Abstract
Myasthenia gravis (MG) and experimental autoimmune myasthenia gravis (EAMG) are antibody-mediated autoimmune diseases in which the nicotinic acetylcholine receptor (AcChoR) is the major autoantigen. The immune response in these diseases is heterogeneous and is directed to a wide variety of T and B cell epitopes of AcChoR. Candidate molecules for specific immunotherapy of MG should, therefore, have a broad specificity. We used recombinant fragments of the human AcChoR, encompassing the extracellular domain of the α-subunit, or shorter fragments derived from it, in experiments to modulate EAMG. We have demonstrated that intranasal administration of these recombinant fragments, which represent a major portion of epitopes involved in MG, prevents the induction of EAMG in rats and immunosuppresses an ongoing disease, as assessed by clinical symptoms, weight loss, and muscle AcChoR content. These effects on EAMG were accompanied by a marked reduction in the proliferative T-cell response and IL-2 production in response to AcChoR, in reduced anti-self AcChoR antibody titers and in an isotype switch of AcChoR-specific antibodies, from IgG2 to IgG1. We conclude that nasal tolerance induced by appropriate recombinant fragments of human AcChoR is effective in suppressing EAMG and might possibly be considered as a therapeutic modality for MG.
Myasthenia Gravis (MG) is a T cell-dependent, antibody-mediated autoimmune disease of the neuromuscular junction in which the nicotinic acetylcholine receptor (AcChoR) is the major autoantigen. Experimental autoimmune MG (EAMG), inducible in various animal species by immunization with AcChoR or by passive transfer of anti-AcChoR antibodies, is a reliable model of the human disease, suitable for the investigation of therapeutic strategies (1, 2).
MG is currently treated mainly by acetylcholinesterase inhibitors and by generalized immunosuppression. These treatments have been effective for both MG and EAMG but are often associated with severe side effects. Ideally, the treatment should be specific and should suppress selectively the immunological reactivity that leads to the neuromuscular disorder without impairing the entire immune system (3). An earlier successful attempt for antigen-specific immunotherapy of EAMG was by the use of a nonpathogenic denatured preparation of AcChoR (4), which could both prevent the induction of EAMG in rabbits and immunosuppress ongoing disease.
The immune response to AcChoR is highly heterogeneous, and a wide variety of T and B cell epitopes have been defined in MG and EAMG (5, 6). Thus, the search for new molecules suitable for treatment of MG should deal with this heterogeneity. Candidate molecules for antigen-specific immunotherapy of MG should share specificities with the native antigen without being pathogenic and should be available in sufficient amounts. Another consideration is their route of administration, which should be easy and safe.
The extracellular portion of the AcChoR α-subunit is the target for the majority of the anti-AcChoR antibodies in MG sera (7). Recombinant proteins corresponding to this region encompass many T and B cell epitopes and can be prepared in large amounts. They therefore represent a potential substitute for the entire antigen, for immunotherapy studies. We have recently shown that recombinant fragments of the extracellular domain of the human AcChoR α-subunit are able to protect AcChoR, in the human cell line TE671 that expresses muscle nicotinic AcChoR, from accelerated degradation induced by monoclonal or polyclonal AcChoR-specific antibodies. Moreover, such recombinant fragments were able to attenuate EAMG passively transferred by pathogenic monoclonal anti-AcChoR antibodies (8, 9).
The observation that mucosal delivery of antigens can induce a state of peripheral immunological tolerance opens new opportunities to investigate antigen-specific immunomodulation of autoimmune diseases. The nasal route for administration of a tolerogen might be especially attractive because it is effective in very low doses and avoids gastric proteolytic degradation of the antigen. There have been some recent studies on oral and nasal administration of Torpedo AcChoR for immunomodulation of EAMG (10–12). However, Torpedo AcChoR would not be suitable for the treatment of human MG because it is from an allogeneic origin, is highly myasthenogenic, and is available in limited amounts.
In this study, we demonstrate that nasal administration of recombinant fragments of the extracellular domain of the human AcChoR α-subunit prevents the onset of EAMG and immunosuppresses an ongoing disease. These results suggest that such recombinant AcChoR fragments can be potentially suitable for antigen-specific immunomodulation of human myasthenia.
MATERIALS AND METHODS
Antigens.
Torpedo AcChoR used for immunizations and in vitro studies was purified from Torpedo electroplax as described (13). Recombinant fragments of the human AcChoR α-subunit were prepared and characterized as reported (8). All recombinant fragments were synthesized by PCR on cDNA prepared from total RNA of TE671 cells, which express human muscle type AcChoR (14). The fragments produced were Hα1-210, corresponding to the entire extracellular domain of the human AcChoR α-subunit, Hα1-121, and Hα122-210. Hα1-121 and Hα1-210 included the p3A exon-encoded region (15) in their preparation, and all three fragments were expressed as fusion proteins with glutathione S-transferase (GST) (8).
Induction of EAMG and Clinical Evaluation.
Female Lewis rats, 6–7 weeks of age, were injected once in the hind foot pads with 40 μg of Torpedo AcChoR emulsified in complete Freund’s adjuvant containing 1 mg/rat Mycobacterium tuberculosis (Difco). EAMG was evaluated as follows: grade 0, no weakness or fatigability; grade 1, weak grip and fatigability; grade 2, weakness, hunched posture at rest, decreased body weight, tremolousness; grade 3, severe weakness, marked decrease in body weight, moribund; grade 4: dead. Animals were weighed and evaluated weekly up to 7–9 weeks after immunization with Torpedo AcChoR. AcChoR content was measured in muscle extracts from treated and control rats as reported (16).
Induction of Nasal Tolerance.
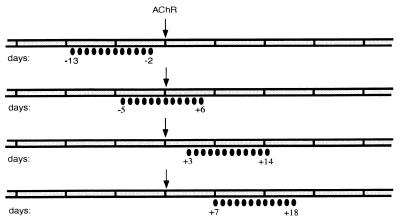
Tolerization with AcChoR fragments was performed either before, during, or after immunization with Torpedo AcChoR. Rats were treated intranasally with the recombinant AcChoR fragments for 12 consecutive days, starting 15 days before, 6 days before, 3 days after, or 7 days after immunization with Torpedo AcChoR (40 μg per rat, in complete Freund’s adjuvant) (Fig. 1). The amount of recombinant protein per dose was 5 μg (dissolved in 60 μl Tris buffer, 30 μl per nostril) for all of the treatment protocols adopted. Control animals were treated with GST or with ovalbumin (5 μg per dose), as indicated.
Figure 1.
Protocols for nasal administration. The black ellipses below the time scale each represent a daily intranasal administration on the specified days. The arrows on day 0 represent immunization with Torpedo AcChoR to induce EAMG.
Lymphocyte Proliferation Assay.
Lymphocyte proliferation assay on cultures of mononuclear cells from draining lymph nodes at the sites of AcChoR immunization was established essentially as described by Melamed and Friedman (17). Proliferation was assessed by measuring [3H] thymidine incorporation during the last 24 hr of a 4-day culture period. Results are expressed as Δcpm (obtained by subtraction of background of unstimulated cultures from that of stimulated lymph node cells).
IL-2 Assay.
The presence of IL-2 in supernatants from lymph node cells cultures after a 24-hr incubation with the indicated antigens was evaluated by their ability to support the proliferation of the IL-2-dependent CTLD cell line, essentially as described by Aharoni et al. (18). Results are expressed as Δcpm.
Antibody and Isotype Assays.
Antibodies against Torpedo AcChoR and recombinant Hα1-210 were assayed by ELISA, essentially as described (8). Bound antibodies were detected by alkaline phosphatase-conjugated goat anti-rat IgG followed by measuring the enzymatic activity of alkaline phosphatase. For assaying the various isotypes (IgG1, IgG2a, IgG2b, and IgG2c), the tested antibodies, which had been absorbed to Hα1-210-coated wells, first were reacted with biotinylated mouse monoclonal antibodies to rat IgG1, IgG2a, IgG2b, and IgG2c (Caltag, Burlingame, CA), for 1 hr at room temperature and, after washing, were reacted with alkaline phosphatase-conjugated Extravidin (Sigma). Results are expressed as OD at 405 nm.
Antibodies to rat AcChoR were measured by reacting the tested antibody with crude rat denervated muscle extract in which the AcChoR had been specifically labeled with I125-α-bungarotoxin, as described (16). Results are expressed as nanomoles of antibody/liter of serum.
RESULTS
Prevention of EAMG by Nasal Pretreatment with Recombinant Fragments.
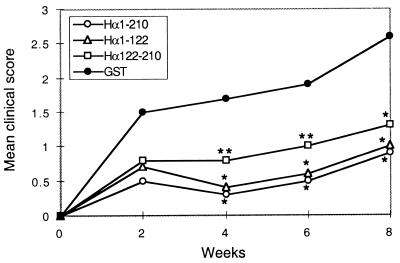
Three recombinant fragments were used in this study: the entire extracellular domain of the human AcChoR α-subunit (Hα1-210) and two shorter fragments derived from it, Hα1-121 and Hα122-210, containing the main immunogenic region (19) and the ligand binding regions, respectively. All three fragments were expressed as fusion proteins with GST (8). For nasal pretreatment (see Fig. 1, first protocol), the fragments were administered daily for 12 consecutive days, each with 5 μg of fragment/rat. Control rats were administered with GST. Two days after the last intranasal application, rats were immunized with Torpedo AcChoR, for induction of EAMG, and were followed for 8 weeks. As shown in Table 1 and Fig. 2, significant protection against EAMG was observed in rats pretreated with either of the AcChoR fragments. At all times tested, the mean clinical score of EAMG in the groups pretreated with each of the AcChoR fragments was lower than that in the control myasthenic group pretreated with GST (Fig. 2). Eight weeks after induction of EAMG, all 10 rats in the control GST-pretreated group had clinical symptoms of EAMG (two died) whereas 67, 56, and 33% of rats in the groups pretreated with Hα1-210, Hα1-121, and Hα122-210, respectively, were completely protected and did not exhibit any symptoms of EAMG (Table 1).
Table 1.
Prevention of EAMG by intranasal treatment with AChoR fragments
| Treatment | Clinical score, no./total*
|
Healthy rats, % | Δ weight 3–7 weeks, g | AChoR content†
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | fmol/mg protein | % | |||
| Control vehicle, GST | 0/10 | 2/10 | 2/10 | 4/10 | 2/10 | 0 | −12.8 ± 9.2 | 17.5 ± 4.1 | 44 |
| Hα122-210 | 3/9 | 3/9 | 1/9 | 1/9 | 1/9 | 33 | +9.2 ± 8.6 | 14.9 ± 1.7 | 38 |
| Hα1-121 | 5/9 | 1/9 | 2/9 | 0/9 | 1/9 | 56 | +13.6 ± 2.5 | 29.6 ± 4.5 | 75 |
| Hα1-210 | 6/9 | 1/9 | 1/9 | 0/9 | 1/9 | 67 | +15.0 ± 6.3 | 35.0 ± 3.4 | 89 |
Evaluated 8 weeks after the induction of EAMG.
Muscle AChoR content in normal age matched rats was 39.5 ± 2.5 fmol/mg protein and was referred to as the 100% value for this experiment.
Figure 2.
Mean EAMG scores at 2, 4, 6, and 8 weeks after immunization with Torpedo AcChoR in rats pretreated nasally with different recombinant fragments of the AcChoR α-subunit. ∗, P < 0.001; ∗∗, P < 0.005 according to Student’s t test, when compared with the control group.
In addition to the clinical evaluation of EAMG, the body weight of the rats and their muscle AcChoR content were followed. Rats with EAMG were usually losing weight, probably because of difficulty reaching food and chewing and swallowing it, because of muscle weakness. Indeed, rats in the control group lost 12.8 ± 9.2 g per rat, between 3 and 7 weeks after induction of EAMG, whereas rats in the groups pretreated intranasally with Hα1-210, Hα1-121, or Hα122-210 gained 15.0 ± 6.3, 13.6 ± 2.5, and 9.2 ± 8.6 g per rat, respectively, during this period (Table 1).
The analysis of AcChoR content in the various groups verified the protective effect of Hα1-210 and Hα1-121. Rats with EAMG, in the control GST-treated group, lost ≈56% of their muscle AcChoR content, as reported (20). On the other hand, there was only a small loss in AcChoR content in rats pretreated with Hα1-210 or Hα1-121 (11 and 25%, respectively). It should be noted that rats pretreated with Hα122-210 exhibited a level of AcChoR loss similar to that of the rats in the control GST-pretreated group, although the clinical score and body weight assessments indicated a protective effect for this fragment as well.
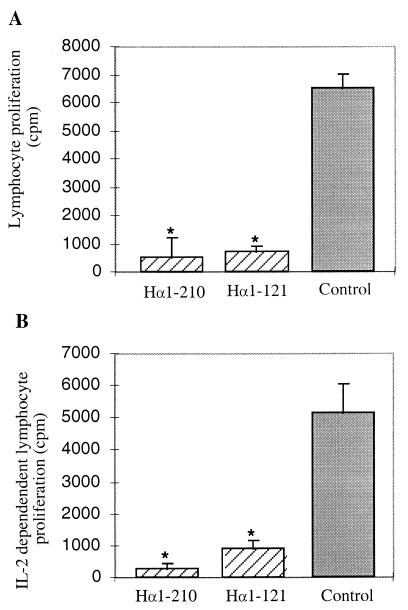
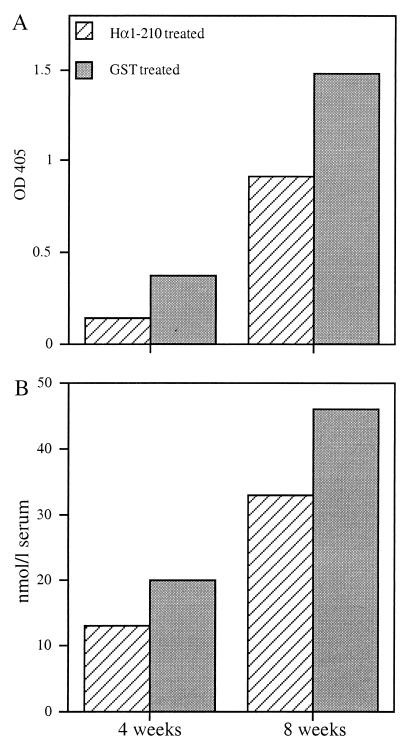
Because the main immunogenic region-containing fragments Hα1-210 and Hα1-121 seemed to have a stronger protective effect than Hα122-210, the following experiments were performed with either Hα1-210 and Hα1-121 or with Hα1-210 only. To get some insight concerning the mode of action of intranasal pretreatment with AcChoR fragments, the effects on AcChoR-specific cellular and humoral immune responses were investigated. There was a marked reduction in the proliferative T-cell response (Fig. 3A) and IL-2 production (Fig. 3B) in response to AcChoR in rats pretreated with either Hα1-210 or Hα1-121. There was also a decrease of up to 40% in the antibody levels against either Hα1-210 or rat muscle AcChoR in the Hα1-210-pretreated group 4 or 8 weeks after immunization with Torpedo AcChoR (Fig. 4).
Figure 3.
Effect of nasal pretreatment with recombinant fragments Hα1-210 and Hα1-121 on in vitro T cell proliferation (A) and on IL-2 production (B) in response to Torpedo AcChoR (0.25 μg/ml). Assays were performed on cells from lymph nodes of individual rats removed 8 weeks after immunization with Torpedo AcChoR, from fragment-treated and control (GST-treated) animals. In vitro T cell responses to Con A were similar in treated and control groups. ∗, P < 0.001 when compared with the control group.
Figure 4.
Antibody levels to Hα1-210 (A) and rat AcChoR (B) in pooled sera from rats pretreated with Hα1-210 or with control vehicle (GST) at 4 and 8 weeks after immunization with AcChoR. This experiment was repeated three times, and the figure represents one typical experiment.
To elucidate the mechanism of the protective effect on EAMG after the intranasal pretreatment, an adoptive transfer experiment was performed. Rats were treated intranasally with Hα1-210 for 12 consecutive days, each with 5 μg of fragment/rat. Spleen cells from the intranasally treated rats were injected i.p. into naive rats (100 × 106 spleen cells per rat). After the adoptive transfer, the rats were injected with Torpedo AcChoR for induction of EAMG. Only 5 of the 12 recipient rats (41%) exhibited symptoms of EAMG 8 weeks after AcChoR injection. All rats in the control groups that received normal spleen cells or spleen cells from rats treated intranasally with BSA were sick or dead at this time (7/7 and 6/6, respectively). These results suggest the involvement of active suppression in the protective effect of nasal tolerance induced by the recombinant fragments.
Therapeutic Effect on EAMG by Nasal Treatment with Hα1-210.
Because the ultimate goal is to treat an ongoing disease rather than preventing its onset, we studied the immunomodulatory effect of intranasal treatment with Hα1-210 when given closer to EAMG induction or after it. We began a regimen of nasal treatment with Hα1-210, similar to the one used for the prevention experiments but starting 5 days before and 3 or 7 days after the induction of EAMG by Torpedo AcChoR (Fig. 1). It should be noted that, on day 7 after AcChoR immunization, most rats display clinical symptoms associated with the first, acute phase of EAMG (21). A suppressive effect on EAMG was observed in all three treatment protocols (Table 2), though the therapeutic effect was lower when nasal treatments were initiated on days 3 or 7 after immunization with AcChoR. Among the rats in the latter two groups, 40 and 30%, respectively, of the rats remained healthy for at least 8 weeks after AcChoR administration. The other rats in these groups had a milder manifestation of EAMG when compared with the rats in the control ovalbumin-treated group. The effect on clinical manifestation was corroborated by the AcChoR content (Table 2) and change in body weight (data not shown). In further recent experiments, we observed that suppression of EAMG has been maintained for at least 3 months after nasal treatment at the acute phase.
Table 2.
Intranasal therapy of EAMG by AChR fragments
| Treatment | Clinical score, no./total*
|
Healthy rats, % | AChoR content†
|
|||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | fmol/mg protein | % | ||
| Control vehicle (ovalbumin; day +3 to +14) | 0/10 | 2/10 | 4/10 | 3/10 | 1/10 | 0 | 11.2 ± 1.3 | 32 |
| Day −5 to +6 | 5/6 | 1/6 | 83 | 21.7 ± 5.8 | 63 | |||
| Day +3 to +14 | 2/5 | 2/5 | 1/5 | 40 | 27.3 ± 10.2 | 79 | ||
| Day +7 to +18 | 3/10 | 3/10 | 3/10 | 1/10 | 30 | 27.4 ± 8.5 | 79 | |
Evaluated 8 weeks after the induction of EAMG.
Muscle AChoR content in normal age matched rats was 34.2 ± 8.5 fmols/mg protein and was referred to as the 100% value for this experiment.
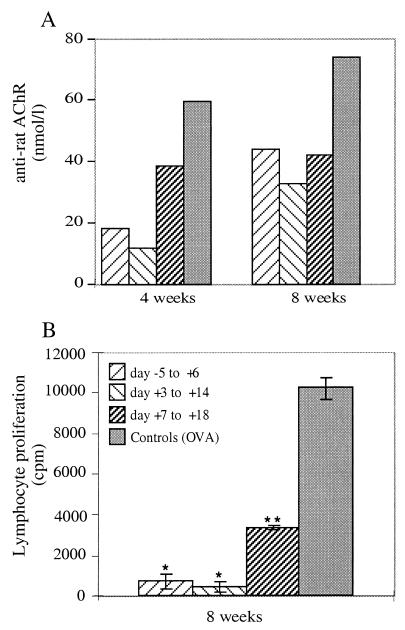
The suppressive effect of the nasal treatments with Hα1-210, starting on days −5, +3, or +7 from AcChoR administration, was accompanied by a reduction in anti-self AcChoR antibody titer (40–80% in the various groups when assayed 4 or 8 weeks after immunization with AcChoR) (Fig. 5A) and by a marked decrease in lymphoproliferative responses to AcChoR (Fig. 5B).
Figure 5.
Effect of nasal treatment with Hα1-210, according to different protocols, on anti-rat AcChoR antibodies 4 and 8 weeks after immunization with AcChoR (A) and on T-cell proliferative responses to Torpedo AcChoR (B), 8 weeks after immunization. ∗, P < 0.001; ∗∗, P < 0.005 when compared with the control group. The figure represents results of one typical experiment of three experiments performed.
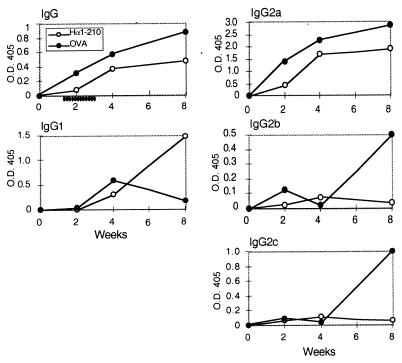
To find out whether the therapeutic effect is accompanied by a switch in IgG isotypes, sera of rats treated with Hα1-210, starting 7 days after disease induction, were analyzed for their anti-AcChoR IgG isotypes (Fig. 6). The homology between the human AcChoR-derived fragment Hα1-210 and its rat homolog is 96%. We have monitored antibodies directed to Hα1-210 that can represent the antibody response toward self rat muscle AcChoR. As depicted in Fig. 6, total IgG levels were lower in the Hα1-210-treated group throughout the 8 weeks of follow-up. Of interest, IgG1 levels in the Hα1-210-treated group increased with time whereas IgG2b and IgG2c remained low in these rats but increased in the control, ovalbumin-treated group. This switch in isotype profile after nasal treatment may reflect a down-regulation of Th1 cells and up-regulation of Th2 cells (22).
Figure 6.
Effect of nasal treatment of EAMG with Hα1-210, on IgG isotypes. Antibodies from pooled sera at 2, 4, and 8 weeks after immunization with Torpedo AcChoR were adsorbed on Hα1-210-coated wells, and the various antibody isotypes were determined as described in Materials and Methods. The schedule of the nasal treatment is depicted under the time scale on the IgG panel. The figure represents one typical experiment of three performed.
DISCUSSION
Antigen-specific inhibition of an autoimmune response is one of the principal goals of research in autoimmunity. Mucosal administration of antigens, either by the oral or nasal route, induces a protective immune response and results in a state of peripheral immunological tolerance. Indeed, mucosal tolerance has been successfully applied in several models of cell-mediated autoimmune diseases (23, 24). EAMG offers a unique opportunity to study the induction of tolerance by mucosal exposure in an antibody-mediated model in which the target autoantigen and its major antigenic epitopes have been well characterized. A caveat to this approach, however, is the possible priming effect exerted by the antigen used for tolerization, especially in the case of a highly immunogenic antigen as AcChoR.
There have been some recent reports on modulation of EAMG induction in rats by oral or nasal administration of the entire AcChoR molecule purified from the Torpedo electric organ (10–12). However, in some of these experiments, antibodies to Torpedo and rat self-AcChoR were markedly increased, and antigen-specific T cell responses were not suppressed (25). The potential expansion of activated autoreactive T cells by the antigen used for tolerization of an ongoing disease must not be underestimated. Indeed, enhancement of the autoimmune response after tolerization has been reported recently in a marmoset model of experimental allergic encephalomyelitis (26) and in a murine model of diabetes (27). These observations suggest that treatment of an ongoing autoimmune disease may require a syngeneic modified antigen with reduced immunogenic potential like the recombinant fragments used in this study. In addition, such fragments corresponding to the human AcChoR can be available in large amounts needed for the long term treatment of MG patients.
The nonpathogenicity of the tolerizing antigen is a key factor for its potential application as a therapeutic agent. There have been some reports on the induction of EAMG symptoms in rats after active immunization with either the recombinant extracellular domain or with the entire recombinant α-subunit (28, 29). We observed mild signs of EAMG in only one of six rats immunized in complete Freund’s adjuvant with high doses (500 μg/rat) of Hα1-210. No signs of EAMG were observed in rats immunized with lower doses of Hα1-210. On the other hand, we did not observe any signs of EAMG in rats followed up to a minimum of 3 months after repeated nasal administrations of Hα1-210 alone. Moreover, antibodies to either Hα1-210 or to Torpedo AcChoR were not detected in sera from these animals.
The detailed mechanism of the suppressive effect by the intranasal treatment with AcChoR fragments is still not worked out. Clinical findings correlated nicely with the in vitro studies. T-cell responses were markedly suppressed in nasally treated animals. The suppression of IL-2 production suggests down-regulation of Th1 cells in this model. Further experiments to evaluate other Th1 and Th2 cytokines are underway. The increase in IgG1 in treated rats (Fig. 6) suggests an isotype switch from a Th1 to a Th2 T-cell response. Adoptive transfer experiments with splenocytes from nasally treated rats suggest that there is a component of active suppression in the tolerizing effect of the recombinant fragments. A more detailed analysis is required to identify the exact cell type(s) involved in this suppression. In conclusion, we have demonstrated that nasal tolerance induced by nonpathogenic recombinant fragments of human AcChoR is efficient and safe in suppressing EAMG and might be considered for application in the treatment of human MG.
Acknowledgments
We thank Profs. A. Ben-Nun, T. Brenner, and O. Abramsky for fruitful discussions and Carmit Bar-Natan for excellent technical assistance. This research was supported by grants from The Association Française contre les Myopathies, The Muscular Dystrophy Association of America, and The Robert Koch-Minerva Center for Research in Autoimmune Diseases at The Weizmann Institute of Science.
ABBREVIATIONS
- AcChoR
acetylcholine receptor
- MG
myasthenia gravis
- EAMG
experimental autoimmune myasthenia gravis
- GST
glutathione S-transferase
References
- 1.Fuchs S. Curr Top Microbiol Immunol. 1979;85:1–29. doi: 10.1007/978-3-642-67322-1_1. [DOI] [PubMed] [Google Scholar]
- 2.Drachman D B. Muscle Nerve. 1996;19:1239–1251. doi: 10.1002/(SICI)1097-4598(199610)19:10<1239::AID-MUS1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs S. In: The Decade of Autoimmunity. Shoenfeld Y, editor. New York: Elsevier; 1999. pp. 255–260. [Google Scholar]
- 4.Bartfeld D, Fuchs S. Proc Natl Acad Sci USA. 1978;75:4006–4010. doi: 10.1073/pnas.75.8.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Protti M P, Manfredi A, Horton R M, Bellone M, Conti-Tronconi B M. Immunol Today. 1993;14:363–368. doi: 10.1016/0167-5699(93)90237-F. [DOI] [PubMed] [Google Scholar]
- 6.Hawke S, Matsuo H, Nicoll M, Malcherek G, Melms A, Willcox N. Immunol Today. 1996;17:307–311. doi: 10.1016/0167-5699(96)10022-0. [DOI] [PubMed] [Google Scholar]
- 7.Tzartos S, Seybold M E, Lindstrom J M. Proc Natl Acad Sci USA. 1982;79:188–192. doi: 10.1073/pnas.79.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barchan D, Asher O, Tzartos S, Fuchs S, Souroujon M C. Eur J Immunol. 1998;28:616–624. doi: 10.1002/(SICI)1521-4141(199802)28:02<616::AID-IMMU616>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 9.Souroujon M C, Barchan D, Fuchs S. Ann N Y Acad Sci. 1998;841:572–575. doi: 10.1111/j.1749-6632.1998.tb10986.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z-Y, Qiao J, Link H. J Neuroimmunol. 1993;44:209–214. doi: 10.1016/0165-5728(93)90045-z. [DOI] [PubMed] [Google Scholar]
- 11.Okumura S, McIntosh K, Drachman D B. Ann Neurol. 1994;36:704–713. doi: 10.1002/ana.410360504. [DOI] [PubMed] [Google Scholar]
- 12.Ma C-G, Zhang G-X, Xiao B-G, Link J, Olsson T, Link H. J Neuroimmunol. 1995;58:51–60. doi: 10.1016/0165-5728(94)00187-s. [DOI] [PubMed] [Google Scholar]
- 13.Aharonov A, Tarrab-Hazdai R, Silman I, Fuchs S. Immunochemistry. 1977;14:129–137. doi: 10.1016/0019-2791(77)90291-9. [DOI] [PubMed] [Google Scholar]
- 14.Schoepfer R, Luther M, Lindstrom J. FEBS Lett. 1988;226:235–240. doi: 10.1016/0014-5793(88)81430-3. [DOI] [PubMed] [Google Scholar]
- 15.Beeson D, Morris A, Vincent A, Newsom-Davis J. EMBO J. 1990;9:2101–2106. doi: 10.1002/j.1460-2075.1990.tb07378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Souroujon M C, Mochly-Rosen D, Gordon A S, Fuchs S. Muscle Nerve. 1983;6:303–311. doi: 10.1002/mus.880060410. [DOI] [PubMed] [Google Scholar]
- 17.Melamed D, Friedman A. Eur J Immunol. 1993;23:935–942. doi: 10.1002/eji.1830230426. [DOI] [PubMed] [Google Scholar]
- 18.Aharoni R, Teitelbaum D, Sela M, Arnon R. Proc Natl Acad Sci USA. 1997;94:10821–10826. doi: 10.1073/pnas.94.20.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tzartos S, Lindstrom J. Proc Natl Acad Sci USA. 1980;77:755–759. doi: 10.1073/pnas.77.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asher O, Neumann D, Witzemann V, Fuchs S. FEBS Lett. 1990;267:231–235. doi: 10.1016/0014-5793(90)80932-9. [DOI] [PubMed] [Google Scholar]
- 21.Lennon V, Lindstrom J, Seybold M. J Exp Med. 1975;141:1365–1375. doi: 10.1084/jem.141.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liblau R S, Singer S H, McDevitt H O. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 23.Weiner H L. Immunol Today. 1997;18:335–343. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 24.Xiao B-G, Link H. Clin Immunol Immunopathol. 1997;85:119–128. doi: 10.1006/clin.1997.4432. [DOI] [PubMed] [Google Scholar]
- 25.Drachman D B, Okumura S, Adams R N, McIntosh K. Ann NY Acad Sci. 1996;778:258–272. doi: 10.1111/j.1749-6632.1996.tb21134.x. [DOI] [PubMed] [Google Scholar]
- 26.Genain C P, Abel K, Belmar N, Villinger F, Rosenberg D P, Linington C, Raine C S, Hauser S L. Science. 1996;274:2054–2057. doi: 10.1126/science.274.5295.2054. [DOI] [PubMed] [Google Scholar]
- 27.Blanas E, Carbone F R, Allison J, Miller J F A P, Heath W R. Science. 1996;274:1707–1709. doi: 10.1126/science.274.5293.1707. [DOI] [PubMed] [Google Scholar]
- 28.Lennon V A, Lambert E H, Leiby K R, Okarma T B, Talib S. J Immunol. 1991;146:2245–2248. [PubMed] [Google Scholar]
- 29.Voltz R, Kamm C, Padberg F, Malotka J, Kerschensteiner M, Spuler S, Tzartos S, Dornmair K. J Neuroimmunol. 1997;80:131–136. doi: 10.1016/s0165-5728(97)00147-1. [DOI] [PubMed] [Google Scholar]