Abstract
Interferon beta (IFN-β) is among the first-line treatment options for patients with multiple sclerosis (MS). A potential caveat of therapy, however, is the development of neutralizing antibodies (NAb) and/or neutralizing activity (NA) non-antibody mediated, although debate is still ongoing as to whether NAb significantly hampers the efficacy of the drug or rather represents an immunologically irrelevant epiphenomenon. In the present study, we describe the effect of NAb on IFN-β-1b through clinical and magnetic resonance imaging (MRI) outcome measures of five relapsing–remitting multiple sclerosis (RRMS) patients who were treated with 250 μg of subcutaneously administered IFN-β-1b every other day and developed NAb at varying titres and times during the course of therapy. Despite the small number of NAb− patients, heterogeneity in MRI/clinical response to IFN-β-1b was identified. Response to IFN-β-1b therapy was observed in the absence or presence of NAb. Also observed was failure to IFN-β-1b coincident with high and sustained NAb titres, but also before NAb development or in the presence of low NAb titres. Multiple MRI and NAb measurements performed within the same individual allow for a better description of the complex heterogeneous response to IFN-β-1b with respect to NAb occurrence.
Keywords: IFN-β, magnetic resonance imaging, multiple sclerosis, neutralizing antibodies
Introduction
Over the past years, several investigations have been performed to estimate the role of neutralizing antibodies (NAb) in hampering the therapeutic efficacy of interferon beta (IFN-β) in patients with multiple sclerosis (MS). Briefly, the first multi-centre clinical trial of IFN-β-1b efficacy in MS patients demonstrated that 38% of patients who underwent therapy developed NAb by the end of the third year. NAb presence in those patients correlated with decreased therapeutic efficacy as measured by an increased exacerbation rate or greater load on T2-weighted (W) magnetic resonance imaging (MRI) [1]. However, later studies did not all confirm that relapse rates and alterations in clinical disability, as measured by the Expanded Disability Status Scale (EDSS) scores [2] or MRI measures of progression, differ significantly between the NAb− and NAb– patients. Results gathered from those many studies were summarized extensively by previous authors in review articles [3–6]. The majority of those studies, while designed in a longitudinal fashion as far as the clinical and MRI data acquisition are concerned, considered different patient groups at single time-points. Therefore, subsequent analyses did not convey the information potentially contained in the longitudinal data acquisition.
In contrast, few longitudinal analyses have explored whether the clinical and MRI profile for a given patient is modified by changes in NAb status. The latter accounts for the intrinsic intrapatient disease variability that is visible over time and hidden by cross-sectional analyses. In recent surveys, it was demonstrated that the switch from NAb– to NAb− status decreased the effectiveness of only low dose of IFN-β in patients with relapsing–remitting (RR) MS regarding the frequency of relapses [7], although NAb were found to neither affect the progression of disability [7] nor the yearly MRI activity over a 2-year study period [7,8]. While this has not been seen consistently in the general secondary progressive (SP) MS population [9], these results were shown to be NAb titres and status definition-dependent in a large cohort of SP MS patients with superimposed relapses undergoing IFN-β-1b therapy [10]. Thus, disagreement remains on the real role of NAb in hampering the therapeutic efficacy of IFN-β.
This is a particularly relevant issue that calls for elucidation aimed at assisting clinicians in their decision-making processes. The importance of this topic and the need for future research were updated recently by a panel of expert neurologists who concluded that the present available information on NAb is insufficient to provide guidelines on the usefulness of NAb testing. Moreover, the same panel of experts argued that the association between NAb occurrence and failure of clinical effectiveness of the drug is only probable [6].
We believe that an appropriate way to determine the effect of NAb on IFN-β therapeutic efficacy is through monitoring NAb and MRI activity with frequent, multiple measurements and describing how these measurements and their relationships evolve over time. Such analyses are extremely relevant because of the high sensitivity of MRI in disclosing even clinically silent disease activity [11], and might aid in reconciling discordant results obtained from previous reports.
To the best of our knowledge, longitudinal analyses evaluating the effects of NAb on MRI disease metrics have not been performed on a monthly basis.
Thus, to provide further insight into this issue, we describe the clinical and MRI outcomes in relation to NAb development in five MS patients who were monitored monthly for 6 months prior to therapy (i.e. natural history phase) and 3 years during IFN-β-1b treatment. Each monthly follow-up included both MRI and a clinical assessment. NAb titres were classified as low-positive and high-positive, as suggested by previous authors [7,9]. Although the sample size is small, thus preventing any type of generalization of our results, we believe that the length of the frequent, longitudinal MRI examinations contributes a unique, novel and powerful description regarding the heterogeneity of the possible roles that NAb may play in individual MS patients undergoing IFN-β therapy.
Patients and methods
Patients and study design
The study was performed at the National Institutes of Health and approved by the National Institute of Neurological Disorders and Stroke Institutional Review Board. Five patients with definite RRMS according to the Poser criteria [12] were enrolled consecutively for IFN-β-1b treatment with monthly MRI follow-ups. Each patient signed informed written consent. The study had a pretherapy phase (PTP) of 6 months followed immediately by a therapy phase (TP) of 36 months. During TP, patients received a 250-μg dose of subcutaneous IFN-β-1b every other day. None of the patients was treated with any type of IFN-β prior to the beginning of the study.
Brain MRIs and clinical neurological examinations, including EDSS measures, were performed monthly throughout the study period. An additional clinical evaluation was performed during the occurrence of a clinical relapse. A clinical relapse was defined as the presence of new symptoms or the exacerbations of previous ones lasting at least 48 h, in the absence of high body temperature. Clinical relapses were treated with either intravenous methylprednisolone at 1 g/day for 3–5 days or prednisone given orally as a tapered dose. Multiple blood collections were performed to evaluate NAb titres, as explained in the session below.
Table 1 summarizes demographic, clinical and MRI characteristics of the five patients at the time of enrolment.
Table 1.
Demographic, clinical and magnetic resonance imaging (MRI) characteristics of patients at enrolment time.
| Patient no. | Age | Gender | Years of MS | EDSS score | No. of CELs |
|---|---|---|---|---|---|
| 1 | 28·0 | Female | 4·0 | 1·5 | 0 |
| 2 | 45·0 | Female | 2·0 | 5·5 | 4 |
| 3 | 23·0 | Female | 4·0 | 5·0 | 13 |
| 4 | 30·9 | Female | 5·9 | 2·0 | 3 |
| 5 | 35·0 | Male | 7·0 | 2·0 | 4 |
| Mean ± s.d. | 32·4 ± 8·3 | 4 F/1 M | 4·6 ± 1·9 | 3·2 ± 1·9 | 4·8 ± 4·9 |
CELs: contrast-enhancing lesions; EDSS: Expanded Disability Status Scale; MS: multiple sclerosis.
MRI acquisition and analysis
Forty-two consecutive MRIs were obtained of each patient, using a 1·5 Tesla scanner (General Electric Medical Systems, Milwaukee, WI, USA) with a standard head coil. The following pulse sequences were used: T2W spin echo (SE) images [echo time (TE) 100 ms, repetition time (TR) 2000 ms], pre- and post-contrast [0·1 mmol/kg (body weight) injection of gadolinium diethylenetriamine pentaacid (Gd-DTPA; Magnevist, Berlex Laboratories, Cedar Knolls, NJ, USA)] T1 W SE (TE 16 ms, TR 600 ms) images were acquired. Twenty-seven contiguous slices, 5 mm thick, 24-cm field of view, 192 × 256 matrix size, were acquired for all MRIs for all patients but one, who was enrolled later in the study when 3-mm slice thickness MRIs were performed. Monthly numbers of total contrast-enhancing lesions (tCELs) were computed twice by a neurologist (FB).
Neutralizing antibodies
NAb were measured only once before the start of therapy (i.e. month 6 of PTP) and every other month thereafter during the TP. However, each patient had some periods when monthly (instead of bimonthly) NAb evaluations during the TP were performed. Specifically, patients 1, 2 and 3 had NAb evaluations for two consecutive months (during months 13, 14–21, 22, 26 and 27), while patient 4 had 23 monthly evaluations (months 12–34) and patient 5 had 24 (months 9–32). NAb measurements were performed using the Myxovirus protein A (MxA) inhibition assay from Berlex Laboratories (Richmond, CA, USA). Details regarding characterization and optimization of the MxA assay used in the study were reported previously [13]. Briefly, all the samples from patients were collected and stored at −20C. Identification numbers were blinded and assigned to the sera, all of which were then titrated at the same time. All samples were analysed in a 1 : 10 dilution and all positive samples were titrated serially at higher dilutions (1 : 2). An equal volume of IFN-β-1b at nominal final concentration of 10 laboratory units (LU)/ml was then added. The mixtures were then incubated for up to 90 min at room temperature. Aliquots of 0·1 ml of these samples were assayed by the 96-well protocol after removal of the seeding medium from the wells. Samples and standards were tested in triplicate with the indicator cells (i.e. human lung carcinoma cell line A549), and each of the three cell lysates was assayed once by immunoassay. In parallel, the IFN preparation was assayed to verify the actual activity used in the neutralization assays. The serum dilution at which 10% residual activity remains was determined. Linear interpolation between two neighbouring serum dilutions determined the dilution because the serum titration curve was linear in the 1 LU/ml region. The neutralization titre was the reciprocal serum dilution that reduced the MxA-inducing activity 10-fold. Assay sensitivities were as follows: limit of detection: 1 : 10, limit of quantification: 1 : 20.
Patients with NAb titres ≥1 : 20 at least once were considered NAb−. NAb− titres were classified further as low (≥ 1 : 20 and < 1 : 400) and high (≥ 1 : 400), as suggested previously [7,9]. Neutralizing activity detected in our samples was not characterized further as immunoglobulin (Ig)-mediated. However, as in previous work performed using this assay, non-Ig-mediated neutralizing activity contributed in very few samples [14] for this study, we referred as NAb− any serum where some neutralizing activity was found.
Statistical analysis
Descriptive statistics [mean and standard deviation (s.d.)] for all demographic, clinical and MRI variables were calculated. The TP was divided into six semesters. The percentage change of tCELs per semester of TP with respect to the PTP was calculated as [(B-A)/A] × 100, where B = the values corresponding to a semester during the TP and A = the values corresponding to the PTP. At each semester of therapy, patients were classified as MRI responders when a decrease in MRI activity by at least two-thirds was observed with respect to the average during PTP, in accordance with previously published NIH data [15].
Results
Table 2 illustrates MRI outcomes at each semester in each patient, whereas changes in EDSS score as well as the occurrence of clinical relapses can be visualized on the graphs in Figs 1–5. Of the five NAb− patients, two (patients 1 and 2) developed NAb activity at low and transient titres, while the remaining three patients exhibited high NAb titres numerous times present only up to the end of the second year of therapy. None of the patients were NAb− before starting therapy.
Table 2.
Percentage change of total contrast-enhancing lesions (tCELs) count each semester relative to pretherapy phase (PTP).
| Patient | PTP tCEL* | Semester 1 | Semester 2 | Semester 3 | Semester 4 | Semester 5 | Semester 6 |
|---|---|---|---|---|---|---|---|
| 1 | 0·7 ± 0·8 | −75% | −50% | 200% | 0% | 50% | 100% |
| 2 | 3·3 ± 2·9 | −75% | −50% | −75% | −65% | −100% | −90% |
| 3 | 7·8 ± 4·2 | −94% | −89% | −98% | −100% | −100% | −98% |
| 4 | 3·3 ± 2·6 | −50% | −40% | 25% | 130% | −15% | 85% |
| 5 | 1·2 ± 1·6 | 0% | 0% | 86% | 143% | 100% | 57% |
Mean ± s.d.
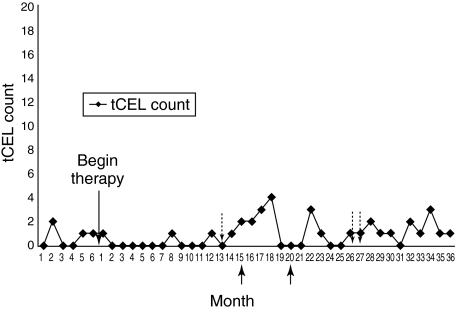
Fig. 1.
The graph represents NAb development in relation to tCELs evolution in patient 1. Neutralizing antibodies (Nab) development and total contrast-enhancing lesions (tCELs) occurrence at each time-point. Each arrow under the x-axis refers to one relapse corresponding to the indicated month. In Figs 1–3, each arrow above the x-axis refers to the month when low NAb titres (i.e. < 1 : 400) were detected. High titres are indicated by text corresponding to the arrow. As NAbs were detected in almost every blood sample collected in patients 4 and 5 at each measurement, titres are indicated with white dots. When a dotted line between two dots is not present, NAbs were not detected. Numbering of the figures corresponds to the numbering of the patients (see text in the results section).
Fig. 5.
The graph represents NAb development in relation to tCELs evolution in patient 5.
Low and transient NAb activity
Patient 1 (data in Fig. 1) showed very low and transient NAb titres. However, her tCEL activity decreased moderately only during the first two semesters of TP, followed by some rise during the subsequent semesters, when NAb continued to be absent or instantaneously present at low titres (Table 2). She suffered her first relapse during semester 3; a second relapse occurred at semester 4. Although her EDSS score was 1·5 throughout the majority of the TP, her MRI response to therapy was still poor, despite the absence of sustained NAb titres.
Patient 2 was enrolled during the progressive phase of MS with superimposed relapses and high levels of tCELs. She also exhibited low, transient but slightly less fluctuating NAb titres (Fig. 2). Her TP clinical disability increased from an EDSS score of 5·5 to 6·5 without superimposed relapses and with a noticeable decrease in tCEL activity (see Table 2). The decreases in tCEL count were evident in both the absence and presence of NAb.
Fig. 2.
The graph represents NAb development in relation to tCELs evolution in patient 2.
Patients 1 and 2 are two different examples of the interaction between low titres of NAb and MRI outcome. While the presence of transient and low NAb titres was temporally coincident with increased tCEL activity in the first patient, who was a poor responder regardless, rather it was associated with a constant decrease in tCELs in the second case.
High but transient NAb activity
Patient 3 had a clinical relapse at months 1 and 2 of PTP. Although biased in part by the associated increase in tCELs with a higher probability of a significant regression to the mean [11], she was a full MRI responder throughout the entire TP (Table 2 and Fig. 3). Two clinical relapses occurred during the TP (semesters 1 and 6), involving the spinal cord with only a low and transient increase in brain tCEL occurrence. NAb activity was detected in months 4–18, ranging from 1 : 31 to 1 : 1044, and coincided with a period of tCEL occurrence. From month 19 until the conclusion of the study, NAb as well as brain tCEL activity were no longer detected and the patient terminated the study with an EDSS of 3·5, which probably reflected a spinal cord exacerbation. The activity of this patient suggests that a temporal coincidence may exist between high NAb activation and MRI disease activity, although even high NAb may not necessarily abrogate completely the MRI IFNβ-1b effect.
Fig. 3.
The graph represents NAb development in relation to tCELs evolution in patient 3.
High and sustained NAb activity
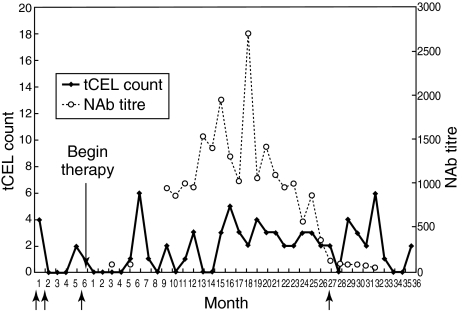
Patients 4 and 5 enrolled with an EDSS of 2·0. Although patient 4 showed twice as many tCELs during the PTP than patient 5, both showed high TP tCEL activity during periods of both high and low NAb activity relative to their PTP. Patient 4 (Fig. 4) exhibited NAb− titres throughout almost the entire course of therapy (ranging from 1 : 24 to 1 : 695, from month 3 until month 34 when the last NAb− titre was detected). No relapses occurred during PTP, but she suffered from four exacerbations during the TP (semesters 1, 3, 5 and 6) with a response to the drug mildly present only during the first semester of TP, when low NAb titres were found (Table 2). However, during periods of high (> 1 : 400) NAb activity (semesters 3 and 4), there was a concurrent spike in patient 4's tCEL activity, thus suggesting a putative role of NAb in increasing the average level of tCEL activity. Interestingly, however, as NAb reverted to low titres, tCELs reverted to the PTP level. She concluded the study with an EDSS of 4·5.
Fig. 4.
The graph represents NAb development in relation to tCELs evolution in patient 4.
Patient 5 experienced three relapses during the PTP and one during the fifth semester of the TP. He also exhibited significant NAb titres ranging from 1 : 48 to 1 : 1956 throughout the study period. Although high NAb activity was concurrent with active lesions, a similar level of tCEL activity was present in the absence of significant NAb titres (Fig. 5). The patient was considered as a non-responder throughout the entire length of the TP (Table 2) and concluded the study with an EDSS of 1·5. Patient 5 exemplifies a poor response to IFN-β-1b regardless of high and sustained antibody titres.
Discussion
Although the study cohort is small, the length of the monthly follow-up allowed the investigators to disclose and describe a high amount of heterogeneity in patterns of IFN-β-1b response in relation to NAb occurrence. As reported previously, NAb started developing as early as the first year of therapy, reached a plateau in some patients during the second year, and started decreasing by the third year. As expected, NAb at low titres were more volatile and disappeared sooner than those at high titres [16,17]. Low titres of NAbs [16,17] may be responsible for its transient detection.
Care needs to be taken when interpreting the biological and clinical value of low titres NAb in our patient cohort. Indeed, it is well known that positive sera at low titres of neutralizing activity may not necessarily contain antibodies. The detected neutralizing activity was not characterized further by the presence of immunoglobulin in our study. As non-IgG-mediated neutralizing activity is known to occur in very few samples [14], if none from patients treated with IFN-β-1b [18], we referred as NAb− any serum where some neutralizing activity was found. However, one cannot exclude the fact that in our low titred samples, other components from the drug manufacture may have contributed in determining the antagonizing effect to IFN-β-1b.
In examining the relationship between NAb presence and MRI activity, with regard to the occurrence of NAb at low and transient titres, we observed that the latter coincided temporally with periods of either decreased or increased tCEL activity with respect to PTP, depending upon the patient. The latter suggests that the response to therapy may occur concurrently with low and transient NAb titres where lower antagonization of the drug (i.e. higher therapeutic efficacy) is expected [19]. Conversely, therapeutic failure may be present even concomitantly with low and instantaneous NAb titres.
Not surprisingly, high titres were more sustained over time. Patients with high NAb titres are less likely to revert to NAb negativity and, if so, longer time is needed [20,21]. When examining patients who developed high NAb titres, different observations were made. One of the patients (patient 3) showed a complete remission of tCEL activity that resumed moderately with the presence of NAb and ceased as NAb titres began to decrease. Although the only time-period when tCELs were observed NAb were also present, the absence of tCEL activity was coincident with high NAb titres for some instantaneous (monthly) observation. It is noteworthy, however, that patient 3 experienced clinical activity during the PTP associated with higher MRI activity, thus indicating a predisposition to a consistent regression toward the mean in the number of tCELs during TP compared to the PTP.
On the contrary, patients 4 and 5 were examples of non-responders with increased tCEL activity in the presence of high NAb titres. Some issues regarding these two cases need to be highlighted in order to appreciate entirely and precisely the heterogeneity in individual behaviours. Both patients exhibited active CELs before positive or high NAb titres were detected. In both patients, high CEL numbers persisted while high NAb decreased to low titres or ceased. However, while in patient 4 a clear burst of CELs was evident only as NAb developed at high titres, thus challenging the argument that NAb are irrelevant with regard to therapy outcome, patient 5 provides evidence that the lack of a temporal association between high titres of NAb and MRI disease exacerbations may occur in some individuals. The latter supports the hypothesis of a predisposition for non-response to IFN-β-1b that may be due to an innately more active immune system prior to NAb occurrence. To what extent NAb further hinders the function of IFN-β-1b during the time of their presence, or merely functions as an epiphenomenon, remains unknown and the two possibilities may be not mutually exclusive. Cumulatively, this finding demonstrates that NAb presence may not necessarily be the direct cause of a diminished or absent positive therapeutic effect in all individuals, and highlights the importance of multiple measurements for disclosing this phenomenon.
Unfortunately, our study lacks measurements of IFN-induced compounds. With such measurements, the argument regarding the cause-and-effect relationship between IFN-β-1b and NAb occurrence in this patient could have been addressed more thoroughly.
Possible limitations of the present study need to be addressed before drawing conclusions. As mentioned previously, this was an open-label study that lacked measurements of IFN-β-induced compounds and considered only a small number of patients. Furthermore, and as a logical consequence of the above-mentioned issues, a systematic analysis of potential effects of steroids given for clinical relapses could not be performed. However, it was demonstrated previously in a large cohort of patients that steroids given for MS exacerbations do not affect the incidence and duration of NAb [22]. Thus, it is unlikely that steroid pulses administered for clinical relapses in our studies may have substantially biased our interpretations in individual cases.
In conclusion, our study provides a careful description of the complex heterogeneity in the relationship between NAb occurrence and MRI outcome among different MS patients or within the same patient during the time of IFN-β-1b therapy. The results highlight the importance of multiple measurements during a large trial when examining the effect(s) of NAb on IFN-β-1b response. Cross-sectional studies or longitudinal analyses with a limited number of measurements run the risk of not capturing the intrapatient heterogeneity in the response to treatment and the consequential potential changes in the relationship between MRI activity and Nab over time. On the contrary, more frequent repeated measures of NAb/tCELs may be useful for clinicians when investigating the role of NAb in individual MS patients undergoing therapy.
Acknowledgments
Drs S. Auh, F. Cantor, A. Gallo and K. Yao are acknowledged for providing important insights into the paper. Dr S. Stuerzebecher is acknowledged for assistance with NAbs testing. Ms D. Glazer-Schoenberg MSc, Editor, from the Office of the Clinical Director, NINDS is acknowledged for revising the manuscript. This research was supported by the Intramural Research Program of the NINDS–NIH. We are sincerely grateful to all our patients for the time and co-operation required to participate into the study.
References
- 1.IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. Neutralizing antibodies during treatment of multiple sclerosis with interferon beta-1b: experience during the first three years. Neurology. 1996;47:889–94. doi: 10.1212/wnl.47.4.889. [DOI] [PubMed] [Google Scholar]
- 2.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 3.Bagnato F, Pozzilli C. Pharmacological methods to overcome IFN-beta antibody formation in the treatment of multiple sclerosis. Expert Opin Invest Drugs. 2003;7:1153–63. doi: 10.1517/13543784.12.7.1153. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen PS, Deisenhammer F, Duda P, et al. Guidelines on use of anti-IFN-β antibodies in multiple sclerosis: a report of an EFNS Task Force on IFN-β in multiple sclerosis. Eur J Neurol. 2005;12:817–27. doi: 10.1111/j.1468-1331.2005.01386.x. [DOI] [PubMed] [Google Scholar]
- 5.Namaka M, Pollitt-Smith M, Gupta A, et al. The clinical importance of neutralizing antibodies in relapsing–remitting multiple sclerosis. Curr Med Res Opin. 2006;22:223–39. doi: 10.1185/030079906X80413. [DOI] [PubMed] [Google Scholar]
- 6.Goodin DS, Frohman EM, Hurwitz B, et al. Neutralizing antibodies to interferon beta: assessment of their clinical and radiographic impact: an evidence report. Neurology. 2007;68:977–84. doi: 10.1212/01.wnl.0000258545.73854.cf. [DOI] [PubMed] [Google Scholar]
- 7.Petkau J, White RA, Ebers GC, et al. Longitudinal analyses of the effects of neutralizing antibodies on interferon beta-1b in relapsing-remitting multiple sclerosis. Mult Scler. 2004;10:126–38. doi: 10.1191/1352458504ms1004oa. [DOI] [PubMed] [Google Scholar]
- 8.Barbero P, Bergui M, Versino E, et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis (INCOMIN Trial) II: analysis of MRI responses to treatment and correlation with Nab. Mult Scler. 2006;12:72–6. doi: 10.1191/135248506ms1247oa. [DOI] [PubMed] [Google Scholar]
- 9.Panitch H, Miller A, Paty D, Weinshenker B in Secondary Progressive MS. Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology. 2004;63:1788–95. doi: 10.1212/01.wnl.0000146958.77317.3e. for the North American Study Group on Interferon beta-1b. [DOI] [PubMed] [Google Scholar]
- 10.Polman C, Kappos L, White R, et al. Neutralizing antibodies during treatment of secondary progressive MS with interferon β-1b. Neurology. 2003;60:37–43. doi: 10.1212/wnl.60.1.37. [DOI] [PubMed] [Google Scholar]
- 11.McFarland HF, Frank JA, Albert PS, et al. Using gadolinium-enhanced magnetic resonance imaging lesions to monitor disease activity in multiple sclerosis. Ann Neurol. 1992;32:758–66. doi: 10.1002/ana.410320609. [DOI] [PubMed] [Google Scholar]
- 12.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 13.Files JG, Gray JL, Do LT, et al. A novel sensitive and selective bioassay for human type I interferons. J Interferon Cytokine Res. 1998;18:1019–24. doi: 10.1089/jir.1998.18.1019. [DOI] [PubMed] [Google Scholar]
- 14.Pungor E, Jr, Files JG, Gabe JD, et al. A novel bioassay for the determination of neutralizing antibodies to IFN-β1b. J Interferon Cytokine Res. 1998;18:1025–30. doi: 10.1089/jir.1998.18.1025. [DOI] [PubMed] [Google Scholar]
- 15.Stone LA, Frank JA, Albert PS, et al. The effect of interferon-beta on blood–brain barrier disruptions demonstrated by contrast-enhanced magnetic resonance imaging in relapsing–remitting multiple sclerosis. Ann Neurol. 1995;37:611–9. doi: 10.1002/ana.410370511. [DOI] [PubMed] [Google Scholar]
- 16.Rice GP, Paszner B, Oger J, et al. The evolution of neutralizing antibodies in multiple sclerosis patients treated with interferon beta-1b. Neurology. 1999;52:1277–9. doi: 10.1212/wnl.52.6.1277. [DOI] [PubMed] [Google Scholar]
- 17.Bellomi F, Scagnolari C, Tomassini V, et al. Fate of neutralizing and binding antibodies to IFN beta in MS patients treated with IFN beta for 6 years. J Neurol Sci. 2003;215:3–8. doi: 10.1016/s0022-510x(03)00173-4. [DOI] [PubMed] [Google Scholar]
- 18.Gilli F, Hoffmann F, Sala F, et al. Qualitative and quantitative analysis of antibody response against IFNb in patients with multiple sclerosis. Mult Scler. 2006;12:738–46. doi: 10.1177/1352458506070968. [DOI] [PubMed] [Google Scholar]
- 19.Pachner AR, Narayan K, Pak E. Multiplex analysis of expression of three IFN beta-induced genes in antibody-positive MS patients. Neurology. 2006;66:444–6. doi: 10.1212/01.wnl.0000196467.71646.72. [DOI] [PubMed] [Google Scholar]
- 20.Herndon RM, Rudick RA, Munschauer FE, III, et al. Eight-year immunogenicity and safety of interferon beta-1a-Avonex® treatment in patients with multiple sclerosis. Mult Scler. 2003;11:409–19. doi: 10.1191/1352458505ms1209oa. [DOI] [PubMed] [Google Scholar]
- 21.Reske D, Walser A, Haupt WF, Petereit HF. Long-term persisting interferon beta-1b neutralizing antibodies after discontinuation of treatment. Acta Neurol Scand. 2004;109:66–70. doi: 10.1034/j.1600-0404.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- 22.Pozzilli C, Antonini G, Bagnato F, et al. Monthly corticosteroids decrease neutralizing antibodies to IFNβ 1b: a randomized trial in multiple sclerosis. J Neurol. 2002;249:50–60. doi: 10.1007/pl00007847. [DOI] [PubMed] [Google Scholar]