Abstract
Tuberculosis is the most frequent co-infection in human immunodeficiency virus (HIV)-infected individuals, and which still presents diagnostic difficulties. Recently we set up an assay based on interferon (IFN)-γ response to region of difference 1 (RD1) peptides selected by computational analysis which is associated with active Mycobacterium tuberculosis replication. The objective of this study was to investigate the response to RD1 selected peptides in HIV-1-infected individuals in a clinical setting. The mechanisms of this immune response and comparison with other immune assays were also investigated. A total of 111 HIV-infected individuals with symptoms and signs consistent with active tuberculosis were enrolled prospectively. Interferon (IFN)-γ responses to RD1 selected peptides and recall antigens were evaluated by enzyme-linked immunospot assay. Results were correlated with CD4− T cell counts, individuals' characteristics, tuberculin skin test, QuantiFERON-TB Gold and T-SPOT.TB. Results from 21 (19%) individuals were indeterminate due to in vitro cell anergy. Among ‘non-anergic’ individuals, sensitivity for active tuberculosis of the assay based on RD1 selected peptides was 67% (24 of 36), specificity was 94% (three of 54). The assay also resulted positive in cases of extra-pulmonary and smear-negative pulmonary active tuberculosis. The response was mediated by CD4− effector/memory T cells and correlated with CD4− T cell counts, but not with plasma HIV-RNA load. Moreover, the RD1 selected peptides assay had the highest diagnostic odds ratio for active tuberculosis compared to tuberculin skin test (TST), QuantiFERON-TB Gold and T-SPOT.TB. RD1 selected peptides assay is associated with M. tuberculosis replication in HIV-infected individuals, although T cell anergy remains an important obstacle to be overcome before the test can be proposed as a diagnostic tool.
Keywords: effector/memory T cells, HIV-TB, IFN-γ, RD1 assays, recall antigen response
Introduction
Human immunodeficiency virus (HIV)-1 infection significantly affects the progression of Mycobacterium tuberculosis infection. In fact, while this infection in immunocompetent hosts usually results in a non-transmissible latent infection (LTBI), individuals co-infected with HIV-1 and M. tuberculosis are at high risk of progressing from LTBI to active disease [1,2]. Moreover, the risk of active tuberculosis also remains elevated among the HIV-infected individuals receiving highly active anti-retroviral therapy (HAART) [3]. Therefore, strategies must be developed to obtain an accurate and rapid diagnosis of LTBI and active tuberculosis to control its spread.
Currently, isolation of M. tuberculosis in culture remains the gold standard for active tuberculosis diagnosis, with a long time for the response [4]. Tuberculin skin test (TST) is used widely to lend support to clinical and radiological findings in the assessment of individuals with suspected tuberculosis. However, TST has significant limitations of low sensitivity and specificity, and suffers from operational drawbacks [5–7]. Moreover, among subjects with HIV infection the likelihood of false negative TST results increases with decreasing CD4− T cell levels [8] as a result of cutaneous anergy [9–10].
A major advance for the diagnosis of tuberculosis was the discovery of the highly immunogenic antigens early secreted antigenic target (ESAT)-6 and culture filtrate protein (CFP)-10 encoded by the region of difference 1 (RD1) genomic segment of M. tuberculosis, which is absent from all M. bovis bacillus Calmette–Guérin (BCG) strains or most environmental mycobacteria [11–15]. Two commercially available tests, QuantiFERON-TB Gold (QTF-G) and T-SPOT.TB, which detect interferon (IFN)-γ responses to overlapping peptides of these RD1 antigens, have been approved for the immune diagnosis of tuberculosis infection [13,16–19]. These tests are rapid and more accurate than TST [20–24]. However, conflicting results have been reported on the performance of these assays in HIV-infected individuals [10,20–25]. Moreover, these tests do not discriminate between active tuberculosis and LTBI.
In this context, we set up an in vitro assay which detects IFN-γ response to ESAT-6 and CFP-10 (RD1) multi-epitopic peptides selected by computational analysis, and we demonstrated that a positive response in this assay is associated with M. tuberculosis replication such as during active tuberculosis disease and/or recent infection [26–28].
The objectives of this pilot study in HIV-infected individuals were: (i) to evaluate whether this RD1 selected peptides assay may help in providing evidence of diagnosis of active tuberculosis in a clinical setting; (ii) to characterize the response to this assay and correlate it with the immune status of the patients; and (iii) to compare the accuracy of this test with that of commercially available tests.
Materials and methods
Patient population and study design
HIV-infected individuals suspected of active tuberculosis, according to the institutional protocol [29], admitted to the infectious diseases ward at the National Institute for Infectious Diseases (INMI) ‘L. Spallanzani’, Rome, Italy, between January 2002 and April 2006 were evaluated for enrolment. None of them were under anti-tuberculosis therapy at the time of enrolment. Individuals underwent TST administered by the Mantoux procedure using 5 IU of purified protein derivative (PPD) (Chiron, Siena, Italy), results were read after 72 h and an induration of at least 5 mm was scored positive [30]. TST and in vitro T cell assays were performed at the same time. Plasma HIV-RNA load was detected by Quantiplex HIV-1 RNA version 3·0 assay (bDNA v3·0; Bayer Diagnostics, Emeryville, CA, USA). Cytomegalovirus (CMV) serology was determined by VIDAS CMV IgG and IgM (bioMérieux sa, Marcy L'Etoile, France).
Enrolled individuals were classified as ‘active tuberculosis’ if the diagnosis was confirmed by a positive M. tuberculosis culture of sputum or bronchoalveolar lavage (BAL) specimens. For those with a diagnosis of extra-pulmonary tuberculosis (three disseminated; three lymph nodal; one lymph nodal and intestinal; one kidney), a polymerase chain reaction (PCR) biopsy was positive for M. tuberculosis. Patients without active tuberculosis were those individuals whose sputum culture or biopsies for M. tuberculosis tested negative, with either a resolution of clinical symptoms and radiographic abnormalities following antibiotic therapy not involving drugs active against M. tuberculosis, or who eventually received an alternative diagnosis (e.g. lung cancer). The study was approved by the Ethics Committee of the Institute and all enrolled individuals gave written informed consent.
Epitope prediction
The selection of human leucocyte antigens (HLA)-class II restricted epitopes of ESAT-6 and CFP-10 M. tuberculosis proteins was performed by quantitative implemented HLA peptide-binding motifs analysis, as described previously [26,27,31,32]. Each selected peptide contained one or more epitopes able to bind at least four different HLA-DR, two different HLA-DP and two different HLA-DQ specificities with a putative binding ability of 80% maximum binding for any allele belonging to an HLA-class II serological specificity. Together, the designed peptide epitopes were able to cover more than 90% of the HLA-class II haplotypes present in different human populations.
Enzyme-linked immunosorbent assays (ELISPOT) based on RD1 selected peptides and other recall antigens
IFN-γ produced by peripheral blood mononuclear cells (PBMC) in control wells [medium or dimethylsulphoxide (DMSO) (10 µg/ml)] and in response to the stimuli was evaluated by ELISPOT, as described previously [26,27,31,32]. Briefly, duplicate cell cultures were incubated with either a pool of the two ESAT-6 peptides (25 µg/ml each), a pool of the three CFP-10 peptides (2 µg/ml each), PPD (batch RT47; Statens Serum Institut, Copenhagen, Denmark) at 10 µg/ml, CMV viral lysate (Strain AD 169, ABI Inc, Columbia, MD, USA) at 2 µg/ml and phytohaemagglutinin (PHA) (Sigma, St Louis, MO, USA) at 5 µg/ml.
Cut-off values, 34 spot-forming cells (SFCs) per million PBMC for RD1 selected peptides, 36 for PPD and CMV and 100 for PHA were determined by constructing a receiver operator characteristic (ROC) curve by means of LABROC-1 software. In vitro anergy was defined as less than 100 SFCs per million PBMC in the PHA-positive control wells. Clinicians were blinded to the results of in vitro assays and laboratory personnel was blinded to the status of the patients.
Phenotypic analysis
PBMC (1 × 106 cells/ml) were cultivated in complete medium in the presence or absence of the stimuli as indicated above. Phorbol-12-myristate-13-acetate (PMA) plus ionomycin (Sigma Aldrich, St Louis, MO, USA) were used as positive controls at 50 ng/ml and 1 µg/ml, respectively. To detect intracellular expression of IFN-γ, 10 µg/ml of brefeldin A (Sigma Aldrich) was used, as described previously [33–34]. Briefly, production of IFN-γ was assessed by staining with appropriate combinations of monoclonal antibodies (MoAb) conjugated directly to fluorochromes: fluorescein isothiocyanate (FITC)-conjugated anti-CD4, allophycocyanine (APC)-conjugated anti-CD45RA MoAb and APC-anti-IFN-γ MoAb (Becton Dickinson, Mountain View, CA, USA) and phycoerythrin (PE)-cyanine 5 (PC5)-conjugated anti-CD27 (Instrument Laboratories, Coulter, Fullerton, CA, USA). Data acquisition and analysis were performed on a FACSCalibur flow cytometer (Becton Dickinson) using CellQUEST software (Becton Dickinson). For all staining procedures, an isotype-matched negative control was processed in parallel.
Commercially available assays
QTF-G (Cellestis Limited, Carnegie, Victoria, Australia) and T-SPOT.TB (Oxford Immunotec, Oxford, UK) assays were performed as indicated by the manufacturers.
Statistical analysis
IFN-γ production in response to antigenic stimulation was expressed as both dichotomous (positive/negative) and continuous (IU/ml) measures. Median and mean (± s.e.) were calculated. The Mann–Whitney U-test was used to compare continuous variables, and χ2 or McNemar tests were used for categorical variables. Analysis was carried out with spss version 14 for Windows (SPSS Italia srl, Bologna, Italy).
Results
Characteristics of enrolled subjects
We enrolled 111 consecutive HIV-infected individuals with suspected active tuberculosis. Among them, a diagnosis of active tuberculosis was confirmed microbiologically in 45 individuals and ruled out in 66. The demographic and clinical features of all enrolled HIV-infected participants are reported in Table 1.
Table 1.
Demographic and clinical characteristics of human immunodeficiency virus (HIV)-infected subjects enrolled in the study.
| Individuals enrolled with suspected active-tuberculosis | |||||||
|---|---|---|---|---|---|---|---|
| Non-anergic* | Anergic* | ||||||
| Active tuberculosis N. 36 | No active tuberculosis N. 54 | Total non-anergic N. 90 | Active tuberculosis N. 9 | No active tuberculosis N. 12 | Total anergic N. 21 | Total N. 111 | |
| Female (%) | 11 (44) | 14 (56) | 25 (28) | 6 (67) | 3 (33) | 9 (43) | 34 (31) |
| Age, median (years) | 38 | 41 | 41 | 41 | 41 | 41 | 41 |
| Ethnicity | |||||||
| West Europe (%) | 18 (33) | 37 (67) | 55 (61) | 3 (25) | 9 (75) | 12 (57) | 67 (60) |
| East Europe (%) | 1 (100) | 0 (0) | 1 (1) | 1 (100) | 0 | 1 (5) | 2 (2) |
| South America (%) | 10 (59) | 7 (41) | 17 (19) | 1 (100) | 0 | 1 (5) | 18 (16) |
| Africa (%) | 6 (38) | 10 (62) | 16 (18) | 4 (57) | 3 (43) | 7 (33) | 23 (21) |
| Asia (%) | 1 (100) | 0 (0) | 1 (1) | 0 | 0 | 0 | 1 (1) |
| BCG vaccinated (%) | 17 (48) | 17 (32) | 34 (38) | 6 (67) | 3 (33) | 9 (43) | 43 (39) |
| TST positive (%) | 14/30** (47) | 16/44** (36) | 30/74** (41) | 0 | 0 | 0 | 30/95** (32) |
| Tuberculosis localization | |||||||
| Pulmonary (%) | 29 (81) | – | 29 (32) | 8 (89) | – | 8 (38) | 37 |
| Extra-pulmonary (%) | 7 (19) | – | 7 (8) | 1 (11) | – | 1 (5) | 8 |
| HIV-RNA, median (cp/ml) | 46 419 | 13 748 | 28 353 | 45 672 | 112 018 | 91 156 | 34 984 |
| CD4− T cells | |||||||
| Median (cells/µl) | 152 | 236 | 201 | 76 | 131 | 78 | 179 |
| 0–100 (%) | 10 (28) | 10 (19) | 20 (22) | 6 (66) | 6 (50) | 12 (57) | 32 (29) |
| 101–200 (%) | 11 (30) | 14 (26 | 25 (30) | 2 (22) | 2 (17) | 4 (19) | 29 (26) |
| 201–300 (%) | 4 (1) | 8 (67) | 12 (13) | 1 (11) | 2 (17) | 3 (14) | 15 (14) |
| ≥ 301 (%) | 11 (30) | 22 (14) | 33 (37) | – | 2 (17) | 2 (10) | 35 (32) |
| HAART treatment (%) | 13 (36) | 33 (61) | 46 (51) | 5 (71) | 2 (29) | 7 (33) | 53 (48) |
In vitro anergy defined as response to mitogen stimulation lower than the cut-off.
Subjects tested over total. BCG: bacille Calmette–Guérin; TST: tuberculin skin test; HAART: highly active anti-retroviral therapy.
Among the 111 patients enrolled, 21 individuals were anergic in vitro, nine with and 12 without active tuberculosis. In vitro anergy was defined as less than 100 SFCs per million PBMC in the PHA-positive control wells. In fact, in the ELISPOT assay, PHA serves as a surrogate marker for anergy as well as a quality control for the assay. As shown in Table 1, none of the anergic individuals were positive to TST, while 14 of 30 (47%) non-anergic individuals who received a TST had a positive response (P = 0·0001). Moreover, the CD4− T cell counts of all anergic individuals were significantly lower than those of the non-anergic (median of CD4 T cells: 78 versus 201; P = 0·0033), and this difference remained significant when individuals with or without active tuberculosis were considered separately (P = 0·04 and (P = 0·03, respectively). No significant differences among anergic and non-anergic patients were observed for the other characteristics considered.
Response to RD1 selected peptides is associated with M. tuberculosis replication
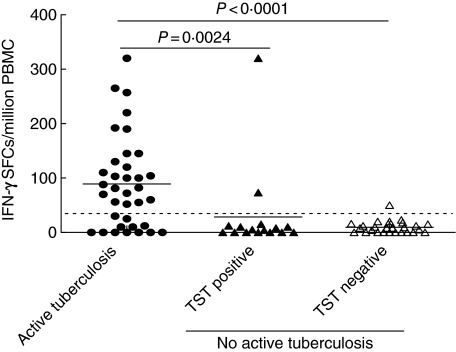
Hereafter, we evaluated data only from those without in vitro anergy. Using RD1 selected peptides in the ELISPOT assay, the mean of IFN-γ SFCs was significantly higher in individuals with active tuberculosis compared to those without active tuberculosis (88 ± 14 versus 16 ± 7, respectively; P < 0·0001). This difference was statistically significant independently of whether the controls were either TST-positive (29 ± 20, P = 0·0006) or TST-negative (10 ± 2, P < 0·0001) (Fig. 1). The response to RD1 selected peptides was scored positive in 24 of 36 (67%) individuals with active tuberculosis and in three of 54 (6%) without active tuberculosis (two of 16 among TST-positive and one of 28 among TST-negative individuals). Thus, in this HIV-infected population, this assay showed a sensitivity for active tuberculosis of 67% and a specificity of 94% (Table 2). Further, we evaluated the performance of the assay in active tuberculosis patients in whom a diagnosis was difficult to obtain: the assay was positive in three of three (100%) cases of smear-negative pulmonary tuberculosis (diagnosis confirmed by BAL culture) and in five of seven (71%) cases of extra-pulmonary tuberculosis.
Fig. 1.
Interferon (IFN)-γ response to region of difference 1 (RD1) selected peptides in HIV-infected individuals with or without active tuberculosis, by enzyme-linked immunospot assay. The highest response to RD1 selected peptides in terms of IFN-γ production is reported as number of spot-forming cells (SFCs) per million peripheral blood mononuclear cells (PBMC) for each individual. Horizontal bars represent the mean of the SFCs per million PBMC for each group of individuals. The P-value denotes the difference between the responders in each group. •, Active tuberculosis; ▴, non-active tuberculosis, tuberculin skin test (TST)-positive; ▵, non-active tuberculosis, TST-negative.
Table 2.
Sensitivity, specificity and diagnostic odds ratio evaluated by region of difference 1 (RD1) selected peptides, tuberculin skin test (TST), T-SPOT.TB and QTF-G assays.
| Assay | Sensitivity (95% CI) | Specificity (95% CI) | Diagnostic OR (95% CI) |
|---|---|---|---|
| RD1 selected peptides | 69·2% (38·6–90·9) | 92·9% (76·5–99·1) | 29·25 (3·65–328·6) |
| TST | 46% (19·2–74·9) | 60·7% (40·6–78·5) | 1·32 (0·29–6·11) |
| T-SPOT.TB | 84·6% (54·5–98·1) | 64·3% (44·1–81·4) | 9·9 (1·6–103·1) |
| QFT-G | 84·6% (54·5–98·1) | 75% (55·1–89·3) | 16·5 (2·5–173·5) |
Response to RD1 selected peptides is mediated by CD4− effector memory T cells and correlates with CD4− T cell counts
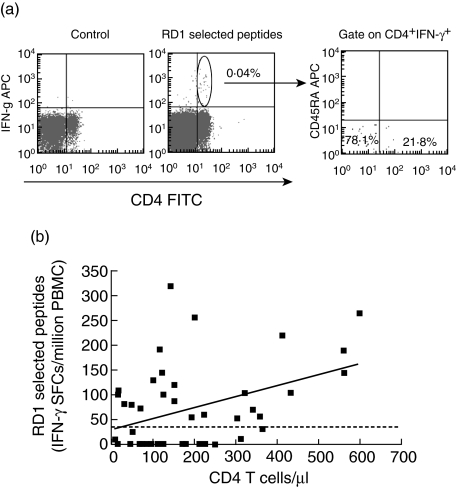
Next we evaluated the phenotypic features of the cells responding to the RD1 antigens in HIV-infected individuals with active tuberculosis. All the individuals analysed responded to the positive control, PMA plus ionomycin (data not shown). An IFN-γ response to RD1 selected peptides was observed for CD4− T cells (Fig. 2a) but not for CD8− T cells (data not shown), and this is plausible, these RD1 epitopes being class II-specific [26]. Most CD4− T cells (78%) responding to RD1 selected peptides had an effector/memory phenotype (defined as CD45RA−CD27−), as shown in Fig. 2a, and as reported previously in individuals without HIV infection [34]. Moreover, a statistically significant positive correlation (r = 0·33; P = 0·047) between the response to RD1 selected peptides and CD4− T cell counts was found (Fig. 2b), while none was observed between the same response and plasma HIV-RNA level (r = −0·18; P = 0·25).
Fig. 2.
CD4− T cell responses to region of difference 1 (RD1) selected peptides. (a) Phenotypic analysis of interferon (IFN)-γ-producing CD4− T cells in human immunodeficiency virus (HIV)-infected individuals with active tuberculosis. Peripheral blood mononuclear cells (PBMC) from active tuberculosis individuals were cultured in the presence of RD1 selected peptides. A negative control staining in the left panel shows no IFN-γ production by CD4− T cells in the absence of specific stimuli. IFN-γ response to RD1 selected peptides is mediated by CD4− T cells (central panel). Gated IFN-γ-producing CD4− T cells were analysed for CD45RA and CD27 expression, and the percentages of cells responding to RD1 selected peptides are shown. Effector memory cells (CD45RA−/CD27−) are represented in the low-left quadrant and the central memory cells (CD45RA−/CD27−) in the low-right quadrant in the right panel. (b) Correlation between the response to RD1 selected peptides and CD4− T cell count in HIV-infected individuals with active-tuberculosis. The highest response to RD1 selected peptides in terms of IFN-γ is shown as SFCs per million PBMC for each individual. The CD4− T cell count is indicated as number of cells per µl.
Evaluation of RD1 selected peptides response among individuals presenting a CMV recall antigen response
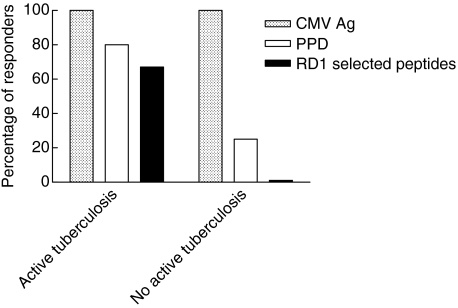
To ascertain whether IFN-γ secretion by CD4− T cells in HIV-infected individuals without active tuberculosis was globally impaired, we evaluated the response to RD1 epitopes in those presenting a response to another recall antigen such as CMV. For this purpose, we selected 25 individuals responsive to CMV antigen in vitro by IFN-γ ELISPOT assay who also had detectable serum IgG antibodies to CMV. Among these 25 individuals, nine had active tuberculosis and 16 had not. Responses to PPD and RD1 selected peptides were analysed. As shown in Fig. 3, among individuals with active tuberculosis, 89% (eight of nine) responded to PPD and 67% (six of nine) to RD1 selected peptides. Among those without active tuberculosis, a positive response to PPD was found in 25% (four of 16), while no response to RD1 selected peptides was observed. Thus, the individuals without active tuberculosis, although not responding to RD1 selected peptides, are able to mount a specific response to recall antigens other than M. tuberculosis.
Fig. 3.
Percentage of responders to purified protein derivative (PPD) and region of difference 1 (RD1) selected peptides among human immunodeficiency virus (HIV)-infected individuals with a positive response to cytomegalovirus (CMV) antigen. Individuals with in vitro responsiveness to CMV antigen by interferon (IFN)-γ enzyme-linked immunospot assay were selected. Among them, nine were with active tuberculosis and 16 without active tuberculosis; their IFN-γ response to PPD and RD1 selected peptides were analysed. The percentage of responders is reported for each group of individuals.
Comparison of response to RD1 selected peptides with that to TST and in vitro assays approved for the immune diagnosis of tuberculosis infection
To substantiate our results, 41 non-anergic (as defined above) individuals, 13 with and 28 without active tuberculosis, were analysed by RD1 selected peptides assay, in vivo TST, T-SPOT.TB and QTF-G, which are based on RD1 overlapping peptides spanning the length of CFP-10 and ESAT-6 proteins. Among active tuberculosis patients, a positive response was found in nine of 13 (69·2%) by RD1 selected peptides assay, in six of 13 (46%) by TST, in 11 of 13 (84·6%) by T-SPOT.TB and in 11 of 13 (84·6%) by QTF-G. Among those without active tuberculosis, two of 28 (7·1%) responded to RD1 selected peptides assay, 10 of 28 (35·7%) to T-SPOT.TB, seven of 28 (25%) to QTF-G and 11 of 28 (39·3%) were TST-positive (Table 2). Therefore, the RD1 selected peptides assay showed a higher specificity for active tuberculosis (92·9%) compared to TST (60·7%) and to the other two assays (64·3% and 75% for T-SPOT.TB and QTF-G, respectively).
In patients with active tuberculosis, the proportion of positive responses to RD1 selected peptides was similar to that observed for the other assays (P > 0·05). Conversely, when considering the subjects without active tuberculosis, the proportion of positive responses to RD1 selected peptides was significantly lower compared to TST (P = 0·004), T-SPOT.TB (P = 0·008) and QFT-G (P = 0·006). Moreover, no statistically significant difference was observed between the two commercial assays (P = 0·5). Based on these results, the RD1 selected peptides assay had the highest diagnostic odds ratio for active tuberculosis (Table 2) compared to QTF-G and T-SPOT.TB.
Discussion
In this study we found that the RD1 selected peptide assay is severely impaired by T cell anergy in HIV-infected individuals with advanced immunosuppression. However, among non-anergic HIV-infected individuals, the IFN-γ response to this assay was associated significantly with M. tuberculosis replication, also in smear-negative pulmonary and extra-pulmonary tuberculosis cases. This response was mediated by CD4− effector/memory T cells and correlated with CD4− T cell counts. Finally, the accuracy of the response to RD1 selected peptides for active tuberculosis, as measured by the diagnostic odds ratio, was higher compared to that obtained by TST and the two commercially available assays used for immune diagnosis of tuberculosis infection, QTF-G and T-SPOT.TB.
There is a relevant concern that the performance of immune assays for tuberculosis, TST included, can be impaired by low sensitivity in patients with advanced immunodeficiency. In the present study, 19% of all enrolled subjects became anergic in the in vitro assay, as they did not show a positive response to PHA. It is noteworthy that this group had a significantly lower CD4− T cell median compared to non-anergic individuals (78 versus 201), and a significant proportion of these anergic individuals had a CD4− T cell number below 100 cell/µl. These data are in agreement with previous studies reporting a high rate of anergy in individuals with CD4− T cells < 100 cell/µl [22,24]. Conversely, others showed that the response to mitogen was not impaired [10,21,35]; however, this was due probably to the selected HIV-infected population characterized by a relatively high median CD4− T cell count, 361 and 392 CD4− T cell/µl, respectively [10,21] and by a high proportion of subjects with CD4− T cell counts higher than 200 cell/µl (87%) compared to our population (45%) [35]. These findings together support the hypothesis that the performance of the IFN-γ assays can be affected negatively in patients with advanced immune suppression who unfortunately in western countries are those at high risk of developing active tuberculosis, also in the HAART era [36].
Limited information is available on the performance of IFN-γ assays for diagnosis of active tuberculosis in HIV-co-infected individuals. A high sensitivity of T SPOT.TB assay has been reported in co-infected adults and children and this sensitivity was significantly higher if compared to TST [20,37]. In the present study, the sensitivity for active tuberculosis of RD1 selected peptides assay was significantly higher than TST, suggesting that the blood tests may be able to better detect M. tuberculosis-specific T cell response in a context of HIV/tuberculosis.
The response to our assay is associated strictly with active M. tuberculosis replication compared to other assays that detect both LTBI and active tuberculosis disease [15,20–25]. These differences may be related to the amount and the composition of epitopes covered by the peptides. For example, the peptides employed in the QTF-G and T SPOT.TB cover the whole CFP-10 and ESAT-6 intact proteins, whereas the peptides used in our assay are few and selected in order to be highly immunogenic [26]. The response to these selected peptides is mediated by CD4− T effector cells that were shown to undergo clonal expansion during M. tuberculosis replication, followed by a contraction phase after efficacious therapy culminating in the generation of CD4− memory T cells [34]. These data are in agreement with those obtained in animal models of M. tuberculosis infection in which a decrease of ESAT-6-specific CD4− T cell effector response has been reported both in the lymph nodes and lungs after the acute phase of infection [38]. This model has been confirmed by studies performed in patients with diseases different from tuberculosis, such as HIV-infected patients undergoing structured treatment interruption, in whom HIV-specific T cell responses have been reported to be higher at the peak of viraemia and then to decrease when therapy is reintroduced [33,39]. Similarly, in patients who had been acutely infected with HCV and who cleared the infection, high frequencies of effector T cells are found soon after infection, whereas higher frequencies of memory T cells appear later on [40].
Comparison among the blood assays for tuberculosis immune diagnosis indicated that our RD1 selected peptides assay had a lower sensitivity compared to QTF-G and T-SPOT.TB. However, its specificity and diagnostic odds ratio for active tuberculosis were significantly higher. Furthermore, we also demonstrated that the lack of response to RD1 selected peptides in individuals without active tuberculosis was not due to the inability of CD4− T cells to secrete IFN-γ, as their ability to mount an antigen-specific response to a non-mycobacterial antigen, such as CMV, was intact. To our knowledge, this is the first study in which a comparison between QTF-G and T-SPOT.TB in HIV-infected individuals with active tuberculosis is performed and no statistically significant difference was observed.
This is a pilot study which presents limitations that will require additional work to overcome. Above all, the study was performed on a relatively small number of individuals, therefore a larger study is needed taking into account CD4− T cell number, type of HAART, HIV-RNA load and concomitant diseases. In addition, the ELISPOT technology used to detect the response to RD1 selected peptides presents difficulties for routine laboratory measurements compared to whole blood ELISA tests that we have demonstrated previously to match the performance of the ELISPOT assay in HIV-uninfected individuals [30].
In conclusion, we demonstrated that in HIV-infected individuals the response to RD1 selected peptides is also associated with M. tuberculosis replication in cases of difficult diagnosis. However, T cell anergy remains an essential problem to be overcome before the test can be proposed as a diagnostic tool, at least in individuals with low CD4− T cell counts.
Acknowledgments
The authors are grateful to all patients and nursing staff who took part in this study. We thank Drs C. Nisii, A. Sacchi and F. Martini for critical review of the paper. The paper was supported by a grant from the Istituto Superiore di Sanità on AIDS research (convenzione 50G.19). D. V., S. C., R. C., E. G. and D. G. have a patent pending on T cell assay based on RD1 selected peptides.
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence and mortality by country. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Nunn P, Williams B, Floyd K, Dye C, Elzinga G, Raviglione M. Tuberculosis control in the era of HIV. Nat Rev Immunol. 2005;5:819–26. doi: 10.1038/nri1704. [DOI] [PubMed] [Google Scholar]
- 3.Girardi E, Sabin CA, D'Arminio Monforte A, et al. Incidence of tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis. 2005;41:1772–82. doi: 10.1086/498315. [DOI] [PubMed] [Google Scholar]
- 4.Watterson SA, Drobniewski FA. Modern laboratory diagnosis of mycobacterial infections. J Clin Pathol. 2000;53:727–32. doi: 10.1136/jcp.53.10.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huebner RE, Schein MF, Bass JB., Jr The tuberculin skin test. Clin Infect Dis. 1993;17:968–75. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 6.Fine PE, Bruce J, Ponnighaus JM, Nkhosa P, Harawa A, Vynnycky E. Tuberculin sensitivity: conversions and reversions in a rural African population. Int J Tuberc Lung Dis. 1999;3:962–75. [PubMed] [Google Scholar]
- 7.Kwamanga DO, Swai OB, Agwanda R, Githui W. Effect of non-tuberculous Mycobacteria infection on tuberculin results among primary school children in Kenya. East Afr Med J. 1995;72:222–7. [PubMed] [Google Scholar]
- 8.Converse PJ, Jones SL, Astemborski J, Vlahov D, Graham NM. Comparison of a tuberculin interferon-gamma with the tuberculin skin test in high-risk adults: effect of human immunodeficiency virus infection. J Infect Dis. 1997;176:144–50. doi: 10.1086/514016. [DOI] [PubMed] [Google Scholar]
- 9.Elzi L, Schlegel M, Weber R, et al. Reducing tuberculosis incidence by tuberculin skin testing, preventive treatment, and antiretroviral therapy in an area of low tuberculosis transmission. Clin Infect Dis. 2007;44:94–102. doi: 10.1086/510080. [DOI] [PubMed] [Google Scholar]
- 10.Dheda K, Lalvani A, Miller RF, et al. Performance of a T cell-based diagnostic test for tuberculosis infection in HIV-infected individuals is independent of CD4 cell count. AIDS. 2005;19:2038–41. doi: 10.1097/01.aids.0000191923.08938.5b. [DOI] [PubMed] [Google Scholar]
- 11.Behr MA, Wilson MA, Gill WP, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–3. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 12.Sorensen AL, Nagai S, Houen G, Andersen P, Andersen AB. Purification and characterization of a low-molecular-mass T cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63:1710–7. doi: 10.1128/iai.63.5.1710-1717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pai M, Riley LW, Colford JM., Jr Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4:761–76. doi: 10.1016/S1473-3099(04)01206-X. [DOI] [PubMed] [Google Scholar]
- 14.Berthet FX, Rasmussen PB, Rosenkrands I, Andersen P, Gicquel B. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10) Microbiology. 1998;144:3195–203. doi: 10.1099/00221287-144-11-3195. [DOI] [PubMed] [Google Scholar]
- 15.Mori T, Sakatani M, Yamagishi F, et al. Specific detection of tuberculosis infection with an interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med. 2004;170:59–64. doi: 10.1164/rccm.200402-179OC. [DOI] [PubMed] [Google Scholar]
- 16.Ravn P, Demissie A, Eguale T, et al. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J Infect Dis. 1999;179:637–45. doi: 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- 17.Ulrichs T, Munk ME, Mollenkopf H, et al. Differential T cell responses to Mycobacterium tuberculosis ESAT-6 in tuberculosis patients and healthy donors. Eur J Immunol. 1998;28:3949–58. doi: 10.1002/(SICI)1521-4141(199812)28:12<3949::AID-IMMU3949>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Doherty TM, Demissie A, Olobo J, et al. Immune responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 signal subclinical infection among contacts of tuberculosis patients. J Clin Microbiol. 2002;40:704–6. doi: 10.1128/JCM.40.2.704-706.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Food and Drug Administration (FDA) FDA approval for the use of synthetic peptide antigens used in the QuantiFERON-TB Gold. Available at: http://www.fda.gov/cdrh/pma/pmadec04.html. P10033/S0006. Last updated 14 April 2005 (accessed 14 April 2007)
- 20.Chapman AL, Munkanta M, Katalin A, et al. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS. 2002;16:2285–93. doi: 10.1097/00002030-200211220-00008. [DOI] [PubMed] [Google Scholar]
- 21.Rangaka MX, Wilkinson KA, Ronnet S, et al. The effect of HIV-1 infection on T cell based and skin test detection of tuberculosis infection. Am J Respir Crit Care Med. 2007;175:514–20. doi: 10.1164/rccm.200610-1439OC. [DOI] [PubMed] [Google Scholar]
- 22.Brock I, Ruhwald M, Lundgren B, Westh H, Mathiesen LR, Ravn P. Latent tuberculosis in HIV positive, diagnosed by the M. tuberculosis specific interferon gamma test. Respir Res. 2006;7:56. doi: 10.1186/1465-9921-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scarpellini P, Tasca S, Galli L, Beretta A, Lazzarin A, Fortis C. Selected pool of peptides from ESAT-6 and CFP-10 proteins for detection of Mycobacterium tuberculosis infection. J Clin Microb. 2004;42:3469–74. doi: 10.1128/JCM.42.8.3469-3474.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luetkemeyer AF, Charlebois ED, Flores LL, et al. Comparison of an interferon-γ release assay to tuberculin skin testing in HIV-infected individuals. Am J Respir Crit Care Med. 2007;175:737–42. doi: 10.1164/rccm.200608-1088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalvani A, Nagvenkar P, Udwadia Z, et al. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J Infect Dis. 2001;183:469–77. doi: 10.1086/318081. [DOI] [PubMed] [Google Scholar]
- 26.Vincenti D, Carrara S, De Mori P, et al. Identification of ESAT-6 Epitopes for the immunodiagnosis of active tuberculosis. Mol Med. 2003;9:105–11. [PMC free article] [PubMed] [Google Scholar]
- 27.Goletti D, Carrara S, Vincenti D, et al. Accuracy of an immune diagnostic assay based on RD1 selected epitopes for active tuberculosis in a clinical setting: a pilot study. Clin Microbiol Infect. 2006;12:544–50. doi: 10.1111/j.1469-0691.2006.01391.x. [DOI] [PubMed] [Google Scholar]
- 28.Goletti D, Parracino MP, Butera O, et al. Isoniazid prophylaxis differently modulates T cell responses to RD1-epitopes in contacts recently exposed to Mycobacterium tuberculosis: a pilot study. Respir Res. 2007;8:5. doi: 10.1186/1465-9921-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmieri F, Girardi E, Petrosillo N, et al. Guidelines for the management of tuberculosis in HIV-infected persons. Giorn It Mal Inf. 2002;8:7–22. [Google Scholar]
- 30.American Thoracic Society. Rapid diagnostic tests for tuberculosis. what is the appropriate use? Am J Respir Crit Care Med. 1997;155:1804–11. doi: 10.1164/ajrccm.155.5.9154896. [DOI] [PubMed] [Google Scholar]
- 31.Goletti D, Vincenti D, Carrara S, et al. Selected Rd1 peptides for active tuberculosis diagnosis: comparison of a gamma interferon whole blood enzyme-linked immunosorbent assay and enzyme-linked immunospot assay. Clin Diagn Lab Immunol. 2005;12:1311–6. doi: 10.1128/CDLI.12.11.1311-1316.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrara S, Vincenti D, Petrosillo N, Amicosante M, Girardi E, Goletti D. Use of a T cell-based assay for monitoring efficacy of anti-tuberculosis therapy. Clin Infect Dis. 2004;38:754–6. doi: 10.1086/381754. [DOI] [PubMed] [Google Scholar]
- 33.D'Offizi G, Montesano C, Agrati C, et al. Expansion of pre-terminally differentiated CD8 T cells in chronic HIV-positive patients presenting a rapid viral rebound during structured treatment interruption. AIDS. 2002;16:2431–8. doi: 10.1097/00002030-200212060-00008. [DOI] [PubMed] [Google Scholar]
- 34.Goletti D, Butera O, Bizzoni F, Casetti R, Girardi E, Poccia F. Region of difference 1 antigen-specific CD4− memory T cells correlate with a favorable outcome of tuberculosis. J Infect Dis. 2006;194:984–92. doi: 10.1086/507427. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann M, Reichmuth M, Fantelli K, et al. Conventional tuberculin skin testing versus T-cell-based assays in the diagnosis of latent tuberculosis infection in HIV-positive patients. AIDS. 2007;21:390–2. doi: 10.1097/QAD.0b013e328012164b. [DOI] [PubMed] [Google Scholar]
- 36.Girardi E, Antonucci G, Vanacore P, et al. for the GISTA-SIMIT Study Group. Tuberculosis in HIV-infected persons in the context of wide availability of highly active antiretroviral therapy. Eur Respir J. 2004;24:11–7. doi: 10.1183/09031936.04.00109303. [DOI] [PubMed] [Google Scholar]
- 37.Liebeschuetz S, Bamber S, Ewer K, Deeks J, Pathan AA, Lalvani A. Diagnosis of tuberculosis in South African children with a T cell-based assay: a prospective cohort study. Lancet. 2004;364:2196–203. doi: 10.1016/S0140-6736(04)17592-2. [DOI] [PubMed] [Google Scholar]
- 38.Lazarevic V, Nolt D, Flynn JL. Long-term control of Mycobacterium tuberculosis infection is mediated by dynamic immune responses. J Immunol. 2005;175:1107–17. doi: 10.4049/jimmunol.175.2.1107. [DOI] [PubMed] [Google Scholar]
- 39.Mollet L, Li TS, Samri A, et al. Dynamics of HIV-specific CD8− T lymphocytes with changes in viral load. J Immunol. 2000;165:1692–704. doi: 10.4049/jimmunol.165.3.1692. [DOI] [PubMed] [Google Scholar]
- 40.Godkin AJ, Thomas HC, Openshaw PJ. Evolution of epitope-specific memory CD4-T cells after clearance of hepatitis C virus. J Immunol. 2002;169:2210–14. doi: 10.4049/jimmunol.169.4.2210. [DOI] [PubMed] [Google Scholar]