Abstract
Cytosolic phospholipase A2 (cPLA2) group IVα is a critical enzyme involved in the liberation of arachidonic acid from cellular membranes. cPLA2−/− mice have reduced allergen-induced bronchoconstriction and bronchial hyperresponsiveness. The goal of this study was to investigate polymorphisms of the (CA)n and (T)n microsatellites and surrounding regions in the cPLA2α gene promoter. We analysed the cPLA2 promoter regions containing (CA)n and (T)n repeats in 87 patients with severe asthma and in 48 control subjects by bidirectional sequencing. Functional studies were performed utilizing reporter genes derived from subjects with varying numbers of these repeats, and on constructs with a series of deletions. We found that the (CA)n and (T)n regions are polymorphic and that constructs with CA or T repeats or CA and T repeats deleted revealed, respectively, a 41·8 ± 7%, 22·3 ± 5% and 100 ± 20% increase in reporter gene activity. A lower number of CA or T repeats caused higher cPLA2 promoter luciferase activity. The group of shorter alleles of the (CA)n microsatellite region (n = 12–18) (Pcor = 0·00006), and the group of shorter alleles of (T)n repeats region (n = 17–38) (Pcor = 0·0039) occurred significantly more often in patients with severe asthma. We also found novel SNPs in positions −292 C > G, −185 A > C, −180 T > C and −165 A > C. Two of them were associated with the severe asthma phenotype: −180T allele (Pcor = 0·03996) and −185 A allele (Pcor = 0·03966). These results demonstrate that (CA)n and (T)n repeats may have an influence on cPLA2 transcription which might play a role in severe asthma pathogenesis.
Keywords: airway inflammation, cytosolic phospholipase A2, gene expression, microsatellites, severe asthma
Introduction
The group IV cytoplasmic 85-kDa phospholipase A2α (cPLA2α) is the major intracellular form of PLA2, expressed constitutively in many tissues, which preferentially hydrolyses membrane phospholipids at the sn-2 position to release arachidonic acid and lysophospholipids. Thus, it represents the rate-limiting enzyme in eicosanoid production [1]. This arachidonic acid-selective event occurs after translocation of cPLA2 from the cytosol to the perinuclear membrane in response to cell activation [2]. The cPLA2-induced release of arachidonic acid from glycerol phospholipids provides a precursor for the synthesis of prostaglandins (PGs), leukotrienes (LTs), hydroxyeicosatetraenoic acids (HETEs) and platelet-activating factor (PAF). These eicosanoids may work as intracellular lipid second messengers or potent inflammatory mediators [3].
It has been shown that eicosanoids play a role in airway inflammation in bronchial asthma [4]. Patients with severe asthma had persistent airway inflammation despite chronic long-term treatment with anti-inflammatory agents including oral glucocorticoids [5]. In patients with severe asthma, urinary cysteinyl leukotrienes levels were increased significantly compared to moderate asthma patients, suggesting up-regulation of arachidonic acid pathways in severe asthma [6].
cPLA2α might play a role in airway inflammation, although this gene has not been well studied in asthma. Critical evidence for the role of cPLA2 in asthma and other inflammatory diseases was delivered by studies on cPLA2 knock-out mice. cPLA2−/− mice had a more rapid recovery from allergen-induced bronchoconstriction and had no airway hyperresponsiveness [7]; as well, they lacked the ability to generate leukotrienes, prostaglandins and PAF from a variety of cell types and stimuli [8–10]. cPLA2 is essential for both the immediate and the delayed phases of eicosanoid generation in mast cells [10]. Other studies have revealed that ovalbumin challenge increased the expression of cPLA2 in lung tissue [11].
The human cPLA2 promoter has been cloned [12,13]. It is TATA-box-less but does contain an initiator element at the transcription start site. It is GC poor and lacks Sp-1 consensus sites. In addition, some regulatory sequences such as Oct (octamer binding sites) were described within its promoter region, raising the possibility of its regulation at the level of transcription [12]. In addition, studies on the functional activity of the cPLA2 promoter revealed that within the 5′-flanking region there are (CA)n and (T)n simple sequence repeats which might act as potential negative regulatory elements [12,14].
Therefore, in order to understand further the transcriptional regulation of the cPLA2 gene, we performed studies on these two possible regulatory regions within the cPLA2 promoter. The aim of our study was, first, to investigate whether (CA)n and (T)n simple sequence repeats are conservative or polymorphic in the cPLA2 gene in humans in order to analyse whether the possible inhibitory effect of these regions depends on the number of (CA)n or (T)n repeats. The final goal of the study was to examine the association of polymorphisms in the analysed regions with the severe asthma phenotype.
Materials and methods
Patients
A group of 135 unrelated subjects living in the central area of Poland was studied. The study group involved 87 patients with severe bronchial asthma selected randomly from a severe asthmatic group treated in the out-patient unit of the department of immunology, rheumatology and allergy in Lodz. Asthma was defined according to criteria consistent with the Global Initiative for Asthma (GINA) 2005 report [15]. The severity of the disease was assessed according to the above guidelines. A patient was included in the severe asthma group if, before the beginning of treatment, any of the following features were observed: daily symptoms, frequent exacerbations, frequent nocturnal asthma symptoms, limitation of physical activities, forced expiratory volume in 1 second (FEV1) or peak expiratory flow (PEF) ≤ 60%, PEF or FEV1 variability > 30%. When the patient had already been on treatment, the classification of asthma severity was performed by daily medication regimen and response to treatment. The sex- and age-matched control group consisted of 48 unrelated subjects with a negative history of atopic diseases and no history of allergy and asthma in parents and siblings. Characteristics of the studied groups are presented in Table 1. The study was approved by the Ethics Committee of the Medical University of Lodz and informed consent was obtained from every subject prior to the study.
Table 1.
Characteristics of groups studied.
| Patients | Controls | |
|---|---|---|
| n | 87 | 48 |
| Median age (range, years) | 41·5 (18–75) | 37 (18–68) |
| Female/male (%) | 55/45 | 56/44 |
| Median FEV1 (range, %) | 71 (36–113) | n.a. |
| Median ICS (range, µg per day)a | 1600 (800–4800)* | 0 |
| Oral GCS (range, mg per day)b | 5 (0–25) | 0 |
| Aspirin intolerance (%) | 13·8 | 0 |
| Chronic rhinosinusitis (%) | 33 | 0 |
| Median eosinophilic count (range, cell/mm3) | 200 (0–1326) | n.a. |
Calculated as budesonide equivalent
calculated as prednisone equivalent.
Some patients received inhaled corticosteroids (ICS) in nebulization. FEV1: forced expiratory volume in 1 second; GCS: glucocorticosteroids.
Tissue culture
A549 cells, a human adenocarcinoma cell line, were obtained from the American Type Culture Collection (ATTC, Manassas, VA, USA) and cultured as described previously [16]. Briefly, cells were grown in Ham's 12K medium (Biosource, Carlsbad, CA, USA) with 10% fetal bovine serum (Biosource) and 2 mM of L-glutamine (Biosource). All experiments were performed when cells were 80–90% confluent.
Genomic DNA preparation
Genomic DNA was extracted from peripheral blood mononuclear cells of the studied subjects using Genomic Maxi AX (A&A, Gdansk, Poland) according to the manufacturer's protocol.
(CA)n and (T)n repeat regions in cPLA2 promoter genotyping
A 349-base pairs (bp) fragment containing the (T)n repeat region was obtained with the following primers: 5′-TTGTGTCTGGGGGAAAGAGATG-3′ (forward) and 5′-GAGACTATCCTGGCTAACACGGTG-3′ (reverse) (Tib MolBiol, Poznan, Poland).
A 422-bp fragment containing the (CA)n repeat region was amplified with the following primers: 5′-CAACACAGGTGTTCTGAGTCTGGAG-3′(forward) and 5′-AGAGGGAGACGCTCTCTTCTCATAG-3′(reverse) (Tib MolBiol). PCR fragments were purified with the Qiaquick Purification Kit (Qiagen, Valencia, CA) and Clean-up (A&A), according to the manufacturers' protocols. These fragments were then labelled using a fluorescently labelled dye terminators technique (Big Dye Terminator Cycle Sequencing version 1·1, Applied Biosystems, Foster City, CA, USA) with specific primers for each direction. To analyse the sequence including the (CA)n repeats the following primers were used: 5′-AGGTTATTCACTGTCTTTTC-3′ (forward), 5′-AGAGGGAGACGCTCTCTTCTCATAG-3′ (reverse) and for the sequence including the (T)n repeats: 5′-CTACCAGTGTGCTCTCTG-3′ (forward) and 5′-AGGTTATTCACTGTCTTTTC-3′ (reverse) (Tib MolBiol), as all samples were sequenced in both directions. The second purification step was performed with the Qiaquick Nucleotide Removal Kit (Qiagen) and ExTerminator (A&A). All sequencing reactions were performed in an ABI Prism 310 capillary sequencer (Applied Biosystems).
Reporter genes construction
A 2180-bp cPLA2 promoter region insert was prepared using a polymerase chain reaction (PCR) technique. DNA was obtained from peripheral blood mononuclear cells of the studied subjects. The following primers were used: the 5′ sense primer: 5′-GAGCTCTGGACAAACCAAGTGGAGAGGG-3′ and the 3′ anti-sense primer: 5′-GCTAGCAGTTCCCAGAGTTACCTGAGAATC-3′. The sense primer had a SacI restriction site added to the 5′ end and the anti-sense primer had a NheI restriction site added to the 5′ end. The product was used in comparative studies of patients with variable numbers of (CA)n and (T)n repeats. Furthermore, it was used as a basic template to generate truncation constructs by PCR. Δ(CA)n and Δ(T)n mutations of the 2180 bp cPLA2 basic promoter template were made using a PCR technique. The deletion of (T)n region was performed utilizing the following primers: the 5′ sense primers: 5′-CTCTCTGCTCCTCTCAACTTGGCTCACTGCAAGCTC-3′, 5′-CAACTTGGCTACTGCAAGCTC-3′ and the 3′ anti-sense primers: 5′-TTGCAGTGAGCCAAGTTGAGAGGAGCAGAGAGCACACTGG-3′, 5′-AGAGGAGCAGAGAGCACACTGG-3′. The deletion of (CA)n region was performed using the 5′ sense primers: 5-CAAGTAGCAATTTCAGACGCGCATATTTTCTGACTTCAAACTCC-3′, 5′-GCATATTTTCTGACTTCAAACTCCTGG-3′, 5′-CAAGTAGCAATTTCAGACGCGAAATCCACAACAGCACTCATGG-3′, GAAATCCACAACAGCACTCATGG-3′ and the 3′ anti-sense primers: 5′-TGAAGTCAGAAAATATGCGCGTCTGAAATTGCTACTTGTCC-3′, 5′-GCGTCTGAAATTGCTACTTGTCC-3′, 5′-TGAGTGCTGTTGTGGATTTCGCGTCTGAAATTGCTACTTGTCC-3′. Subsequently, the simultaneous deletion of (CA)n and (T)n regions (Δ(CA)n-(T)n constructs) was prepared utilizing both sets of these primers.
The amplified PCR products were verified by gel electrophoresis and cloned into pCRII TOPO vector (Invitrogen, Carlsbad, CA, USA). The inserts were removed from the vector by digestion with SacI and NheI and isolated by gel electrophoresis on a 2% agarose gel. Then, recovered inserts were subsequently subcloned into linearized pGL3 basic vector (Promega, Madison, WI, USA) using a T4 DNA ligase reaction (Promega). The identity and orientation of the inserts were confirmed by full bidirectional sequencing.
Transient transfection and reporter activity assessment
A549 cells were transfected with 1·5 μg of reporter gene and 0·5 μg of Renilla/pRL-cytomegalovirus (CMV) vector as a control for transfection efficiency. Cells were transfected in six-well dishes (PGS Scientific, Bethesda, MD, USA) using Lipofectamine Plus reagent (Invitrogen) for 4 h in serum-free Ham's F-12K medium (containing 2 mm l-glutamine). After transfection, medium was replaced with standard Ham's F-12K medium containing 10% fetal bovine serum and 2 mM glutamine and cells were maintained for 16 h.
Cells were washed three times in ice-cold phosphate-buffered saline and lysed using passive lysis buffer (Promega). Cell lysate was frozen at −80°C. Luciferase activity was measured using a double luciferase assay system (Promega) with a Turner TD20 luminometer (Promega).
Statistical analysis
Genotype and allele frequencies were compared between patients and controls using a χ2 Yates' test for independence and Fisher's test when appropriate. Hardy–Weinberg equilibrium was determined using the test provided by the Institute of Human Genetics, Technical University Munich, Germany (http://ihg.gsf.de/cgi-bin/hw/hwal.pl/). A two-tailed P-value < 0·05 was considered to indicate statistical significance. In functional deletion studies, data are expressed as mean ± standard error of the mean (s.e.m.) of the relative luciferase activity from at least six different experiments. Student's t-test was used to compare the difference between promoter activities. The difference was considered statistically significant when P < 0·05. To correct for incidental significance, the P-value was multiplied by the number of independent comparisons performed (Pcor). Thus, the P-values in the case–control studies were multiplied by six, because we analysed two microsatellite regions and four SNPs.
Results
Functional analysis of (CA)n and (T)n regions
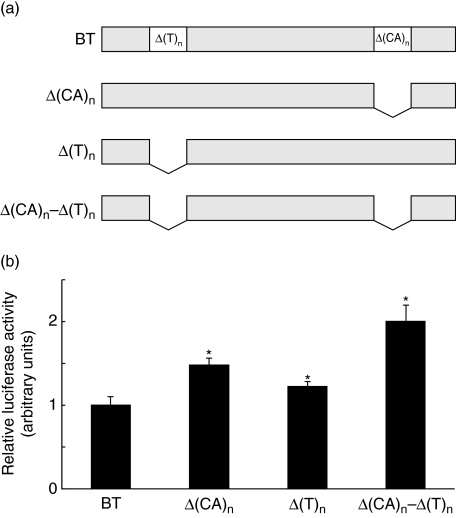
Direct sequencing of the cPLA2 promoter revealed the presence of one mononucleotide (T)n and one dinucleotide (CA)n repeats region. The dinucleotide purine–pyrymidine sequence consists mainly of (CA)n repeats and is located around the −256 to −221 bp position from the transcription start site. The mononucleotide sequence centred on the −1305 bp position consists of (T)n repeats. These regions appeared to be highly polymorphic in the studied population. To elucidate whether the variable number of (CA)n and (T)n repeats may effect cPLA2 gene expression, we performed luciferase reporter gene studies. First, we prepared a basic reporter gene construct [basic template (BT)] from the whole 2180 bp region of the cPLA2 promoter. It included the longest (T)n microsatellite repeats region and the longest (CA)n microsatellite repeats region. A series of promoter deletions was then performed employing a PCR technique: Δ(CA)n − the construct with the (CA)n repeats region deleted, Δ(T)n − the construct without the (T)n region and Δ(CA)n–Δ(T)n − the construct with both of these regions deleted. These constructs were transfected to the A549 cell line and a double luciferase assay was performed to evaluate promoter activity. Figure 1 presents the results obtained. Constructs without the (CA)n repeats region revealed a 41·8 ± 7% increase in reporter gene activity (P < 0·05). Constructs with the deletion of the (T)n repeats region revealed a 22·3 ± 5% increase in reporter gene activity (P < 0·05). Promoter constructs with the simultaneous deletion of the (CA)n and (T)n repeats regions revealed a twofold increase in reporter gene activity compared to the basic template (100% ± 20%) (P < 0·05).
Fig. 1.
Cytosolic phospholipase A2 (cPLA2) reporter gene activity. Schematic diagram of prepared constructs with the whole region (CA)n, (T)n or (CA)n and (T)n region deleted (a) and effect of these deletions on cPLA2 reporter gene activity compared to the basic template (BT) (b). (a) The 2180 bp cPLA2 promoter region insert was prepared using a polymerase chain reaction technique from DNA obtained from peripheral blood mononuclear cells of the studied subjects. It was used as a BT to generate truncation constructs by polymerase chain reaction. Δ(CA)n − construct with the (CA)n repeats region deleted, Δ(T)n − construct without the (T)n region and Δ(CA)n–Δ(T)n − construct with both of these regions deleted. (b) Constructs were transfected to the A549 cell line and a double luciferase assay was performed to evaluate promoter activity. Δ(CA)n revealed a 41·8 ± 7% increase in reporter gene activity (P < 0·05). Δ(T)n revealed a 22·3 ± 5% increase in reporter gene activity (P < 0·05). Promoter constructs Δ(CA)n–(T)n revealed a twofold increase in reporter gene activity compared to BT (100 ± 20%) (P < 0·05). Data are expressed as mean ± standard error of the mean of the relative luciferase activity from at least six different experiments. *P < 0·05 as compared to BT.
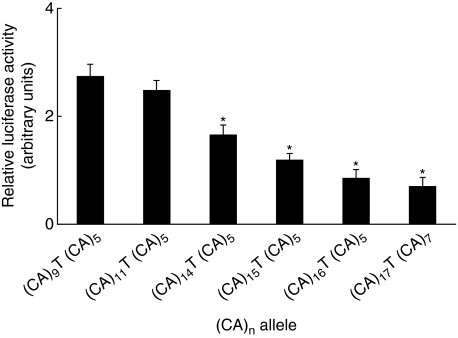
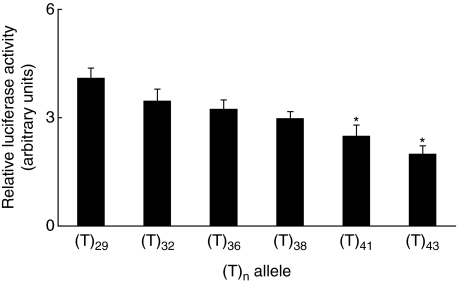
Further, considering that none of the studied patients and controls had either the whole (CA)n microsatellite region or (T)n region deleted, we performed luciferase reporter gene studies with the promoter constructs derived from polymorphic individuals. The results are shown in Figs 2 and 3. A lower number of CA or T repeats found in the cPLA2 promoter derived from those individuals was associated with a higher cPLA2 reporter gene activity, whereas a higher number of these repeats was associated with a decreased reporter gene activity. In case of the (CA)n microsatellite region, a significant decrease in relative luciferase activity appeared with n = 19 and more CA repeats in comparison to the shorter (CA)n regions. In the case of the (T)n microsatellite region, the presence of n = 41 and more T nucleotides caused a significant decrease in relative luciferase activity in comparison to the shorter (T)n repeats regions.
Fig. 2.
Cytosolic phospholipase A2 (cPLA2) reporter gene activity depends on the length of the (CA)n repeats region. cPLA2 reporter gene activity in promoter constructs derived from individuals polymorphic in the number of (CA)n repeats. The cPLA2 promoter region insert was prepared using a polymerase chain reaction technique from DNA obtained from peripheral blood mononuclear cells of the studied subjects. A lower number of CA repeats resulted in a greater cPLA2 reporter gene activity, whereas a higher number of these repeats decreased reporter gene activity. *P < 0·05 compared to (CA)9T (CA)5. Data are expressed as mean ± standard error of the mean of the relative luciferase activity (n = 5–6).
Fig. 3.
Cytosolic phospholipase A2 (cPLA2) reporter gene activity depends on the length of the (T)n repeats region. cPLA2 reporter gene activity in promoter constructs derived from individuals polymorphic in the number of (T)n repeats. The cPLA2 promoter region insert was prepared using a polymerase chain reaction technique from DNA obtained from peripheral blood mononuclear cells of the studied subjects. Constructs with shorter forms of the (T)n repeats revealed a significant increase in reporter gene activity compared to longer forms of the (T)n repeats (n = 41 and more). *P < 0·05 compared to (T)29. Data are expressed as mean ± standard error of the mean of the relative luciferase activity (n = 4–5).
Analysis of the (CA)n microsatellite region
Analysis of the number of CA repeats
The (CA)n repeats region located around −256 to −221 bp in the cPLA2 promoter appears to be highly polymorphic, although we found only the homozygotic variants of this fragment in the studied populations. In the patient group, the structure of this sequence varied from (CA)12T to (CA)17T(CA)7, varying in the number of (CA)n repeats. In the control group, this sequence differed from (CA)13T(CA)5 to (CA)13T(CA)10. In relation to our functional gene expression data, within this microsatellite region we studied two groups of alleles: the shorter (n = 12–18) and the longer (n = 19–24) ones. The comparison between the patients and controls revealed that indeed the group of shorter alleles of the (CA)n microsatellite region (n = 12–18) occurred significantly more often in patients with severe asthma (96·65%) than in the control group (67·67%) (Pcor = 0·00006, OR = 14·0). To correct for incidental significance, here the P-value was multiplied by six because we analysed independently two microsatellite regions and four SNPs. Thus, the structure of the (CA)n microsatellite region in patients with severe asthma seemed to be significantly shorter than in the control group.
Novel SNPs around the (CA)n microsatellite region of the cPLA2 promoter
The direct bidirectional sequencing analysis of the whole −351 to −74 bp fragment of the cPLA2 promoter revealed that not only the (CA)n region was polymorphic in the number of CA repeats but there were also some novel single nucleotide polymorphisms (SNPs) in the close neighbourhood of this region. We found novel SNPs in positions: −292 C > G, −185 A > C, −180 T > C and −165 A > C. Frequencies of these alleles and genotypes are shown in Table 2. To determine whether there is an association between these novel SNPs and bronchial severe asthma we analysed whether each of the genotypes at each locus was consistent with Hardy–Weinberg equilibrium and then we performed association studies. We found that −180T allele [Pcor = 0·03996, OR = 3·917, CI = (1·385 11·079)], as well as the −185 A allele [Pcor = 0·03966, OR = 3·675, CI = (1·374 9·826)] were present significantly more often in patients with severe asthma. To correct for incidental significance, here the P-values were multiplied by six. We found no significant difference in distribution of the other newly described polymorphisms between the patient and the control group.
Table 2.
Genotype frequencies of cytosolic phospholipase A2 (cPLA2) promoter single nucleotide polymorphisms (SNPs) in groups of studied patients and controls.
| Pcor for | |||||
|---|---|---|---|---|---|
| SNP and sample | Allele and frequency | Genotype and frequency | Allele test | Genotype test | |
| −292 C > G | C | G | CC/CG/GG | n.s. | n.s. |
| Patients | 0·71 | 0·29 | 0·26/0·74/0·00 | ||
| Controls | 0·72 | 0·28 | 0·13/0·87/0·00 | ||
| −185 A > C | A | C | AA/AC/CC | 0·03966 | 0·0201 |
| Patients | 0·93 | 0·07 | 0·86/0·14/0·00 | ||
| Controls | 0·78 | 0·22 | 0·57/0·43/0·00 | ||
| −180 T > C | T | C | TT/TC/CC | 0·03996 | 0·02214 |
| Patients | 0·94 | 0·06 | 0·88/0·12/0·00 | ||
| Controls | 0·80 | 0·20 | 0·60/0·40/0·00 | ||
| −165 A > C | A | C | AA/AC/CC | n.s. | n.s. |
| Patients | 0·63 | 0·37 | 0·42/0·58/0·00 | ||
| Controls | 0·57 | 0·43 | 0·43/0·57/0·00 | ||
Analysis of the (T)n microsatellite region
Analysis of the number of T repeats
Amplification and bidirectional sequencing of the amplified 349 bp fragment revealed that the (T)n region is highly polymorphic, but also in a homozygotic manner, in the studied populations. We found that the number of (T)n repeats varied both in the patient and the control group from 17 to 46. Here, also in relation to our functional data, two groups of alleles were isolated. The comparison between patients and controls revealed that the group of shorter alleles of (T)n repeats region (n = 17–38) occurred significantly more often in the patient group than in the control group (77·1% in patients versus 44·9% in controls; Pcor = 0·0039, OR = 4·14). The P-value here was also multiplied by six.
Discussion
The 85-kDa cPLA2α is a key enzyme in mediating agonist-induced arachidonic acid release and regulating transcription of some inflammatory genes such as interleukin-8 (IL-8) and cyclooxygenase-2 (COX-2) in human lung cells [16]. Together with the current effectiveness of anti-leukotriene therapy in severe asthma and of the cPLA2α inhibitor (Efipladib®) in a Phase II clinical trial (Wyeth Annual Review 2005, http://ccbn.mobular.net/ccbn/7/1372/1467/index.html), these data suggest that the cPLA2 and cPLA2-dependent arachidonate metabolism might play an important role in inflammation.
The studies performed by Wu et al. [12] and Dolan-O'Keefe et al. [14] revealed that the CA repeat region in cPLA2 may have a suppressor effect on cPLA2 gene transcription. Deletion of this region from the promoter resulted in a 20–30% [12] or a 40–50% [14] increase in cPLA2 promoter activity. The results of our functional studies with the promoters of severe asthmatic patients, with a variable number of CA repeats, provide new evidence for a regulatory function of the CA repeats. We showed that even the difference of five dinucleotide CA repeats may change luciferase activity significantly. More (CA)n repeats in the cPLA2 promoter resulted in less relative luciferase activity. The deletion of the whole CA repeats region in our study caused a 41·8 ± 7% increase in reporter gene activity, which confirms previous results. There is also some evidence that not only cPLA2 gene transcription might be regulated by a different number of (CA)n repeats. The first intron of the interferon-γ (IFN-γ) gene contains a CA microsatellite repeat that is highly polymorphic, with up to six alleles [17]. Allele 2 with 12 CA repeats was shown to be associated with a high level of IFN-γ production in vitro [17]. In the oestrogen receptor beta (ESRB) gene, the number of (CA)n repeats was associated with androgen and sex steroid-hormone binding globulin (SHBG) [18]. When we compared the results in patients with severe asthma to the control group, we found a significant difference in the number of (CA)n repeats in the cPLA2 gene promoter between these groups. A lower number of (CA)n repeats occurred significantly more often in patients with severe asthma than in the control group.
Moreover, the whole region enclosing the (CA)n repeats appeared to be highly polymorphic in our studied populations as we also found novel single nucleotide polymorphisms (SNPs). Two of them −185 A > C and −180 T > C appeared to be associated with severe asthma. The −180T as well as the −185 A allele were present significantly more often in patients with severe asthma. The analysis of this region with a transcription factor database suggests that a potential octamer binding site – ATTTACAT might exist in the position of −181 bp to −174 bp. Transcription factors which bind specifically to these motifs are called octamer binding proteins (Oct). The family of these transcription factors consists of proteins such as Oct-1, Oct-2 and Oct-4, which are expressed ubiquitously and involved in the transcription regulation of numerous genes [19,20]. Oct proteins can therefore act as a repressor or an activator of transcription. Although there is no evidence for the influence of Oct proteins on cPLA2 transcription, authors describing the cPLA2 5′-flanking region have reported two octamer binding sites within the cPLA2 promoter [12,21,22]. Therefore, our finding that the existence of polymorphisms within this sequence may be associated with the severe asthma phenotype might support the hypothesis of the importance of Oct transcription factors in the regulation of cPLA2 expression.
Deletion studies performed by Dolan-O'Keefe et al. [14] revealed that the −2271 bp promoter construct is characterized by slightly lower luciferase activity in comparison to the −1294 bp construct, which lacks the (T)n region. However, their results did not reach statistical significance. Here, we showed that constructs with (T)n repeats deleted revealed a 22·3 ± 5% increase in reporter gene activity. Moreover we demonstrated, similar to (CA)n repeats, that cPLA2 reporter gene activity depends on the number of (T)n repeats. Promoter constructs with shorter forms of the (T)n repeats revealed a significant increase in reporter gene activity in comparison to longer forms of the (T)n repeats. This observation indicates that the mononucleotide (T)n repeats in the cPLA2 promoter region might exert some inhibitory effect on the transcription of the cPLA2 gene. Further elucidation of this observation revealed that not only was the group of (CA)n shorter alleles associated with the severe asthma phenotype, but also the group of shorter alleles of (T)n repeats region (n = 17–38) occurs significantly more often in patients.
We are aware that the groups enclosed to our study are relatively small and could lack power to measure accurately an association. Moreover, the asthma severity criteria used in GINA 2005 [15] were sometimes difficult to establish. When the patient had already been on treatment, the classification of asthma severity was performed by a daily medication regimen and response to treatment. It was also consistent with GINA 2005 [15] recommendations. However, there was a potential for misclassification and bias in our studied populations. This might be the reason why, in the latest guidelines, other issues such as the level of asthma control are introduced. Furthermore, an intriguing issue that should be investigated further is the lack of heterozygosity of (T)n and (CA)n repeats regions in our results, which was confirmed by bidirectional sequencing. Nevertheless, the results of our case–control study, together with the higher cPLA2 transcription in these patients, might suggest an important role of cPLA2 in asthma pathogenesis or severity. However, studies involving larger populations and perhaps different geographic and ethnic groups may be needed to address this phenomenon.
In conclusion, there are two important observations from this study. First, the (T)n repeats and (CA)n repeat regions may exert an effect on the level of cPLA2 gene transcription. Secondly, there might be an association of these regions, either by SNPs around the (CA)n repeats region or by different-length variants of the (CA)n and the (T)n repeats regions with severe asthma. Nevertheless, further studies are needed to elucidate the role of the described polymorphisms in the cPLA2 promoter in the development of different asthmatic phenotypes.
Acknowledgments
The authors thank Marzena Kraska for the preparation of genomic DNA, Anna Lewandowicz for technical assistance in sequence analysis and Tomasz Adamusiak for help with statistical analysis. This work was supported by the Medical University of Lodz grants 502-11-248 and 503-00-261 and Polish Government grants 3P04A 02124 and N401 191 32/4009.
References
- 1.Dennis EA. Phospholipase A2 in eicosanoid generation. Am J Respir Crit Care Med. 2000;161:S32–5. doi: 10.1164/ajrccm.161.supplement_1.ltta-7. [DOI] [PubMed] [Google Scholar]
- 2.Schievella AR, Regier MK, Smith WL, Lin LL. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J Biol Chem. 1995;270:30749–54. doi: 10.1074/jbc.270.51.30749. [DOI] [PubMed] [Google Scholar]
- 3.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–6. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 4.Park GY, Christman JW. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol. 2006;290:L797–805. doi: 10.1152/ajplung.00513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vachier I, Kumlin M, Dahlen SE, Bousquet J, Godard P, Chanez P. High levels of urinary leukotriene E4 excretion in steroid treated patients with severe asthma. Respir Med. 2003;97:1225–9. doi: 10.1016/s0954-6111(03)00253-1. [DOI] [PubMed] [Google Scholar]
- 6.Green SA, Malice MP, Tanaka W, Tozzi CA, Reiss TF. Increase in urinary leukotriene LTE4 levels in acute asthma: correlation with airflow limitation. Thorax. 2004;59:100–4. doi: 10.1136/thorax.2003.006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sapirstein A, Bonventre JV. Specific physiological roles of cytosolic phospholipase A2 as defined by gene knockouts. Biochim Biophys Acta. 2000;1488:139–48. doi: 10.1016/s1388-1981(00)00116-5. [DOI] [PubMed] [Google Scholar]
- 8.Uozumi N, Kume K, Nagase T, et al. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 1997;390:618–22. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- 9.Bonventre JV, Huang Z, Taheri MR, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–5. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 10.Nakatani N, Uozumi N, Kume K, Murakami M, Kudo I, Shimizu T. Role of cytosolic phospholipase A2 in the production of lipid mediators and histamine release in mouse bone-marrow-derived mast cells. Biochem J. 2000;352:311–17. [PMC free article] [PubMed] [Google Scholar]
- 11.Chung YW, Oh HY, Kim JY, Kim JH, Kim IY. Allergen-induced proteolytic cleavage of annexin-1 and activation of cytosolic phospholipase A2 in the lungs of a mouse model of asthma. Proteomics. 2004;4:3328–34. doi: 10.1002/pmic.200400895. [DOI] [PubMed] [Google Scholar]
- 12.Wu T, Ikezono T, Angus CW, Shelhamer JH. Characterization of the promoter for the human 85 kDa cytosolic phospholipase A2 gene. Nucl Acids Res. 1994;22:5093–8. doi: 10.1093/nar/22.23.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tay A, Maxwell P, Li Z, Goldberg H, Skorecki K. Isolation of promoter for cytosolic phospholipase A2 (cPLA2) Biochim Biophys Acta. 1994;1217:345–7. doi: 10.1016/0167-4781(94)90299-2. [DOI] [PubMed] [Google Scholar]
- 14.Dolan-O'Keefe M, Chow V, Monnier J, Visner GA, Nick HS. Transcriptional regulation and structural organization of the human cytosolic phospholipase A2 gene. Am J Physiol Lung Cell Mol Physiol. 2000;278:L649–57. doi: 10.1152/ajplung.2000.278.4.L649. [DOI] [PubMed] [Google Scholar]
- 15.Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) Available at: http://www.ginasthma.org[accessed on 1 December 2006]
- 16.Pawliczak R, Logun C, Madara P, et al. Cytosolic phospholipase A2 Group IValpha but not secreted phospholipase A2 Group IIA, V, or X induces interleukin-8 and cyclooxygenase-2 gene and protein expression through peroxisome proliferator-activated receptors gamma 1 and 2 in human lung cells. J Biol Chem. 2004;279:48550–61. doi: 10.1074/jbc.M408926200. [DOI] [PubMed] [Google Scholar]
- 17.Pravica V, Asderakis A, Perrey C, Hajeer A, Sinnott PJ, Hutchinson IV. In vitro production of IFN-γ correlates with CA repeat polymorphism in the human IFN-gamma gene. Eur J Immunogenet. 1999;26:1–3. doi: 10.1046/j.1365-2370.1999.00122.x. [DOI] [PubMed] [Google Scholar]
- 18.Westberg L, Baghaei F, Rosmond R, et al. Polymorphisms of the androgen receptor gene and the estrogen receptor beta gene are associated with androgen levels in women. J Clin Endocrinol Metab. 2001;86:2562–8. doi: 10.1210/jcem.86.6.7614. [DOI] [PubMed] [Google Scholar]
- 19.Franke S, Scholz G, Scheidereit C. Identification of novel ubiquitous and cell type-specific factors that specifically recognize immunoglobulin heavy chain and kappa light chain promoters. J Biol Chem. 1994;269:20075–82. [PubMed] [Google Scholar]
- 20.Wu GD, Lai EJ, Huang N, Wen X. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter. The role of Oct-1 as a transcriptional repressor. J Biol Chem. 1997;272:2396–403. [PubMed] [Google Scholar]
- 21.Morri H, Ozaki M, Watanabe Y. 5′-flanking region surrounding a human cytosolic phospholipase A2 gene. Biochem Biophys Res Commun. 1994;205:6–11. doi: 10.1006/bbrc.1994.2621. [DOI] [PubMed] [Google Scholar]
- 22.Cowan MJ, Yao XL, Pawliczak R, et al. The role of TFIID, the initiator element and a novel 5′-TFIID binding site in the transcriptional control of the TATA-less human cytosolic phospholipase A2α promoter. Biochim Biophys Acta. 2004;1680:145–57. doi: 10.1016/j.bbaexp.2004.09.006. [DOI] [PubMed] [Google Scholar]