Abstract
To elucidate the structural and energetic basis of attractive protein interactions in the aging lens, we investigated the binding of destabilized mutants of βB1-crystallin to the lens chaperones, α-crystallins. We show that the mutations enhance the binding affinity to αA- but not αB-crystallin at physiological temperatures. Complex formation disrupts the dimer interface of βB1-crystallin consistent with the binding of a monomer. Binding isotherms obtained at increasing concentrations of βB1-crystallin deviate from a classic binding equilibrium and display cooperative-like behavior. In the context of βB1-crystallin unfolding equilibrium, these characteristics are reflective of the concentration-dependent change in the population of a dimeric intermediate that has low affinity to αA-crystallin. In the lens, where α-crystallin binding sites are not regenerated, this may represent an added mechanism to maintain lens transparency.
Keywords: α-crystallin, β-crystallin, chaperone, small heat-shock protein, cataract
1. Introduction
Lens aging leads to changes in its optical properties that partly reflect the evolution of protein-protein interactions in the fiber cells [1,2]. These quiescent, organelle-free cells contain high concentrations of three families of water-soluble proteins, the crystallins. Age-long accumulation of protein damage, truncations and side-chain modifications [3-6] in a low protein turn-over environment change the solubility and stability of the crystallins which can lead to formation of protein aggregates.
The major protein component, α-crystallin, is an oligomer of two subunits that are related to the small heat-shock protein (sHSP) superfamily. α-crystallin plays a central role in delaying the loss of refractivity and transparency through binding of aggregation-prone proteins [7]. The interaction of α-crystallin with its substrates, referred to as chaperone activity, is energetically driven by thermodynamic destabilization of the substrate [8]. Thus, this activity towards modified β- and γ-crystallin is a major contributor to protein associations in the aging lens. Consistent with this model, old and cataractous lenses have co-aggregates of unfolded and cross-linked crystallins [9].
In contrast to the extensive studies of α-crystallin chaperone activity using model substrates [10-12], relatively little is known concerning the structural and energetic aspects of β- and γ-crystallins binding to α-crystallin. Previous work predominantly utilized suppression of crystallins thermal aggregation at temperatures that exceed the melting temperature of the chaperone and the substrate [13,14]. We have developed a steady state assay for quantitative assessment of sHSP binding to substrates at physiological temperatures [8]. Using this approach, we demonstrated that a phosphorylation mimic of αB-crystallin “senses” βB2-crystallin thermodynamic stability binding the more destabilized mutants to a higher level [15]. Analysis of the interaction of α-crystallin with β- or γ-crystallin is not only relevant to the molecular understanding of lens transparency but has implications for the etiology of cataracts. In a mouse cataract model associated with a mutation in γ-crystallin, α-crystallin forms co-aggregates with this protein at a temperature well below its unfolding temperature [16].
In the lens, seven β-crystallins are found as polydisperse homo- and hetero-oligomers although recombinant βB1- and βB2-crystallin form dimers in vitro [2]. One of the building blocks is βB1-crystallin, a protein that undergoes extensive truncation as well as deamidation during lens aging [5]. Contrary to expectation, βB1- and βB2- crystallin have different dimerization motifs despite extensive sequence similarity and essentially identical secondary structure topology [17].
In this paper, we show that the multi-state unfolding equilibrium of βB1-crystallin [18] leads to anomalous concentration dependence and cooperative-like shape of the binding isotherms to αA-crystallin. We propose that an unfolding intermediate, which has marginal affinity to α-crystallin, buffers the changes in stability by reducing the population of the unfolded state compared to a two-state equilibrium. In the lens, this may be significant in delaying the formation of stable complexes with α-crystallin.
2. Materials and methods
2.1. Protein expression and purification
βB1-crystallin mutants were expressed, purified and labeled as previously described [18]. αA- and αB-crystallin were expressed and purified as previously described [8,11]. Bimane-labeled mutants of βB1-crystallin are referred to by the suffix B2.
2.2. Binding isotherms of βB1-crystallin to α-crystallin
Binding isotherms were constructed at fixed concentrations of bimane-labeled βB1-crystallin. For each isotherm, samples containing a fixed concentration of βB1-crystallin and varying amounts of αA-crystallin were incubated for two hours at 37°C. The fluorescence emission of the samples was obtained using a PTI L-format spectrofluorometer equipped with an RTC2000 temperature controller. The bimane probe was excited at 380 nm and the fluorescence emission recorded in the 420 nm – 500 nm range. The bimane intensity at 470 nm was plotted versus the molar ratio of αA- to βB1-crystallin to construct a binding isotherm. Analysis of the binding isotherms was carried out using non-linear least squares as described previously [8].
3. Results
3.1. General methodology
The goal of this paper is to define the energetic threshold for the formation of a stable complex between α- and β-crystallins. Our approach uses site-directed mutagenesis to reduce the substrate free energy of unfolding and then spectroscopically monitor the partitioning of the substrate between folded in solution and stably bound to α-crystallin. Binding is driven by the dynamic population of unfolded states and its level depends on the energetic balance between binding to the chaperone and refolding [8].
Two destabilized mutants of βB1-crystallin that target the domain packing interface are used in this study, L206A and E208L. To detect binding, a bimane label was introduced either at the native C79 or at site K117C in a background where C79 was replaced with a valine. Analysis of the unfolding of these mutants and the effects of the introduction of the probe has been reported [18]. Binding isotherms are constructed at a fixed βB1-crystallin concentration by monitoring the change in bimane emission intensity in the presence of α-crystallin. The binding levels can be manipulated by changing the fixed concentration of βB1-crystallin in a range defined by the dissociation constant.
3.2. Destabilized βB1-crystallin mutants bind αA-crystallin and the phosphorylation mimic of αB-crystallin
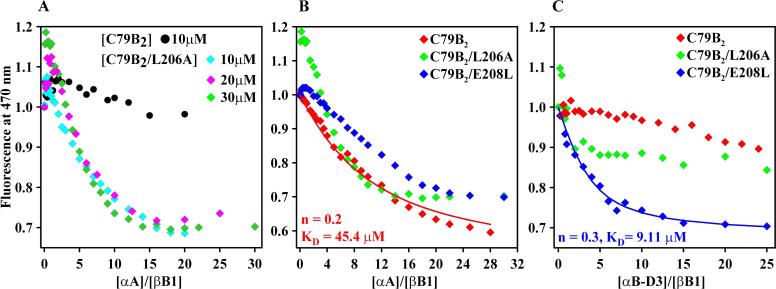
Binding curves in Figure 1A and 1B show that at physiological pH and temperature, αA-crystallin binds destabilized mutants of βB1-crystallin. Addition of αA-crystallin leads to a reduction in the emission intensity of a bimane label attached at site 79 of βB1-crystallin and analysis by size exclusion chromatography confirms the formation of a stable complex (data not shown). Binding does not substantially shift the wavelength of maximum emission suggesting subtle changes in the polarity of the bimane environment. As expected, at similar concentrations the most destabilized mutant L206A binds extensively while the labeled WT (C79B2) shows little change in the bimane intensity (Figure 1A). Increasing the fixed concentration of βB1-crystallin leads to incipient biphasic shape suggestive of two-mode binding. At low molar ratios of αA-to βB1-crystallin the fluorescence of the bimane increases while at high molar ratio it decreases (Figure 1A). Previous studies using the model substrate T4L have demonstrated that highly destabilized mutants activate a low affinity but high capacity binding mode [8].
Figure 1.
(A) Differential binding of bimane-labeled βB1-WT and βB1-L206A. At higher concentrations, L206A shows clear evidence of two-mode binding. (B) Binding of βB1-crystallin and its mutants in the C79B2 background to αA-crystallin at 37°C. The concentration of βB1 was 30 μM. (C) Binding of βB1-crystallin and its mutants to αB-D3 at 37°C. The concentration of βB1 was 20 μM. The solid lines in panels A and C are a non-linear least-squares fit assuming a single mode of binding. n is the number of βB1 binding sites per αA-crystallin subunit. KD is the dissociation constant.
At concentrations where significant binding of C79B2 is observed, both L206A and E208L display evidence of biphasic behavior (Figure 1B). The limited contribution of low-affinity binding hinders the use of non-linear least squares to fit the L206A isotherms. Therefore, the analysis was carried out for selected curves that appear to be predominantly monophasic. For C79B2, the parameters obtained are consistent with the predominance of high-affinity binding previously correlated with the binding of four α-crystallin subunit to one substrate molecule [8].
Binding to WT αB-crystallin is marginal at these concentrations (data not shown). However, Figure 1C shows that the bimane-labeled βB1-crystallins bind the phosphorylation mimic of αB-crystallin, αB-D3, where three serines (S19, S45, S59) were replaced with aspartates. This is consistent with previous analysis demonstrating that phosphorylation of αB-crystallin is required to activate substrate binding [8,15]. Binding leads to blue shift in the wavelength of maximum emission consistent with a transition of the bimane to a more hydrophobic environment (data not shown).
3.3. Structural features of bound βB1-crystallin
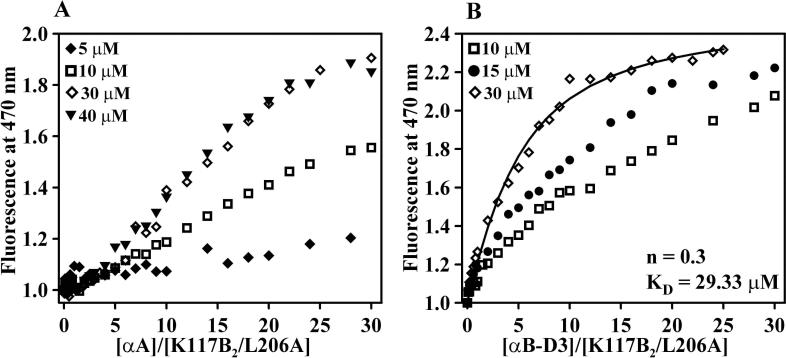
To probe the structure of bound βB1-crystallin, we monitored the dimer interface through the attachment of a bimane probe at site K117. In the crystal structure, this residue is in contact with tryptophan 174 from the symmetry-related subunit. Tryptophans quench bimane emission in a distance-dependent manner [19]. Unfolding relieves the quenching of the bimane at site K117 through the separation of the two monomers [18]. Thus, the trp/bimane pair provides a fingerprint of the dimer interface.
At position K117, complex formation with αA-crystallin or αB-D3 induces a bimane signal change of opposite sign compared to Figure 1. The increase in the emission intensity reflects the separation of the trp and bimane upon binding (Figure 2). This result indicates extensive structural rearrangements at the dimer interface and is consistent with dissociation of the dimer and binding of βB1-crystallin in a monomeric form.
Figure 2.
Binding of βB1-L206A in the C79V/K117B2 background to (A) αA-crystallin and (B) αB-D3. The solid line in panel B is a non-linear least squares fit assuming a single mode of binding.
3.4. Cooperative-like binding isotherms and differential binding by αA-crystallin and αB-D3
The shape of the binding curves for αA-crystallin deviates from simple monophasic behavior for all mutants in the K117B2 background. At low molar ratio of αA to βB1-L206A (Figure 2A), the curve is flat suggesting lack of binding or alternatively a small contribution to the signal from the bound species. Similarly, increasing the fixed concentration of βB1-E208L (Figure 3A) leads to anomalous changes in the shape of the binding curves. The curves are expected to be left shifted at concentrations of the binding partners comparable to the dissociation constant. Figure 3 shows that increasing the fixed concentration of the βB1-crystallin does not significantly change the signal intensity at low molar ratio and the shape of the binding curve is similar to that of a classic cooperative transition. Efficient binding requires a critical ratio of αA-to βB1-crystallin. The critical molar ratio appears correlated to the overall stability; it has a smaller value for K117B2-L206A than K117B2. The cooperative-like shape is not observed in the binding of αB-D3 and the curves could be fit assuming a single mode of binding (Figure 2B). Furthermore, the curves obtained at higher fixed concentrations of βB1-crystallin display the expected left shift.
Figure 3.
Cooperative-like binding isotherms of (A) βB1-K117B2/E208L and (B) βB1-K117B2 to αA-crystallin.
There are two possible origins of the shape changes in the binding isotherms of the destabilized mutants. First, binding may occur in two modes, high- and low-affinity, with opposite fluorescence signal changes that cancel each other. In this case, extensive binding is expected for K117B2 in the flat region. Analysis of mixtures of αA and mutant βB1-crystallin by size exclusion chromatography shows a substantial fraction of unbound βB1-crystallin (data not shown). Furthermore, the curves could not be fit using a two-mode binding model [8].
Because the flat region depends on the fixed concentration of βB1-crystallin, a more likely origin is the reported concentration-dependent reduction in the fractional population of the unfolded state [18]. The unfolding curves of βB1-crystallin show a change in the midpoint of the transition over the concentration range where the anomalous binding curves are observed. This suggests that increasing the fixed concentration of βB1-crystallin reduces the population of the species that bind α-crystallin and that the intermediate has low binding affinity to αA-crystallin.
4. Discussion
Scheme 1
| [1] |
| [2] |
| [3] |
The results presented in this paper can be interpreted using the thermodynamic model of chaperone activity previously proposed for sHSP [20]. Three coupled equilibria model the dependence of binding on the substrate free energy of unfolding (equation 1) and on the activation state of the chaperone (equation 2). Equation 1 was modified to reflect the multi-state equilibrium of βB1-crystallin [18]: D is the native dimer and I2 is a dimeric intermediate. The binding-induced increase in the emission intensity of the bimane at site K117C suggests that bound βB1-crystallin is monomeric. For βB1-crystallin, loss of dimeric structure is coupled to unfolding; therefore we assume that the stably bound state is extensively unfolded (equation 3). A systematic study of the bound structure of T4L monitoring multiple trp/bimane pairs reveals that the bound conformation is extensively unfolded (Claxton D.P., and Mchaourab, H.S., unpublished results).
αA-crystallin affinity and capacity to βB1-crystallin follows the trends observed for T4L. Levels of binding correlate with effective ΔGunf of the mutants with two-mode binding observed for the most destabilized mutant L206A. Nevertheless, there are substantial differences in the shape and concentration dependence of the binding isotherms between T4L and βB1-crystallin. The origin of these specific characteristics can be rationalized using eqs. 1-3. The coupling of unfolding and dissociation implies that the fractional population of the unfolded state (fu) is inversely related to the total protein concentration (Pt) while the fractional population of the intermediate (fI) increases with higher Pt. The equilibrium constant that describes the transition from the intermediate (I2) to the unfolded state is given by Keq = [Pt] fu2/fI [21]. Consequently, the decrease in the level of binding at higher βB1-crystallin concentration with binding of βB1 in an unfolded conformation and suggests that the intermediate (I) has no measurable affinity to αA-crystallin.
Conversely, selective binding to the unfolded state implies that an increase in concentration of the αA-crystallin (the x-axis in Figure 3A) leads to an effective decrease in the apparent stability of the intermediate similar to the one induced by lower protein concentration. The model predicts, therefore, that the shape of the binding curve will reflect the cooperative nature of the dissociation of the dimeric intermediate.
The lack of the anomalous concentration dependence in the C79B2 background can be explained by its higher stability relative to the K117B2 background [18]. In the latter, the concentration range over which the dimer intermediate is stabilized corresponds to the range used for binding isotherms. For C79B2, the effect of the intermediate is implicit and is manifested by the overall moderate affinity to αA-crystallin (Figure 1A).
Destabilized mutants of βB1- and βB2-crystallin [15] marginally bind αB-crystallin. This may suggest a limited role of αB-crystallin in preventing the aggregation of βB-crystallins. Binding reported by previous studies occurred under strongly denaturing conditions such as high temperature where the favorable free energy of equation 1 drives the coupled equilibrium thereby overriding the unfavorable activation state of αB-crystallin. The lack of a cooperative-like shape for the binding isotherms to αB-D3 may reflect either its higher affinity to βB1-crystallin or a fundamental difference at the mechanistic level including binding to the dimeric intermediate. Analysis using a larger set of mutants will be necessary to distinguish between these two possibilities.
There are two consequences of the exceedingly high protein concentration in the lens. First, even with dissociation constants in the micromolar range, our coupled model predicts substantial binding for relatively stable proteins. Second, crowding may act to enhance the dissociation constants effectively pulling the coupled equations to the right [22]. However, the findings in this paper suggest that for βB1-crystallin, these effects are counteracted by the concentration dependence of the intermediate states stability which serves to reduce the overall affinity to α-crystallin.
Acknowledgments.
This work was supported by grant R01−12018 from the National Eye Institute, NIH. The authors thank Derek P. Claxton for critical reading of the manuscript.
Abbreviations
- sHSP
small heat-shock proteins
- T4L
T4 lysozyme
- αB-D3
αB-crystallin S19D/S45D/S59D
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Progress in Biophysics & Molecular Biology. 2004;86:407–85. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Jaenicke R, Slingsby C. Lens Crystallins and their Microbial Homologs: Structure, Stability and Function. Critical Reviews in Biochemistry and Molecular Biology. 2001;36:435–499. doi: 10.1080/20014091074237. [DOI] [PubMed] [Google Scholar]
- 3.Ajaz MS, Ma Z, Smith DL, Smith JB. Size of human lens beta-crystallin aggregates are distinguished by N-terminal truncation of betaB1. Journal of Biological Chemistry. 1997;272:11250–5. doi: 10.1074/jbc.272.17.11250. [DOI] [PubMed] [Google Scholar]
- 4.Lampi KJ, et al. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Experimental Eye Research. 1998;67:31–43. doi: 10.1006/exer.1998.0481. [DOI] [PubMed] [Google Scholar]
- 5.Lampi KJ, Oxford JT, Bachinger HP, Shearer TR, David LL, Kapfer DM. Deamidation of human βB1 alters the elongated structure of the dimer. Experimental Eye Research. 2001;72:279–88. doi: 10.1006/exer.2000.0950. [DOI] [PubMed] [Google Scholar]
- 6.Ma Z, Hanson SR, Lampi KJ, David LL, Smith DL, Smith JB. Age-related changes in human lens crystallins identified by HPLC and mass spectrometry. Experimental Eye Research. 1998;67:21–30. doi: 10.1006/exer.1998.0482. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz J. The function of α-crystallin in vision. Seminars in Cell and Developmental Biology. 2000;11:53–60. doi: 10.1006/scdb.1999.0351. [DOI] [PubMed] [Google Scholar]
- 8.Sathish HA, Stein RA, Yang G, Mchaourab HS. Mechanism of chaperone function in small heat-shock proteins. Fluorescence studies of the conformations of T4 lysozyme bound to alphaB-crystallin. J. Biol. Chem. 2003;278:44214–44221. doi: 10.1074/jbc.M307578200. [DOI] [PubMed] [Google Scholar]
- 9.Truscott RJW. Age-related nuclear cataract-oxidation is the key. Experimental Eye Research. 2005;80:709–25. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Carver JA, Lindner RA, Lyon C, Canet D, Hernandez H, Dobson CM, Redfield C. The interaction of the molecular chaperone alpha-crystallin with unfolding alpha-lactalbumin: a structural and kinetic spectroscopic study. Journal of Molecular Biology. 2002;318:815–27. doi: 10.1016/S0022-2836(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 11.Mchaourab HS, Dodson EK, Koteiche HA. Mechanism of Chaperone Function in Small Heat-Shock Proteins.Two-Mode Binding of the Excited States of T4 Lysozyme Mutants by aA-Crystallin. Journal of Biological Chemistry. 2002;277:40557–40566. doi: 10.1074/jbc.M206250200. [DOI] [PubMed] [Google Scholar]
- 12.Rao PV, Horwitz J, Zigler JS., Jr. Alpha-crystallin, a molecular chaperone, forms a stable complex with carbonic anhydrase upon heat denaturation. Biochemical & Biophysical Research Communications. 1993;190:786–93. doi: 10.1006/bbrc.1993.1118. [DOI] [PubMed] [Google Scholar]
- 13.Horwitz J. α-crystallin can function as a molecular chaperone. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:10449–53. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinreb O, van Rijk AF, Dovrat A, Bloemendal H. In vitro filament-like formation upon interaction between lens alpha-crystallin and betaL-crystallin promoted by stress. Investigative Ophthalmology & Visual Science. 2000;41:3893–7. [PubMed] [Google Scholar]
- 15.Sathish HA, Koteiche HA, McHaourab HS. Binding of destabilized betaB2-crystallin mutants to alpha-crystallin: the role of a folding intermediate. Journal of Biological Chemistry. 2004;279:16425–32. doi: 10.1074/jbc.M313402200. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, et al. Crystallin {gamma}B-I4F mutant protein binds to {alpha}-crystallin and affects lens transparency. Journal of Biological Chemistry. 2005;280:25071–8. doi: 10.1074/jbc.M502490200. [DOI] [PubMed] [Google Scholar]
- 17.Van Montfort RLM, Bateman OA, Lubsen NH, Slingsby C. Crystal structure of truncated human betaB1-crystallin. Protein Science. 2003;12:2606–12. doi: 10.1110/ps.03265903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koteiche HA, Kumar MS, Machaourab HS. Analysis of βB1-crystallin unfolding equilibrium by spin- and fluorescence labeling. Evidence of a dimeric intermediate. FEBS LETTERS. 2007 doi: 10.1016/j.febslet.2007.04.004. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansoor SE, McHaourab HS, Farrens DL. Mapping proximity within proteins using fluorescence spectroscopy. A study of T4 lysozyme showing that tryptophan residues quench bimane fluorescence. Biochemistry. 2002;41:2475–84. doi: 10.1021/bi011198i. [DOI] [PubMed] [Google Scholar]
- 20.Koteiche HA, McHaourab HS. Mechanism of a Hereditary Cataract Phenotype: Mutations in αA-crystallin activate substrate binding. Journal of Biological Chemistry. 2006;281:14273–9. doi: 10.1074/jbc.M512938200. [DOI] [PubMed] [Google Scholar]
- 21.Grimsley JK, Scholtz JM, Pace CN, Wild JR. Organophosphorus hydrolase is a remarkably stable enzyme that unfolds through a homodimeric intermediate. Biochemistry. 1997;36:14366–74. doi: 10.1021/bi971596e. [DOI] [PubMed] [Google Scholar]
- 22.Minton AP. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. Journal of Biological Chemistry. 2001;276:10577–80. doi: 10.1074/jbc.R100005200. [DOI] [PubMed] [Google Scholar]