-chain (mAb clone S4–56C9, 12.5 μg/ml; Coulter), c-kit (polyclonal antibody Ab-1, 2 μg/ml, Oncogene), and the human nuclear cell proliferation-associated antigen Ki-67 (mAb MIB-1, dilution 1:1; Dianova, Hamburg, Germany, ref. 22) as primary antibodies (overnight incubation at 4°C) and the streptavidin-biotin detection system (Histostain-Plus kit, Zymed) as described (4, 16–18). MC proliferation was further quantified by 3H-thymidine (4) and BrdUrd incorporation following the manufacturer’s instructions (Boehringer Mannheim). BrdUrd was added to the cells at the beginning of culture, and the BrdUrd incorporation rate was quantified after different culture times by counting the positive MC on cytospins.
Flow Cytometry.
For each condition, 5–10 × 104 MC (purity >96%) were cultured for 20 days, washed (400 g, 4 min), and resuspended in 100 μl of PBS supplemented with 0.1% BSA, 0.1% sodium acid, and 250 μg/ml of rabbit IgG to inhibit unspecific binding of the mAbs to Fc receptors. Cells were labeled by using primary antibodies directed against IL-4R α-chain (5 μg/ml, Coulter), IgE receptor α-chain (mAb 22E7, 2 μg/ml, Hoffmann–La Roche), and SCF receptor c-kit (CD 117-PE, clone 104D2, dilution 1:50, Becton Dickinson) and appropriate secondary antibodies. Fluorescence-activated cell sorter analysis was performed by using the FACSCalibur system (Becton-Dickinson).
MC Development from Peripheral Blood Mononuclear Cells (PBMC).
PBMC were isolated from normal donors by density centrifugation using Ficoll-Hypaque (Amersham Pharmacia). They were cultured for up to 9 weeks in 96-well culture plates at an initial density of 2.5 × 105 cells per well as described (6). Culture conditions were identical to those described for intestinal MC.
Statistics.
All data in text and figures are expressed as mean ± SD if not indicated otherwise. The significance of differences was assessed by using the Wilcoxon test. P < 0.05 was considered to be statistically significant.
RESULTS
Effect of IL-4 on Human MC Survival and Proliferation.
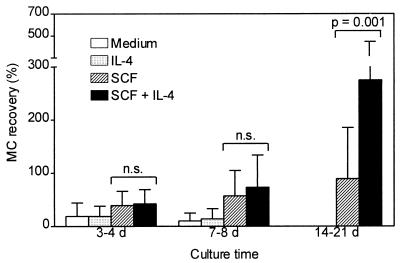
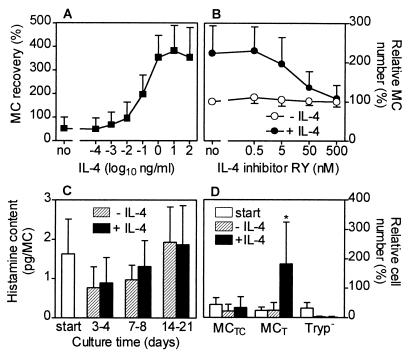
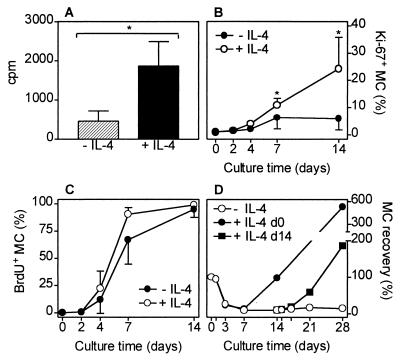
Enriched human intestinal MC were cultured up to 3 weeks in medium alone or in medium supplemented with IL-4, SCF, or both cytokines. IL-4 by itself had no visible effect on human intestinal MC survival or proliferation. In the presence or absence of IL-4 all MC died within 4–9 days of culture. Confirming previous data, MC could be maintained in culture for several weeks if supplemented with SCF and MC purity was enhanced, because almost all contaminating cells died (4). Most interestingly, simultaneous addition of IL-4 and SCF to the culture system caused a pronounced enhancement of MC numbers, compared with SCF alone, suggesting a synergistic effect of IL-4 and SCF on human MC proliferation (Fig. 1). During culture, MC purity (65 ± 21% at day 0, mean ± SD, n = 12) increased depending on the cytokine supplementation (with SCF: 82 ± 19% after 3–4 days, 90 ± 11% after 7–8 days, 97 ± 4% after 14–21 days; with IL-4 and SCF: 81 ± 16% after 3–4 days, 92 ± 5% after 7–8 days, 99 ± 1% after 14–21 days). The IL-4-dependent MC proliferation occurred in a dose-dependent fashion, the ED50 of IL-4 being about 100 pg/ml (Fig. 2A). The effect of IL-4 on MC proliferation could be totally blocked by the competitive IL-4 inhibitor RY (Fig. 2B), an IL-4 mutant protein without agonistic activity that blocks the effect of wild-type IL-4 (19). The IL-4 inhibitor did not change MC numbers in cultures supplemented with SCF. The histamine content per MC was not changed by IL-4 (Fig. 2C). IL-4 induced a selective increase of tryptase-positive MC whereas the number of tryptase/chymase double-positive MC remained almost unchanged (Fig. 2D). The IL-4-dependent proliferation of purified MC was confirmed by an enhanced incorporation of 3H-thymidine and by an increase of MC expressing the proliferation marker Ki-67 (Figs. 3 A and B and 4A). The high percentage of cells that incorporated BrdUrd after 7 and 14 days of culture (Figs. 3C and 4B) indicates that almost all MC undergo cell division in the presence of SCF, and, perhaps even more rapidly, in the presence of SCF and IL-4. Experiments shown in Fig. 3D demonstrate that a delayed addition of IL-4 to the MC cultured in the presence of SCF at day 14, when MC are already almost pure, shortens the time interval between addition of IL-4 and onset of its effects on MC proliferation.
Figure 1.
Effect of SCF and IL-4 on human intestinal MC proliferation. Enriched MC preparations obtained by positive selection of c-kit-expressing cells were cultured in medium alone or supplemented with IL-4 (10 ng/ml), SCF (25 ng/ml), or both. MC recovery, expressed in percent (start of culture = 100%), is shown (means + SD of 12 experiments performed in duplicate). Analogous results were obtained with unpurified cell preparations containing 2–8% MC (not shown). Statistical analysis by Wilcoxon test; n.s., not significant.
Figure 2.
Concentration-dependency of IL-4 and IL-4 inhibitor effects on MC growth and effect of IL-4 on histamine content and MC subtypes. (A) Concentration-dependent effect of IL-4 on MC proliferation (culture time 19–21 days, n = 4). (B) Inhibition of the IL-4 (1 ng/ml) effect on MC recovery by RY, a competitive IL-4 inhibitor (expressed in percent, MC number without IL-4 and without RY was set 100%, n = 3). (C) Total cellular histamine content per MC determined after cell lysis (n = 8) in MC cultured for different time periods. (D) MC subtypes assessed by immunocytochemistry (MCT, tryptase-positive MC; MCTC, tryptase/chymase-double-positive MC; Tryp−, tryptase-negative cells). Total cell numbers at start of culture were set 100%. Data obtained after culture (17–21 days, ± IL-4) are expressed in percent of total cell number at time of start of culture, n = 5; ∗, P < 0.05 compared with percentages at culture start and after culture without IL-4).
Figure 3.
Proliferation of purified human intestinal MC cultured with or without IL-4. (A) 3H-thymidine incorporation. Radioactivity (6 × 105 cpm/well) was added at day 14 of culture (2 × 104 MC/well at day 0, 99% MC purity at day 14). Cells were harvested and washed three times, and radioactivity was measured at day 18 (n = 6). (B) Percentage of purified MC (>93% purity at day 4 and later) staining positive for Ki-67, a nuclear cell proliferation-associated antigen (n = 5; ∗, P < 0.05 compared with culture without IL-4). (C) Percentage of enriched MC that incorporated BrdUrd. BrdUrd was added at day 0, and positive cells were counted at different time points indicated (since day 4, MC purity was always >94%, n = 3). (D) Recovery (100% = start of culture) of enriched MC (66% purity at day 0, >98% purity at days 14–28) cultured in the presence of SCF for 28 days with (closed symbols) or without (open symbols) IL-4 that was added at day 0 (circles) or day 14 (squares). One of two experiments performed in duplicate is shown.
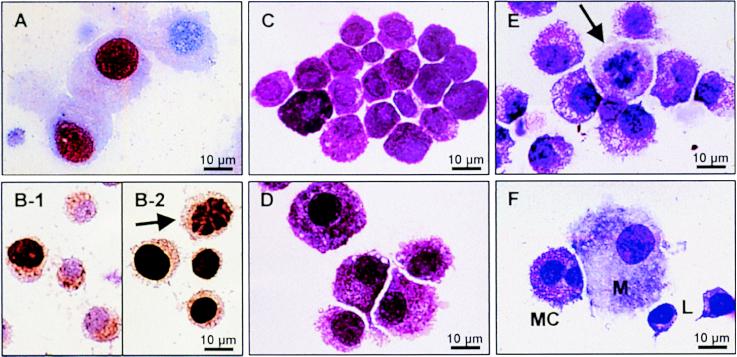
Figure 4.
Ki-67 expression, BrdUrd incorporation, and morphology of human MC. (A) Immunostaining of MC (purity 98%) cultured with IL-4 and SCF for 14 days. The nucleus of MC that express the proliferation marker Ki-67 is colored red. (B) BrdUrd incorporation by MC (dark brown staining) cultured for 4 days (B-1) or 14 days (B-2, arrow indicates a mitotic figure) in the presence of IL-4 and SCF. (C) Isolated human intestinal MC before culture (MC purity 81%). (D) Same cells as in C after 14 days of culture with SCF and IL-4. MC were enlarged and had more abundant cytoplasmatic projections. MC purity increased to 99%. No morphological differences were observed between MC cultured with SCF alone (not shown) or MC cultured with SCF and IL-4. (E) Occasionally, mitotic figures (arrow) could be detected in cultures supplemented with SCF alone or SCF and IL-4. (F) Double-lobed MC developed in vitro from PBMC cultured as described in the text. The MC are neighbored by a macrophage and two lymphocytes. Cells were stained with May-Grünwald/Giemsa (C–F).
Effect of IL-4 on MC Phenotype.
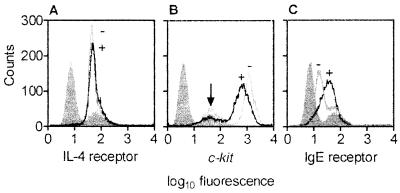
Fig. 4 C and D shows that after culture MC were enlarged and had more abundant cytoplasmatic projections. Occasionally, mitotic figures could be detected in cell cultures with SCF alone or SCF and IL-4 (Fig. 4 B and E). However, no morphological differences were observed between MC cultured with SCF alone (not shown) and MC cultured with SCF and IL-4. By using flow cytometry (Fig. 5A) and immunocytochemistry (not shown) we found that human intestinal MC express the IL-4R α-chain at any time of culture (day 0–21) and that the IL-4R expression is not altered by the addition of IL-4 to the culture. These findings were confirmed on the mRNA level by RT-PCR in pure MC preparations (data not shown). However, IL-4 down-regulated expression of c-kit, the SCF receptor (Fig. 5B), and enhanced the α-chain of the Fcɛ receptor (Fig. 5C).
Figure 5.
Expression of (A) IL-4R α-chain, (B) SCF receptor (c-kit), and (C) high-affinity IgE receptor α-chain on cultured MC measured by flow cytometry. MC (99% purity) were cultured in medium supplemented with SCF without IL-4 (−) or with IL-4 (+) for 20 days. Isotype control staining is shown in gray (left most peak), which yielded almost identical results for all conditions. Arrow indicates area of dead cells. One representative experiment (of three) is shown.
Effect of IL-4 on Mediator and Cytokine Release by Human MC.
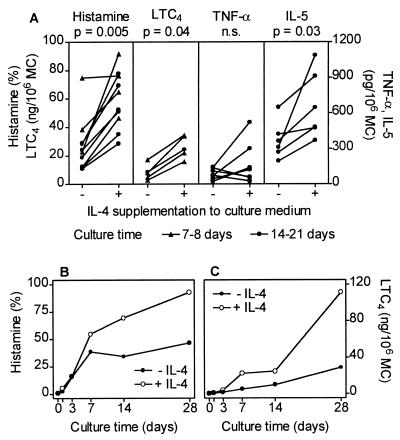
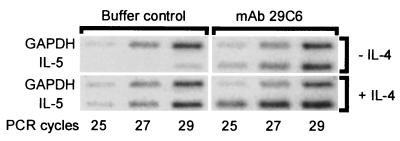
We reported previously that after 6-day culture in the presence of SCF, the release of histamine and LTC4 by MC triggered with mAb 29C6 is by far more pronounced compared with that in freshly isolated MC (4). Here we show that the additional supplementation of the culture medium with IL-4 further enhanced the release of mediators in response to IgE-receptor crosslinking (Fig. 6A). Moreover, we found that IL-5 production in MC also was enhanced by IL-4 in all experiments, whereas TNF-α release was increased only in four of eight experiments (Fig. 6A). Interestingly, we also could detect spontaneous release of IL-5 in MC cultured with IL-4 and SCF, but not in cultures supplemented with SCF alone. We found 137 ± 94 pg IL-5 per 106 MC in culture medium of MC cultured for 17–21 days in the presence of IL-4 and SCF (n = 3). In cell preparations cultured without IL-4, spontaneous IL-5 production was not detected (n = 6). Spontaneous release of TNF-α (detection limit: 5 pg/106 MC) was found only in two of 16 experiments (one of eight with IL-4 and one of eight without IL-4 supplementation). The IL-4-induced enhancement of mediator release after IgE-receptor crosslinking was time dependent, starting after 7 days of culture and being more pronounced after 28 days (Fig. 6 B and C). In contrast to SCF (4), IL-4 failed to trigger MC directly for mediator release (data not shown). The TNF-α and IL-5 protein release data shown in Fig. 6A were confirmed on the mRNA level. Whereas the IL-4 effects on TNF-α protein release and mRNA expression (not shown) were inconsistent, we found a clear enhancement of IL-5 mRNA expression in MC cultured with SCF and IL-4 compared with MC cultured with SCF alone (Fig. 7). By semiquantitative RT-PCR, we could show that not only the IL-5 mRNA expression induced by mAb 29C6 was enhanced through IL-4, but also the spontaneous IL-5 mRNA expression, which was hardly detectable in MC cultured without IL-4 (Fig. 7). These data demonstrate that IL-5 production by human MC also can be induced by IgE receptor-independent mechanisms.
Figure 6.
Effect of IL-4 on MC mediator release and cytokine production induced by IgE receptor crosslinking. (A) MC were cultured in medium supplemented with SCF ± IL-4 for 7–21 days, washed, and then challenged for 30 min (histamine, LTC4) or 6 hr (TNF-α, IL-5) with mAb 29C6 (100 ng/ml) directed against the α-chain of the high-affinity IgE receptor. Specific mediator release into supernatants is shown. Each symbol represents one experiment performed in duplicate. Data derived from the same experiment (±IL-4) are connected by lines. Time course of IL-4 in altering (B) histamine release and (C) LTC4 production in MC. Cells were cultured with or without IL-4 for different time periods and then triggered for 30 min by IgE receptor crosslinking (one of two experiments performed in duplicate is shown).
Figure 7.
IL-5 mRNA production by MC (purity >98%) cultured with SCF in the absence or presence of IL-4 for 20 days, and subsequently challenged to buffer control or mAb 29C6 for 6 hr. mRNA was measured semiquantitatively by duplex RT-PCR (number of PCR cycles are indicated, GAPDH primers were added three cycles later than the IL-5 primers).
Endogenous IL-4 Production in Cell Cultures.
In medium derived from MC cultures that have been cultured with SCF for 14–21 days, a time period after which MC are almost pure (>98% MC purity), no IL-4 protein was detectable by ELISA, indicating that neither MC nor contaminating cells that were mainly fibroblasts produced IL-4 (n = 12). Also after stimulation of the cells with 100 ng/ml of mAb 29C6 for 6 hr, no IL-4 was detected. These data were confirmed by RT-PCR analysis in purified MC, immunostaining of MC cytospins, and intracellular IL-4 staining measured by flow cytometry, all yielding negative results (data not shown).
Effect of IL-4 on MC Development from PBMC.
If PBMC were cultured in vitro in the presence of SCF, MC-like cells were observed after approximately 2 weeks of culture. These cells showed metachromasia and were tryptase positive and c-kit positive. In the presence of SCF (25 ng/ml), 7.0 ± 6.8 × 103 MC-like cells developed after 9 weeks of culture from 2.5 × 105 PBMC (mean ± SD, n = 3). Confirming previous studies (5–7), we found that the addition of IL-4 (10 ng/ml) at day 0 of the culture caused a reduction to 2.4 ± 1.4 × 103 MC-like cells. The IL-4-induced reduction of MC-like cells was less pronounced if IL-4 was added at day 21 (4.1 ± 3.9 × 103 MC-like cells) or day 42 (4.6 ± 2.8 × 103 MC-like cells). The morphology and the functional properties of these blood-derived MC-like cells were different from intestinal MC. About 20–40% of the MC obtained from PBMC cultures showed nuclear atypia such as poly-lobed or double-lobed nuclei (Fig. 4F), which may indicate aberrant cell development and has been observed by other investigators (23).
DISCUSSION
In previous studies, SCF has been recognized as an important regulator of human MC functions (1–4). SCF promotes survival and a weak proliferation of human MC (4, 16). Moreover, SCF enhances IgE receptor-dependent mediator release (3, 4). We found that IL-4 alone, in contrast to SCF, has virtually no effect on human MC, but in the presence of SCF, IL-4 strongly affects multiple MC functions such as proliferation, release of proinflammatory mediators, e.g., histamine and LTC4, and production of cytokines, e.g., IL-5. Therefore, we conclude from our data that IL-4, in synergism with SCF, seems to be an important regulator of human MC function.
Our data raise the question of whether IL-4 is capable of inducing proliferation in mature MC or whether the observed effects of IL-4 are caused by the proliferation of a subpopulation of immature MC that may be contained in the MC preparations used in our study. Indeed ultrastructural studies revealed that the intestine contains about 10–15% immature MC (24). However, the cells used for the present experiments, which were isolated from human intestinal tissue, showed the typical morphology, phenotype, and functional properties of mature human MC. After culture with or without IL-4, more than 99% of the MC had an oval unlobed nucleus (in contrast to MC developed in vitro from progenitor cells), all cells were densely granulated, contained histamine and tryptase, expressed the high-affinity IgE receptor, and released mediators and cytokines in response to IgE receptor crosslinking. Such features have been proposed to be typical for mature MC (1, 24, 25). Moreover, the BrdUrd experiments indicate that after 14 days of culture time almost all purified MC underwent cell division in the presence of IL-4 and SCF, making it rather unlikely that only a small subpopulation of MC progenitors is proliferating in response to IL-4 and SCF. These data suggest that IL-4, in combination with SCF, renders mature human MC to proliferate.
The notion that intestinal MC proliferate in response to SCF and IL-4 is supported by the observation that IL-4 does not enhance proliferation in immature MC derived from different origins such as peripheral blood or fetal liver cells (5–7). This functional difference between MC isolated from intestinal tissue and MC developed in vitro from progenitor cells, as well as their distinct morphology, indicates that these two kinds of MC are clearly different, possibly because MC generated in vitro from progenitor cells undergo aberrant or incomplete MC maturation. Apart from SCF and IL-4, other yet-unknown growth factors seem to be involved in the maturation of human tissue MC (25, 26). The data suggest that IL-4 might inhibit MC development on a very early stage, but promotes the proliferation and mediator release of mature MC. The observation that a delayed addition of IL-4 to PBMC cultured for 3 or 6 weeks with SCF, when MC-like cells are already visible, also failed to induce proliferation of MC could be because these MC-like cells generated in vitro, although they exert some characteristics of mature MC, are still immature (5–10, 23).
Several lines of evidence indicate that IL-4 affects human intestinal MC directly without involvement of other cell types. First, we could demonstrate that human intestinal MC express the IL-4R α-chain. Second, the IL-4-dependent MC proliferation occurred at particularly low IL-4 concentrations in a dose-dependent fashion. The minimal IL-4 concentration enhancing MC proliferation (1 pg/ml) was at least 40-fold lower than those required for the induction of IgE synthesis in B cells (13). Such low IL-4 concentrations are found in peripheral blood (27), and it is likely that they also might occur in tissue. Third, purified human MC preparations were used. Variations of MC purity at day 0 (start of culture when IL-4 and SCF were added) from 40% to 90% did not influence the IL-4 effect on proliferation or mediator release (not shown). Also in experiments in which IL-4 was added after 2 weeks of culture, when MC purity was almost 100%, IL-4 was similarly effective as in partially purified MC preparations to which IL-4 was added at start of culture.
Apart from proliferation, mediator release in human MC seems to be regulated by IL-4. We found that culture of intestinal MC with IL-4 strongly enhanced IgE receptor-mediated release of preformed histamine and de novo synthesized LTC4 and IL-5. Moreover, IL-4, together with SCF, rendered MC capable of producing IL-5 in an IgE receptor-independent fashion. The results extend previous reports showing that IL-4 promotes intercellular adhesion molecule-1 expression in human lung MC (28), enhances IL-3 and IL-8 mRNA expression in HMC-1 cells (29), and increases IgE receptor-mediated IL-13 production in MC developed from cord blood cells (23). These data indicate that IL-4 might be an important regulator of mature human MC effector functions.
Extending previous data in MC generated in vitro from progenitor cells (5, 15) and in other cell types such as human colorectal carcinoma cells (30), we found that IL-4 down-regulates expression of c-kit, the SCF receptor, on human intestinal MC, excluding the possibility that the IL-4-induced MC proliferation is caused by an increased SCF receptor expression. By contrast, the number of IgE receptors on intestinal MC is enhanced by IL-4, as shown earlier in progenitor cell-derived MC and mature nasal MC (7, 9, 31). However, it is unlikely that the enhanced mediator release by MC cultured with IL-4 is entirely the result of an increased IgE receptor expression, because our own unpublished observations indicate that IL-4 also enhances IgE receptor-independent mediator release.
According to our data, it is unlikely that MC are a source of IL-4, which may act in an autocrine fashion. In contrast to previous reports (31, 32), we did not detect any IL-4 synthesis in purified MC, neither spontaneously nor after stimulation by IgE receptor crosslinking, by using different detection methods such as RT-PCR, ELISA, immunocytochemistry, and flow cytometry. Furthermore, maximally effective concentrations of the IL-4 inhibitor RY, which blocked the effects of exogenous IL-4 totally, did not change MC numbers in cultures supplemented with SCF, largely excluding the possibility that IL-4 is produced endogenously in the cultures. However, because all MC used in our studies were isolated from nonallergic donors, we cannot exclude an autocrine amplification of MC functions by their own IL-4 production in allergic disease (31), but more likely, IL-4 that affects human MC functions is produced by other cells such as Th2 lymphocytes or basophils (12, 14).
During culture, MC underwent morphologic and functional changes. It may be questioned whether the properties of these MC are comparable to those of MC in vivo, or whether a rather artificial type of MC was examined. Confirming our own previous studies we found that cultured MC release much higher amounts of mediators in response to IgE receptor crosslinking than freshly isolated MC, which is most likely related to the fact that the isolation procedure and the purification steps cause a reversible damage of the cells that reduces their functional capacities (4). During culture, the decreased functional qualities might be restored, suggesting that cultured MC reflect more accurately the functional properties of in vivo MC (33). This assumption is confirmed by the observation that MC cultured for a few days without SCF supplementation, but not freshly isolated MC, release histamine and LTC4 after a short incubation with SCF (4). Similar findings were made in vivo, because SCF causes a pronounced weal and flare reaction when injected into human skin (34). The fact that pure human MC derived from mucosal tissues can be obtained only if they were cultured in the presence of cytokines further emphasizes the value of the culture system used here for the study of human MC functions.
Our findings extend the range of biological effects of IL-4, which is known to control B cell class switching to IgE-producing plasma cells, and T cell maturation to IL-4- and IL-5-producing Th2 cells (12–14). The data suggest that IL-4, besides SCF, regulates local MC proliferation and enhances mediator release in tissue, but on the other hand, IL-4 may suppress a further recruitment and maturation of MC progenitors from the blood stream (5–7). Moreover, our results might explain on a molecular level the suggested link between the specific immune system, namely Th2 type lymphocytes, and MC. In conclusion, the data further emphasize the outstanding role of IL-4 in the pathogenesis of allergic reactions, parasitic infections, and other inflammatory diseases characterized by a Th2-type immune response.
Acknowledgments
We thank Dr. E. Liehl (Novartis Research Institute, Vienna, Austria) for IL-4, Dr. R. Chizzonite (Hoffmann-La Roche, Nutley, NJ) for mAb 29C6, and Gisela Weier and Nicole Abraham for excellent technical assistance. This work was supported by the German Research Association Deutsche Forschungsgemeinschaft (Grant SFB280-C8 to S.C.B.).
ABBREVIATIONS
- SCF
stem cell factor
- MC
mast cells
- GAPDH
glycerinalde-hydephosphate-dehydrogenase
- LTC4
leukotriene C4
- RT-PCR
reverse transcription–PCR
- TNF
tumor necrosis factor
- IL-4R
IL-4 receptor
- PBMC
peripheral blood mononuclear cells
References
- 1.Metcalfe D D, Baram D, Mekori Y A. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 2.Galli S J, Zsebo K M, Geissler E N. Adv Immunol. 1994;55:1–96. doi: 10.1016/s0065-2776(08)60508-8. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff S C, Dahinden C A. J Exp Med. 1992;175:237–244. doi: 10.1084/jem.175.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bischoff S C, Schwengberg S, Raab R, Manns M P. J Immunol. 1997;159:5560–5567. [PubMed] [Google Scholar]
- 5.Nilsson G, Miettinen U, Ishizaka T, Ashman L K, Irani A A, Schwartz L B. Blood. 1994;84:1519–1527. [PubMed] [Google Scholar]
- 6.Sillaber C, Sperr W R, Agis H, Spanblöchl E, Lechner K, Valent P. Int Arch Allergy Immunol. 1994;105:264–268. doi: 10.1159/000236767. [DOI] [PubMed] [Google Scholar]
- 7.Xia H Z, Zhongmin D, Craig S, Klisch G, Noben-Trauth N, Kochan J P, Huff T H, Irani A A, Schwartz L B. J Immunol. 1997;159:2911–2921. [PubMed] [Google Scholar]
- 8.Yanagida M, Fukamachi H, Ohgami K, Kuwaki T, Ishii H, Uzumaki H, Amano K, Tokiwa T, Mitsui H, Saito H, et al. Blood. 1995;86:3705–3714. [PubMed] [Google Scholar]
- 9.Toru H, Ra C, Nonoyama S, Suzuki K, Yata J, Nakahata T. Int Immunol. 1996;8:1367–1373. doi: 10.1093/intimm/8.9.1367. [DOI] [PubMed] [Google Scholar]
- 10.Toru H, Eguchi M, Matsumoto R, Yanagida M, Yata J, Nakahata T. Blood. 1998;91:187–195. [PubMed] [Google Scholar]
- 11.Irani A A, Craig S S, DeBlois G, Elson C O, Schechter N M, Schwartz L B. J Immunol. 1987;138:4381–4386. [PubMed] [Google Scholar]
- 12.Brunner T, Heusser C H, Dahinden C A. J Exp Med. 1993;177:605–611. doi: 10.1084/jem.177.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Classen J L, Levine A, Buckley R H. J Immunol. 1990;144:2123–2130. [PubMed] [Google Scholar]
- 14.Romagnani S. J Clin Immunol. 1995;15:121–129. doi: 10.1007/BF01543103. [DOI] [PubMed] [Google Scholar]
- 15.Sillaber C, Strobel H, Bevec D, Ashman L K, Butterfield J H, Lechner K, Maurer D, Bettelheim P, Valent P. J Immunol. 1991;147:4224–4228. [PubMed] [Google Scholar]
- 16.Bischoff S C, Sellge G, Schwengberg S, Lorentz A, Manns M P. Int Arch Allergy Immunol. 1999;118:104–107. doi: 10.1159/000024041. [DOI] [PubMed] [Google Scholar]
- 17.Bischoff S C, Lorentz A, Schwengberg S, Weier G, Raab R, Manns M P. Gut. 1999;44:643–652. doi: 10.1136/gut.44.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorentz A, Schwengberg S, Mierke C, Manns M P, Bischoff S C. Eur J Immunol. 1999;29:1496–1503. doi: 10.1002/(SICI)1521-4141(199905)29:05<1496::AID-IMMU1496>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Tony H P, Shen B J, Reusch P, Sebald W. Eur J Biochem. 1994;225:659–665. doi: 10.1111/j.1432-1033.1994.00659.x. [DOI] [PubMed] [Google Scholar]
- 20.Riske F, Hakimi J, Mallamaci M, Griffin M, Pilson B, Tobkes N, Lin P, Danho W, Kochan J, Chizzonite R. J Biol Chem. 1991;266:11245–11251. [PubMed] [Google Scholar]
- 21.Bischoff S C, Dahinden C A. Blood. 1992;79:2662–2669. [PubMed] [Google Scholar]
- 22.Key G, Becker M H G, Baron B, Duchrow M, Schlüter C, Flad H D, Gerdes J. Lab Invest. 1993;68:629–636. [PubMed] [Google Scholar]
- 23.Toru H, Pawankar R, Ra C, Yata J, Nakahata T. J Allergy Clin Immunol. 1998;102:491–502. doi: 10.1016/s0091-6749(98)70140-x. [DOI] [PubMed] [Google Scholar]
- 24.Craig S S, Schechter N M, Schwartz L B. Lab Invest. 1989;60:147–157. [PubMed] [Google Scholar]
- 25.Dvorak A M, Mitsui H, Ishizaka T. Clin Exp Allergy. 1994;24:649–659. doi: 10.1111/j.1365-2222.1994.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 26.Dvorak A M, Furitsu T, Kissell-Rainville S, Ishizaka T. J Leukocyte Biol. 1992;51:557–569. doi: 10.1002/jlb.51.6.557. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto K, Taki F, Miura M, Matsuzaki M, Takagi K. Chest. 1994;105:681–686. doi: 10.1378/chest.105.3.681. [DOI] [PubMed] [Google Scholar]
- 28.Valent P, Bevec D, Maurer D, Besemer J, Di Padova F, Butterfield J H, Speiser W, Majdic O, Lechner K, Bettelheim P. Proc Natl Acad Sci USA. 1991;88:3339–3342. doi: 10.1073/pnas.88.8.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buckley M G, Williams C M M, Thompson J, Pryor P, Ray K, Butterfield J H, Coleman J W. Immunology. 1995;84:410–415. [PMC free article] [PubMed] [Google Scholar]
- 30.Lahm H, Amstad P, Yilmaz A, Borbenyi Z, Wyniger J, Fischer J R, Suardet L, Givel J C, Odartchenko N. Cell Growth Differ. 1995;6:1111–1118. [PubMed] [Google Scholar]
- 31.Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. J Clin Invest. 1997;99:1492–1499. doi: 10.1172/JCI119311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradding P, Feather I H, Howarth P H, Mueller R, Roberts J A, Britten K, Bews J P A, Hunt T C, Okayama Y, Heusser C H, et al. J Exp Med. 1992;176:1381–1386. doi: 10.1084/jem.176.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor A M, Galli S J, Coleman J W. Immunology. 1995;86:427–433. [PMC free article] [PubMed] [Google Scholar]
- 34.Costa J J, Demetri G D, Harrist T J, Dvorak A M, Hayes D F, Merica E A, Menchaca D M, Gringeri A J, Schwartz L B, Galli S J. J Exp Med. 1996;183:2681–2686. doi: 10.1084/jem.183.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]