Abstract
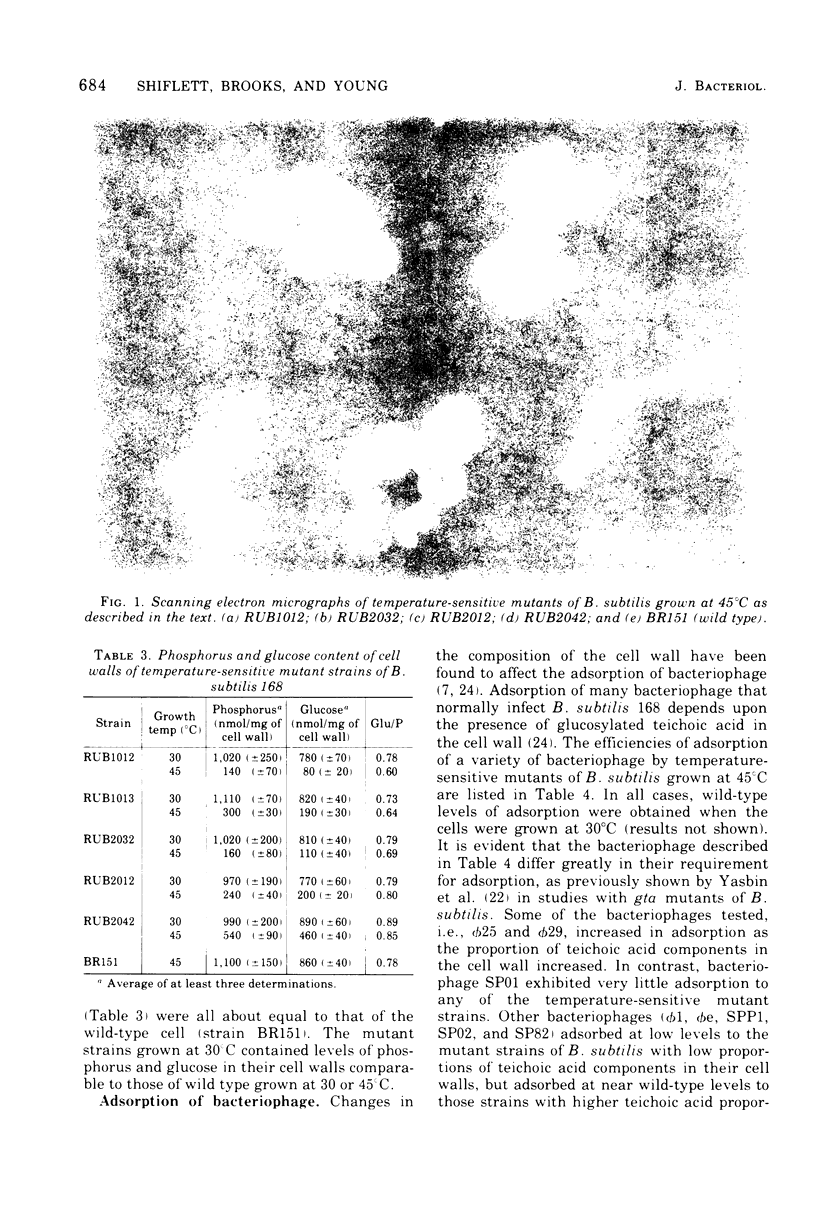
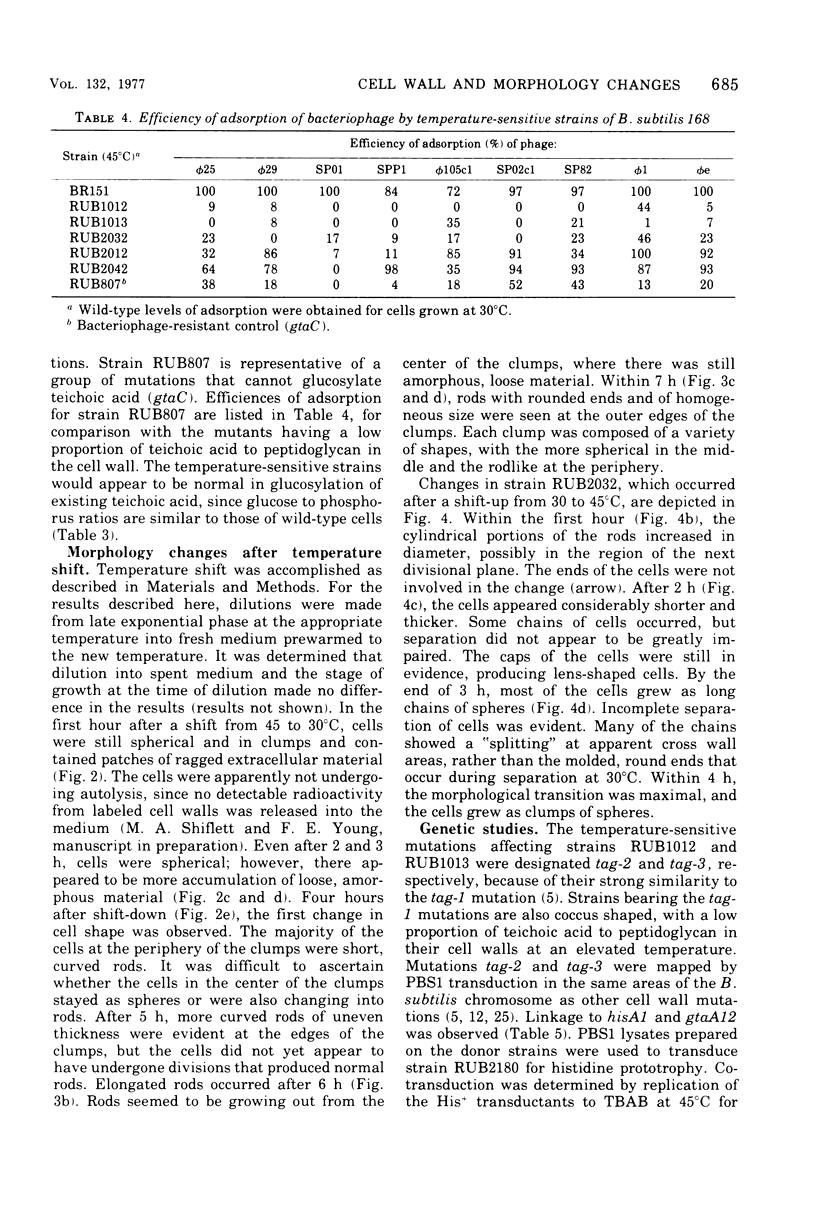
Bacillus subtilis RUB1012 and RUB1013 have the following phenotype when grown at 45°C: no growth on tryptose blood agar base, growth as clumps of spheres in broth culture, a slow autolysis rate, and a low proportion of teichoic acid to peptidoglycan. Revertants of strain RUB1012 (RUB2032, RUB2012, and RUB2042) that could grow on tryptose blood agar base were isolated. Each revertant had a different proportion of teichoic acid to peptidoglycan. The nanomoles of phosphorus per milligram of cell wall at the nonpermissive temperature were 141, 160, 236, and 541 for strain RUB1012 and revertants RUB2032, 2012, and 2042, respectively, as compared with 1,100 for the parent strain. With most bacteriophage tested, plating efficiency was related to the amount of glucosylated teichoic acid. Scanning electron microscopy was used to study strain RUB2032 during a shift from growth at 30°C to growth at 45°C. The change from rod to sphere began with the thickening of the cylindrical portion of the cell. Caps of the cells appeared to be immune to the thickening process. During growth, the cells became progressively shorter and thicker, and cell separation was inhibited. When cells of strain RUB2032 were shifted from growth at 45°C to growth at 30°C, accumulation of an amorphous material on the outer surfaces of the cells preceded the change from sphere to rod morphology. Cells remained clumped, with rods appearing at the periphery of the clumps. Analysis by DNA-mediated transformation and PBS1-mediated transduction indicated that strains RUB1012 and RUB1013 have multiple mutations mapping in the same region as other cell wall mutations.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald A. R., Coapes H. E. Bacteriophage SP50 as a marker for cell wall growth in Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1195–1206. doi: 10.1128/jb.125.3.1195-1206.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H., Brooks D., Young F. E. Regulation of the bacterial cell wall: analysis of a mutant of Bacillus subtilis defective in biosynthesis of teichoic acid. J Bacteriol. 1972 Apr;110(1):281–290. doi: 10.1128/jb.110.1.281-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan R. J., Mendelson N. H. Initial characterization of a temperature-sensitive rod--mutant of Bacillus subtilis. J Bacteriol. 1969 Dec;100(3):1316–1321. doi: 10.1128/jb.100.3.1316-1321.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. C., Wilson C. R., Lukehart S., Young F. E., Shiflett M. A. Analysis of autolysins in temperature-sensitive morphological mutants of Bacillus subtilis. J Bacteriol. 1976 Jan;125(1):166–173. doi: 10.1128/jb.125.1.166-173.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N. Use of bacteriophage-resistant mutants to study the nature of the bacteriophage receptor site of Staphylococcus aureus. J Bacteriol. 1969 May;98(2):519–527. doi: 10.1128/jb.98.2.519-527.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. M., Popkin T. J., Boylan R. J., Mendelson N. H. Ultrastructure of a temperature-sensitive rod- mutant of Bacillus subtilis. J Bacteriol. 1970 Sep;103(3):793–810. doi: 10.1128/jb.103.3.793-810.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman B. E., Beckman M. M. Cell wall turnover at the hemispherical caps of Bacillus subtilis. J Bacteriol. 1974 Mar;117(3):1330–1334. doi: 10.1128/jb.117.3.1330-1334.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M., Cunningham W. P. Ultrastructural studies on a mutant of Bacillus subtilis whose growth is inhibited due to insufficient autolysin production. J Bacteriol. 1972 Mar;109(3):1247–1257. doi: 10.1128/jb.109.3.1247-1257.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Wyrick P. B., Ward J. B., Rogers H. J. Effect of phosphate limitation on the morphology and wall composition of Bacillus licheniformis and its phosphoglucomutase-deficient mutants. J Bacteriol. 1973 Feb;113(2):969–984. doi: 10.1128/jb.113.2.969-984.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamata D., McConnell M., Rogers H. J. Mapping of rod mutants of Bacillus subtilis. J Bacteriol. 1972 Jul;111(1):73–79. doi: 10.1128/jb.111.1.73-79.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson N. H. Helical growth of Bacillus subtilis: a new model of cell growth. Proc Natl Acad Sci U S A. 1976 May;73(5):1740–1744. doi: 10.1073/pnas.73.5.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E W, Schafer M, Lederberg J. Gene Linkage in DNA Transfer: A Cluster of Genes Concerned with Aromatic Biosynthesis in Bacillus Subtilis. Genetics. 1963 Apr;48(4):529–551. doi: 10.1093/genetics/48.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Layered distribution, according to age, within the cell wall of bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1139–1147. doi: 10.1128/jb.125.3.1139-1147.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., McConnell M., Burdett I. D. Cell wall or membrane mutants of Bacillus subtilis and Bacillus licheniformis with grossly deformed morphology. Nature. 1968 Jul 20;219(5151):285–288. doi: 10.1038/219285a0. [DOI] [PubMed] [Google Scholar]
- Rogers H. J., Thurman P. F., Taylor C., Reeve J. N. Mucopeptide synthesis by rod mutants of Bacillus subtilis. J Gen Microbiol. 1974 Dec;85(2):335–349. doi: 10.1099/00221287-85-2-335. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilby M. J. Helical shape and wall synthesis in a bacterium. Nature. 1977 Mar 31;266(5601):450–452. doi: 10.1038/266450a0. [DOI] [PubMed] [Google Scholar]
- Yasbin R. E., Ganesan A. T., Young F. E. Bacteriophage interference in Bacillus subtilis 168. J Virol. 1974 Apr;13(4):916–921. doi: 10.1128/jvi.13.4.916-921.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Maino V. C., Young F. E. Bacteriophage resistance in Bacillus subtilis 168, W23, and interstrain transformants. J Bacteriol. 1976 Mar;125(3):1120–1126. doi: 10.1128/jb.125.3.1120-1126.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Fractionation and partial characterization of the products of autolysis of cell walls of Bacillus subtilis. J Bacteriol. 1966 Oct;92(4):839–846. doi: 10.1128/jb.92.4.839-846.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E., Smith C., Reilly B. E. Chromosomal location of genes regulating resistance to bacteriophage in Bacillus subtilis. J Bacteriol. 1969 Jun;98(3):1087–1097. doi: 10.1128/jb.98.3.1087-1097.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]






