Abstract
In a series of 84 head and neck patients, a statistically significant correlation was observed between high serum soluble interleukin (IL)-2 receptor alpha (sIL-2Rα) (P = 0·034) and metalloproteinase-9 (MMP-9) concentrations (P = 0·036) at diagnosis and a shorter survival of these patients. As MMP-9 has been shown to mediate cleavage of IL-2Rα (CD25) by preactivated T cells, we looked for a relationship between MMP-9 expression and soluble IL-2Rα serum concentrations in these cancer patients. We did not find any correlation between intratumoral expression of MMP-9 or serum MMP-9 concentrations and serum sIL-2Rα levels. These results led us to reassess the role of MMP-9 in the release of sIL-2Rα. Treatment of Kit225 leukaemic cells with recombinant MMP-9 slightly decreased membrane CD25 expression and was associated with an increased concentration of sIL-2Rα in the supernatants. However, using a selective inhibitor of MMP-9 we did not succeed in specifically inhibiting the release of sIL-2Rα by the Kit225 cell line or by phytohaemagglutinin (PHA)-activated peripheral blood mononuclear cells. In addition, in a preclinical mouse model, basal serum sIL-2Rα concentrations and sIL-2Rα production by activated cells were not altered in MMP-9-deficient mice compared to wild-type mice. Interestingly, a broad spectrum metalloproteinase inhibitor inhibited the release of sIL-2Rα by PHA-activated peripheral blood mononuclear cells, suggesting that in contrast with current views concerning the major role of MMP-9 in the cleavage of membrane IL-2Rα, other proteases are involved in the shedding of sIL-2Rα. MMP-9 and sIL-2Rα appear therefore as independent prognostic markers in head and neck cancers.
Keywords: cytokine, head and neck cancer, MMP-9, prognostic marker, soluble IL-2 receptor
Introduction
Metalloproteinases (MMPs) are cysteine proteases with zinc-ion-dependent proteolytic activity [1,2]. To date, more than 20 MMPs have been identified in mammals and they are currently grouped according to structural features. The majority of MMPs are secreted into the extracellular matrix (ECM) and a subset are membrane-bound by virtue of a transmembrane or phosphatidyl–inositol-linked domain [3]. MMPs can degrade all components of the ECM, but they also cleave and regulate the activity of growth factors, chemokines and cytokines as well as cell surface adhesion receptors and proteoglycans involved in intercellular communication and tumour cell migration. These properties explain their direct role in tumour progression and metastasis.
MMP-9, a member of the MMP family also known as gelatinase B or 92 kDa type IV collagenase, is the major structural component of basement membrane. MMP-9 is released from cells as a 92-kDa latent pro-enzyme that is cleaved proteolytically by exogenous proteases to generate the active 84 kDa form [4,5]. MMP-9 digests type IV collagen (the key component of basement membrane) and promotes tumour angiogenesis in various preclinical models [6]. In most cases, MMP-9 behaves as a tumour growth-promoting factor. Transfection of tumorigenic rat cell line with an MMP-9 expression vector increased the metastatic dissemination of these cells [7]. In glioma, transfection of anti-sense MMP-9 inhibited their ability to form tumours in nude mice [8].
Cancer cells were less able to colonize the lungs of MMP-9-deficient mice than the lungs of wild-type mice, and MMP-9 null mice developed fewer cancers than wild-type mice [9,10].
In two transgenic models of tumour progression, the K14-HPV16 skin cancer model [11] and the RIPI-Tag2 insulinoma model [12], cancer cell proliferation was decreased in tumours from MMP-9-deficient mice compared to wild-type mice, indicating that MMP-9 generated a growth-promoting signal.
The high-affinity interleukin (IL)-2 receptor (IL-2R) is a complex of three associated polypeptide chains designated IL-2Rα (CD25), IL-2Rβ (CD122) and IL-2Rγ (CD132). Only the α and β chains can bind to IL-2. Both β and γ chains are required for transduction of proliferative signal. The γ chain is shared by other receptors belonging to this family (IL-4R, IL-7R, IL-9R, IL-15R, IL-21R, and so on). The heterodimeric IL-2Rβγ complex is expressed constitutively on resting T cells. Once activated by IL-2, IL-15 or other stimuli, T cells synthesize IL-2Rα, but up-regulation of IL-2Rα after activation is a feature of many cell types (for example, natural killer (NK) cells, B cells, monocytes).
Soluble IL-2Rα is produced by a membrane proteolytic mechanism [13]. Cleavage of IL-2Rα is observed in parallel with activation of T cells, and in vitro activated human T cells release soluble IL-2Rα (sIL-2Rα) in culture supernatants.
Significantly elevated serum levels of sIL-2Rα and MMP-9 have been found in patients with renal cell carcinoma, B chronic lymphocytic leukaemia and non-small cell lung carcinoma compared to healthy controls [14–19].
High serum concentrations of sIL-2Rα or MMP-9 are correlated with adverse prognosis in patients with colorectal cancer, breast cancer and melanoma [20–27].
With respect to head and neck cancer patients, an elevated serum concentrations of sIL-2Rα and MMP-9 were found prior to any therapy compared to healthy controls [28–31]. We have reported previously a correlation between high serum sIL-2Rα levels at the time of diagnosis and a high risk of local or regional recurrence and poor survival in patients with head and neck squamous cell carcinoma (HNSCC) [32,33]. An overexpression of MMP-9 was observed in head and neck cancer tissues, with a frequency ranging from 20% to 52% of cases [34–36] and different groups found relationships between positive MMP-9 immunostaining or increased intratumoral MMP-9 activity in head and neck cancers and poor survival rates [35–38].
In this study, we addressed the prognostic value of serum levels of MMP-9 in head and neck cancer patients and showed a significant correlation between high MMP-9 serum concentrations and a shorter survival of these patients. Because it has been shown that MMP-9 mediated the cleavage of IL-2Rα by preactivated T cells [39], we also looked for a relationship between these two markers and reviewed the role of MMP-9 in the cleavage of membrane IL-2Rα.
Patients and methods
Patients
Eighty-four newly diagnosed untreated patients with primary histologically proven HNSCC were included in this prospective study. Patient characteristics are presented in Table 1.
Table 1.
Patient characteristics.
| Characteristics | No. patients |
|---|---|
| Sex | |
| Male | 76 |
| Female | 8 |
| Primary tumour site | |
| Oral cavity | 14 |
| Oropharynx | 20 |
| Hypopharynx | 15 |
| Epilarynx | 15 |
| Larynx | 19 |
| n.d. | 1 |
| Tumour (T) staging | |
| T1 | 9 |
| T2 | 28 |
| T3 | 24 |
| T4 | 23 |
| Lymph node involvement | |
| N0 | 40 |
| N1 | 12 |
| N2 | 29 |
| N3 | 3 |
| M0 | 82 |
| M1 | 2 |
n.d.: Not determined because of diffuse carcinose.
Each patient's disease was staged according to the fifth edition of the International Union Against Cancer/American Joint Committee on Cancer (UICC/AJCC) system for head and neck cancer [40].
Treatment modalities consisted of surgery, alone or combined with radiotherapy and chemotherapy. Fifty-eight patients (69%) received induction chemotherapy before surgery.
This study was conducted in accordance with French law and after approval by the local ethics committee.
Cells
The human T cell chronic lymphocytic leukaemia-derived, IL-2-dependent, Kit225 cell line was provided by Dr J. Bertoglio (Chatenay Malabry, France) and was established initially as described [41]. Cells were maintained in RPMI-1640 culture medium containing 2 mM l-glutamine, 0·1 mg/ml streptomycin, 100 U/ml penicillin, 2% sodium pyruvate, 10% fetal calf serum (FCS), supplemented with 0·5 nM recombinant human IL-2 (Proleukin; Chiron, Emeryville, CA, USA).
Mice
MMP-9-deficient mice on a C57BL/6 background and control littermates were provided by B. Lelongt (Hôpital Tenon, Paris, France) and were generated as described previously [42,43].
Flow cytometry
Kit225 cells were stained with directly labelled anti-CD25 monoclonal antibody (mAb) (Becton Dickinson, Mountain View, CA, USA) for 30 min at 4°C in the dark and then resuspended in 1% paraformaldehyde phosphate-buffered saline (PBS).
Isotype-matched control antibodies were included in each experiment. The cells were analysed with fluorescence activated cell sorter (FACScalibur) (Becton Dickinson).
Assays
Plasma was obtained from all patients at the time of diagnosis and stored at −80°C prior to assay. No patients received chemotherapy or anti-inflammatory drugs during the 2 weeks preceding blood sampling. Human MMP-9, sIL-2Rα and interferon (IFN)-γ concentrations were determined using enzyme-linked immunosorbent assay (ELISA) kits purchased from R&D Systems Europe (Lille, France), Beckman Coulter-Immunotech (Marseille, France) and Diaclone (Besançon, France), respectively.
As reported previously [32,33], the sIL-2Rα assay measured both free sIL-2Rα and sIL-2Rα complexed to IL-2. A cut-off value of 70 pm corresponding to limits outside the 95-percentile ranges in healthy subjects was used to divide patients into two groups with high or low values.
MMP-9 ELISA allows the determination of both human active and pro-matrix metalloproteinase 9
A sandwich ELISA was developed to measure mouse sIL-2Rα based on previous publications [44,45]. Briefly, 96-well plates were coated with 10 µg/ml of rat IgG1 mAb anti-mouse CD25 (clone PC61·5; eBioscience, San Diego, CA, USA) diluted in carbonate buffer (pH = 9·6). Plates were incubated overnight at 4°C, and washed twice with PBS containing 0·05% Tween 20.
After saturation of wells with PBS 1% bovine serum albumin for 2 h at room temperature, 50 µl of serum were added and incubated for 2 h at 37°C. After washings, biotinylated IgM anti-mouse CD25 (5 µg/ml) (clone 7D4; BD Biosciences, Franklin Lakes, NJ, USA) was added to each well and incubated for 2 h at 37°C. After washings, 100 µl/well streptavidin peroxidase (0·15 µg/ml) (Diaclone) diluted in 0·1% PBS-Tween 20 and 1% bovine serum albumin was added and the plate was incubated for 20 min at room temperature. Then, 100 µl of ready-to-use peroxidase substrate (TMB) (Diaclone) was added and incubated for 15 min in the dark. Optical density was read at 450 nm.
The standard source of murine soluble IL-2Rα used was derived from supernatants of murine splenocytes stimulated with concanavalin A (ConA) for 72 h. An arbitrary value of 1000 was defined for the concentrations of this supernatant. A serial 1 : 2 dilution was performed to generate a standard curve.
Effect of recombinant MMP-9 on the cleavage of IL-2Rα and the release of sIL-2Rα
The Kit225 cell line was treated with 1 µg/ml of recombinant active MMP-9 (R&D) or MMP-3 (Calbiochem VWR International, Fontenay-sous-Bois, France). Supernatants and cells were collected 6 h after addition of the enzymes.
Effect of MMP-9 inhibitor on the release of sIL-2Rα
The Kit225 cell line was activated or not with IL-2 (0·5 nM) and treated or not with various concentrations of MMP-9 inhibitor I. Supernatants were collected 24 h after seeding.
Peripheral blood mononuclear cells (PBMC) were activated with 10 µg/ml of phytohaemagglutinin (PHA). Six hours after the start of activation, MMP-9 inhibitor I (Calbiochem VWR International) or TAPI-0, a broad-spectrum metalloproteinase inhibitor (Calbiochem), were added at various concentrations. Supernatants were collected 24 or 72 h after stimulation.
Release of sIL-2Rα by activated mouse splenocytes
Splenocytes derived from wild-type C57BL/6 or MMP-9-deficient mice were stimulated with ConA (10 µg/ml) (Sigma-Aldrich, Saint Quentin Fallavier, France). Supernatants were collected 72 h after activation, and mouse sIL-2Rα was assayed by ELISA.
Statistical analysis
Serum sIL-2Rα concentrations were expressed as mean ± standard deviation (s.d.) in pM units or divided into two groups (high and low) based on a cut-off value of 70 pM corresponding to limits outside the 95-percentile ranges in healthy subjects as previously described [33]. As MMP-9 concentrations were distributed exponentially, they were expressed as geometric means and their associated confidence intervals in pg/ml or divided into two groups defined by a cut-off concentration of 95 ng/ml, corresponding to the median MMP-9 concentration.
Survival functions were estimated by the Kaplan–Meier method and compared between the low and high sIL-2Rα and MMP-9 level groups by the log-rank test. To confirm these analyses performed on derived categorical variables, a Cox's proportional hazard model was applied on sIL-2Rα and MMP-9 concentrations (results not shown when similar). The same semiparametric model was performed for multivariate analysis. Overall survival was defined as the time from initial diagnosis until death or until last follow-up (right censored data). The median follow-up for the overall population was 20 months.
Correlations between sIL-2Rα and MMP-9 concentrations were expressed as scatter plots and Pearson's correlation coefficients. Student's t-test was used for comparison of MMP-9 expression and serum sIL-2Rα concentrations.
All analyses were performed with SAS statistical software (version 8·2). A P-value less than 0·05 was considered to be significant. All confidence intervals were calculated using the normal approximation.
The Mann–Whitney U-test was also used to assess whether two samples of observations come from the same distribution.
Results
High serum levels of MMP-9 are associated with poor survival in head and neck cancer patients
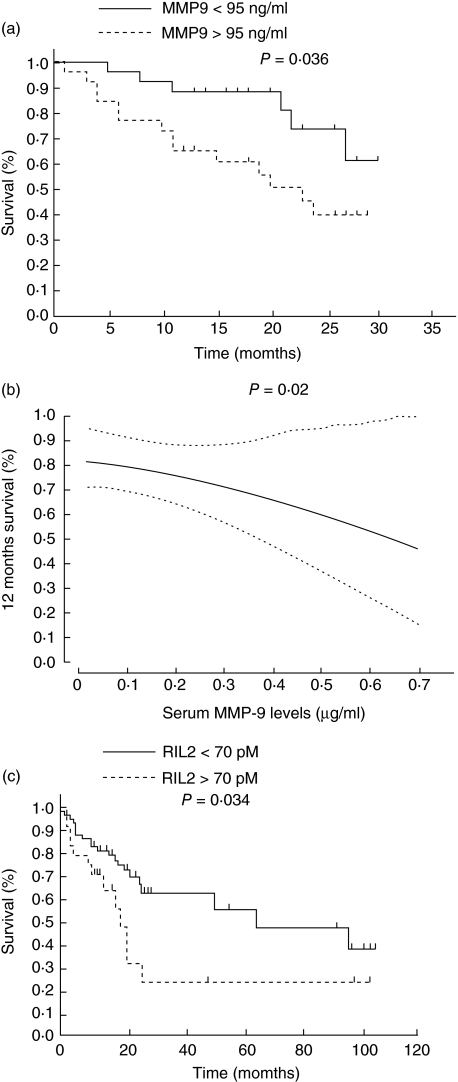
High serum MMP-9 concentrations at diagnosis in head and neck cancer patients were associated statistically with shorter survival (Fig. 1a,b) Overall survival at 24 months was 73·7% (95% CI: 52·4–95) in patients with low serum MMP-9 levels. Survival decreased to 40% (95% CI: 19·2–60·9) for patients with high serum MMP-9 concentrations (P = 0·036) (Fig. 1a,b).
Fig. 1.
Survival of patients with head and neck squamous cell carcinoma in relation to serum metalloproteinase-9 (MMP-9) (a,b) and soluble interleukin (IL)-2 receptor alpha (sIL-2Rα) (c) and levels. (b) Dashed line indicates the upper or lower 95% CI (confidence interval).
Adjusted on tumour classification (T), lymph node involvement (N) and presence or absence of chemotherapy, subjects with high serum MMP-9 levels had a 2·2-fold higher risk of death (95% CI: 0·81–6·01) than subjects with low serum MMP-9 levels. This is the first study demonstrating a prognostic value of serum MMP-9 levels in head and neck cancers.
As expected, high serum sIL-2Rα concentrations at diagnosis were also associated with shorter survival (Fig. 1c), and when adjusted on tumour classification (T) and lymph node involvement (N), subjects with high serum sIL-2Ra levels had a 2·1-fold higher risk of death (95% CI: 1·01–4·38) than subjects with low serum sIL-2Rα levels. Although not statistically significant, a trend towards longer survival (P = 0·06) and better locoregional control (P = 0·06) was observed in patients with low sIL2Rα serum levels in a multivariate analysis, including tumour note metastasis (TNA). No similar association between MMP-9 concentrations and clinical outcome was found in this study (data not shown).
As these two parameters overlapped as prognostic markers in head and neck cancer patients and it has been shown that MMP-9 mediated the cleavage of IL-2Rα, we looked for a relationship between these two markers.
Absence of relationship between serum levels of soluble IL-2Rα and MMP-9
As observed in the scatter plot, no significant linear relationship was observed between serum sIL-2Rα and MMP-9 concentrations (P = 0·35) (Fig. 2). When sIL-2Rα was selected as a discrete variable, MMP-9 concentrations were not significantly different between the two groups with high or low sIL-2Rα concentrations, as the geometric mean serum MMP-9 concentrations in the group of patients with high serum sIL-2Rα was 121·6 pg/ml (95% CI: 76–128), while this value decreased to 98·7 pg/ml (95% CI: 76·1–194·3) in the group with low sIL-2Rα values (P = 0·4). We also found no correlation between serum sIL-2Rα concentrations and MMP-9 expression in tumour (data not shown).
Fig. 2.
Absence of relationship between serum soluble interleukin (IL)-2 receptor alpha (sIL-2Rα) and metalloproteinase-9 (MMP-9) levels in head and neck cancer patients. sIL-2Rα and MMP-9 were measured by enzyme-linked immunosorbent assay. Pearson's correlation test was used to analyse the correlation between serum MMP-9 and sIL-2Rα concentrations.
Effect of MMP-9 on cleavage of IL-2Rα
These results led us to reassess the role of MMP-9 in the cleavage of membrane CD25. The human T cell chronic lymphocytic leukaemia-derived, IL-2-dependent Kit225 cell line spontaneously expresses and releases CD25.
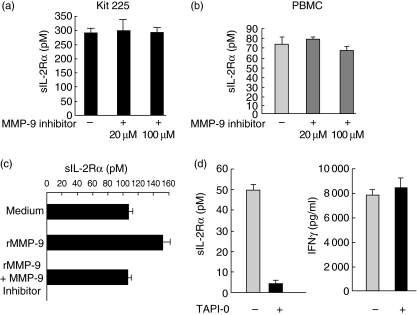
Treatment of the Kit225 cell line with recombinant MMP-9 slightly decreased membrane CD25 expression (Fig. 3). This effect was not observed when cells were treated with recombinant MMP-3 (Fig. 3). This down-regulation of membrane CD25 in the presence of MMP-9 was associated with an increased concentration of sIL-2Rα in the supernatants compared to untreated or MMP-3-treated cells (Fig. 3). This activity was observed only at high MMP-9 concentrations (1 µg/ml) and the activity was not increased at 3 µg (data not shown).
Fig. 3.
Effect of metalloproteinase-9 (MMP-9) on cleavage of interleukin-2 receptor alpha (IL-2Rα). The Kit225 cell line was incubated for 6 h with medium, MMP-9 (1 µg/ml) or MMP-3 (1 µg/ml). Cells were then stained with CD25 monoclonal antibody. Isotype control-matched antibodies were included in each experiment. (b) Soluble interleukin (IL)-2 receptor alpha (sIL-2Rα) concentrations were measured in the supernatants by enzyme-linked immunosorbent assay. *The Mann–Whitney U-test was used for statistical analysis.
Inhibition of MMP-9 did not block release of soluble IL-2 receptor
Using a selective inhibitor of MMP-9, we were unable to inhibit specifically the release of soluble IL-2 receptor in two different models.
Incubation of the Kit225 cell line with high concentrations of MMP-9 inhibitor did not influence the basal release of sIL-2Rα in the supernatants (Fig. 4a). Similar negative results were observed when cells were stimulated with IL-2 during the experiments (data not shown). In line with these results, no change in sIL-2Rα release was observed when PBMC were stimulated with PHA in the presence or absence of the MMP-9 inhibitor (Fig. 4b).
Fig. 4.
Metalloproteinase-9 (MMP-9)-specific inhibitor did not influence the release of soluble interleukin (IL)-2 receptor alpha (sIL-2Rα). (a) The Kit225 cell lines or (b) 6 h PHA stimulated peripheral blood mononuclear cells (PBMC) were incubated with a specific MMP-9 inhibitor at various concentrations. Supernatants were collected after 24 h and sIL-2Rα was assayed by enzyme-linked immunosorbent assay. (c) The Kit225 cell line was incubated for 6 h with medium, MMP-9 (1 µg/ml) alone or a mix of MMP-9 (1 µg/ml) and MMP-9 inhibitor (100 µM). sIL-2Rα concentrations were measured in the supernatants by enzyme-linked immunosorbent assay (ELISA); (d) 6 h phytohaemagglutinin-stimulated PBMC were incubated or not with TAPI-0 (10 µg/ml). Supernatants were collected after 24 h and sIL-2Rα (left) or interferon-γ (right) were assayed by ELISA.
The inhibitory concentration (IC50) of the MMP-9 inhibitor used was 5 nM and no change in the sIL-2Rα concentration in the supernatant was observed with doses of up to 100 µM. To control the activity of the MMP-9 inhibitor used in these experiments, we showed that it was able to inhibit the increase of sIL-2Rα released by Kit225 treated with recombinant active MMP-9 (Fig. 4c).
In the various models, the presence of MMP-9 inhibitor did not affect CD25 membrane expression levels by the Kit225 cell line or by stimulated PBMC (data not shown).
Interestingly, we demonstrated a strong and almost complete inhibition of sIL-2Rα release when PHA-stimulated PBMC were treated with TAPI-0, a broad metalloproteinase inhibitor (Fig. 4d, left). To control the absence of toxicity of this inhibitor, we showed that the secretion of IFN-γ was not decreased in the presence of this inhibitor (Fig. 4d, right). TAPI-0 also inhibited the release of sIL-2Rα by the Kit225 cell line (data not shown).
Baseline serum sIL-2Rα concentrations and sIL-2Rα production by activated T cells were not altered in MMP-9-deficient mice compared to wild-type mice
Murine serum sIL-2Rα concentrations were measured by ELISA in wild-type or MMP-9-deficient mice. The median baseline serum sIL-2Rα concentrations was 1248 units in wild-type mice and 1798 units in MMP-9-deficient mice. Therefore, in contrast to expected results, MMP-9-deficient mice did not exhibit decreased serum sIL-2Rα concentrations (Fig. 5a).
Fig. 5.
Basal murine serum soluble interleukin (IL)-2 receptor alpha (sIL-2Rα) concentrations or soluble IL-2Rα release after concanavalin A (ConA) stimulation are not decreased in metalloproteinase-9 (MMP-9)-deficient mice compared to wild-type mice. (a) Serum IL-2Rα concentrations were measured by enzyme-linked immunosorbent assay (ELISA) in wild-type C57BL/6 mice or MMP-9-deficient mice in a C57BL-6 background. (b) Splenocytes from wild-type or MMP-9-deficient mice were stimulated with ConA (10 µg/ml). Seventy-two hours after activation supernatants were collected and mouse sIL-2Rα concentrations were measured by ELISA. Using the Mann–Whitney test no significant correlation (P < 0·05) was found between the wild-type and MMP-9-deficient mice for both serum IL-2Rα concentrations and IL-2Rα concentrations in supernatants from activated splenocytes.
As the release of soluble IL-2Rα may be different at homeostasis or during immune stimulation, we activated splenocytes from wild-type or MMP-9-deficient mice with ConA. Non-stimulated splenocytes did not release detectable levels of sIL-2Rα (data not shown), whereas sIL-2Rα release was demonstrated in the supernatants of both wild-type and MMP-9-deficient mice (Fig. 5b). The mean level of sIL-2Rα secreted in the supernatants of ConA-stimulated splenocytes was 771 units in wild-type mice and 1280 units in MMP-9-deficient mice. Using the Mann–Whitney U-test, no significant correlation (P < 0·05) was found between the wild-type and MMP-9-deficient mice for both serum IL-2Rα concentrations and IL-2Rα concentrations in supernatants from activated splenocytes. When rIL-2 was administered to mice, a similar increase of sIL-2Rα was also observed in wild-type and MMP-9-deficient mice (data not shown).
To exclude any bias in the interpretation of these results, we confirmed the absence of detection of MMP-9 in serum and supernatants of activated cells derived from MMP-9-deficient mice (data not shown).
On the basis of these results obtained in MMP-9-deficient mice, MMP-9 does not appear to be essential for sIL-2Rα release.
Discussion
This study confirms and extends previous reports about the prognostic value of serum sIL-2Rα in head and neck cancer [32,33] and reports, for the first time, the association between low MMP-9 serum concentrations prior to therapy and longer survival of head and neck cancer patients (Fig. 1a,b). However, although various arguments (cleavage of membrane IL-2Rα by MMP-9 [39] (Fig. 3), increased concentrations of serum sIL-2Rα or intratumoral CD25 and MMP-9 in the same group of cancer patients [14–19,46] and also in asthmatic patients [47] the overlapping role of the two parameters as prognostic factors in cancer patients [20,21,26,27]) has suggested a possible link between expression of MMP-9 and release of sIL-2Rα into serum, we did not find any relationships between these two factors.
Various parameters have to be taken into consideration in the interpretation of our results. Our ELISA method measured both the active and precursor forms of MMP-9 and it may be that only active MMP-9 levels are related to the release of sIL-2Rα. However, antibodies able to discriminate the pro-enzyme and the active form are not yet available. However, the bias due to the absence of discrimination between the active and zymogen forms of MMP-9 may be more theoretical than real, as Ikebe et al. have demonstrated that zymography-detected gelatinolytic activity of MMP-9 was correlated significantly with the degree of total MMP-9 detected in frozen sections of the same biopsy specimens [48]. The active form of MMP-9 in serum is difficult to detect because it is present at very low concentrations, but a correlation has been demonstrated between the concentrations of active and inactive forms of MMP-9 [49].
Furthermore, the presence of active MMP-9 does not inevitably mean that this MMP-9 mediates proteolysis of its substrates, as MMP-9 activity is balanced by the presence of specific inhibitors of MMP-9 such as the tissue inhibitor of MMP (TIMP-1) [50] or reversion-inducing cysteine-rich protein with kazal motifs (RECK) [51,52] and by the broad-spectrum protease inhibitor α2-macroglobulin [3]. While α2-macroglobulin is the primary regulator of MMPs in the fluid phase, TIMPs and RECK are considered to be key inhibitors in tissue [53]. In addition, in some cases, MMP-9 is also complexed to the neutrophil gelatinase-associated lipocalin (NGAL), which protects them from degradation and consequently enhances the MMP-9 activity [54]. The ratio between MMP-9 and its inhibitors or regulators may therefore be more relevant to assess accurately the potential activity of MMP-9. In the follow-up of steroid therapy in asthmatics, the serum MMP-9 : TIMP-1 ratio has been shown to be a more useful indicator of responsiveness than the determination of MMP-9 alone [55]. As discussed above, an ELISA test to detect free active MMP-9 in serum is not yet available and if biochemistry techniques could be applied to characterize proteins complexed to MMP-9 in situ, they would be unsuitable for quantitative analysis.
As recommended, we have measured MMP-9 in plasma and not in serum, because MMP-9 measurement in serum reflects primarily release of proteases by leucocytes during the clotting process in the blood collection tube [56,57]. Our tubes were stored at – 80°C before MMP-9 assay. However, the stability of MMP-9 at −80°C is a matter of debate [58,59].
With respect to the measurement of sIL-2Rα, our assay was able to detect both free sIL-2Rα and sIL-2Rα complexed to IL-2 [32].
Although a number of biases could explain the absence of correlation between serum MMP-9 and sIL-2Rα concentrations, we decided to conduct functional assays to confirm preliminary data concerning the direct role of MMP-9 in cleavage of membrane IL-2Rα. Recombinant MMP-9 promotes cleavage of IL-2Rα, as reflected by down-regulation of membrane CD25 and increased release of sIL-2Rα after incubation of cells with MMP-9. This activity was observed only at high MMP-9 concentrations (1 µg/ml), confirming previous results [39]. However, in two models, a leukaemic cell line which expressed and spontaneously released CD25 and PBMC activated by PHA, we failed to demonstrate a physiological role of MMP-9 in the cleavage of IL-2Rα using a specific inhibitor of MMP-9 at doses up to 800 times the IC50 of the inhibitor. A previous study by Junghans et al. also reported that zinc chelators known to inhibit metalloproteinase activity [60] did not influence shedding of sIL-2Rα [61]. The absence of change in sIL-2Rα release in MMP-9-deficient mice also supports these results, as although it does not formally exclude a physiological role of MMP-9 in the regulation of sIL-2Rα, it demonstrates that other proteases may replace MMP-9 for cleavage of sIL-2Rα. We have indeed demonstrated that a broad metalloproteinase inhibitor inhibited the release of sIL-2Rα (Fig. 4d). Although we reproduced some of the data reported by Sheu et al. [39], our results do not appear to support the main role of MMP-9 in the cleavage of IL-2Rα. However, even in the pioneer paper, the efficiency of the cleavage by MMP-9 was low, as high doses of MMP-9 had to be added to observe this effect compared to other studies [62,63]. MMP-9 had only a partial effect on cleavage and high doses of MMP-9 inhibitor were also required to inhibit this cleavage.
Overall, this study demonstrates a predictive value of serum sIL-2Rα and MMP-9 concentrations for the survival of patients with head and neck cancers. However, we did not find any correlation between MMP-9 and sIL-2Rα concentrations. The partial effect of MMP-9 on the release of sIL-2Rα shown by Sheu et al. [39], the dramatic inhibition of sIL-2Rα release by a broad spectrum metalloproteinase and the normal production of sIL-2Rα observed in MMP-9-deficient mice suggest strongly that other proteases are involved in the shedding of sIL-2Rα.
Acknowledgments
This work was supported by grants from Association pour la Recherche sur le Cancer, Fondation de France, Cancéropole d'Ile de France, Ligue Nationale contre le Cancer, Institut National du Cancer (INCA) and Pole de Competitivité Ile de France: Projet Immucan. B. Vingert is a fellow of the Fondation de France.
References
- 1.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–64. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 2.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 3.Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in tumor angiogenesis. Int J Cancer. 2005;115:849–60. doi: 10.1002/ijc.20945. [DOI] [PubMed] [Google Scholar]
- 4.Shute J. Matrix metalloproteinase-9: marker or mediator of tissue damage in asthma? Clin Exp Allergy. 2002;32:168–71. doi: 10.1046/j.1365-2222.2002.01302.x. [DOI] [PubMed] [Google Scholar]
- 5.St-Pierre Y, Van Themsche C, Esteve PO. Emerging features in the regulation of MMP-9 gene expression for the development of novel molecular targets and therapeutic strategies. Curr Drug Targets Inflamm Allergy. 2003;2:206–15. doi: 10.2174/1568010033484133. [DOI] [PubMed] [Google Scholar]
- 6.Huang S, Van Arsdall M, Tedjarati S, et al. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;94:1134–42. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- 7.Bernhard EJ, Gruber SB, Muschel RJ. Direct evidence linking expression of matrix metalloproteinase 9 (92-kDa gelatinase/collagenase) to the metastatic phenotype in transformed rat embryo cells. Proc Natl Acad Sci USA. 1994;91:4293–7. doi: 10.1073/pnas.91.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kondraganti S, Mohanam S, Chintala SK, et al. Selective suppression of matrix metalloproteinase-9 in human glioblastoma cells by antisense gene transfer impairs glioblastoma cell invasion. Cancer Res. 2000;60:6851–5. [PubMed] [Google Scholar]
- 9.Itoh T, Tanioka M, Matsuda H, et al. Experimental metastasis is suppressed in MMP-9-deficient mice. Clin Exp Metastasis. 1999;17:177–81. doi: 10.1023/a:1006603723759. [DOI] [PubMed] [Google Scholar]
- 10.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–90. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin LA, Jay G, Nelson DL. The released interleukin 2 receptor binds interleukin 2 efficiently. J Immunol. 1986;137:3841–4. [PubMed] [Google Scholar]
- 14.Semenzato G, Foa R, Agostini C, et al. High serum levels of soluble interleukin 2 receptor in patients with B chronic lymphocytic leukemia. Blood. 1987;70:396–400. [PubMed] [Google Scholar]
- 15.Yano T, Fukuyama Y, Yokoyama H, et al. Interleukin-2 receptors in pulmonary adenocarcinoma tissue. Lung Cancer. 1996;16:13–19. doi: 10.1016/s0169-5002(96)00608-3. [DOI] [PubMed] [Google Scholar]
- 16.Ostenstad B. Soluble interleukin-2 receptor levels in patients with malignant melanoma and renal cell cancer. Acta Oncol. 1992;31:413–15. doi: 10.3109/02841869209088281. [DOI] [PubMed] [Google Scholar]
- 17.Lein M, Jung K, Laube C, et al. Matrix-metalloproteinases and their inhibitors in plasma and tumor tissue of patients with renal cell carcinoma. Int J Cancer. 2000;85:801–4. doi: 10.1002/(sici)1097-0215(20000315)85:6<801::aid-ijc11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 18.Bauvois B, Dumont J, Mathiot C, Kolb JP. Production of matrix metalloproteinase-9 in early stage B-CLL: suppression by interferons. Leukemia. 2002;16:791–8. doi: 10.1038/sj.leu.2402472. [DOI] [PubMed] [Google Scholar]
- 19.Iizasa T, Fujisawa T, Suzuki M, et al. Elevated levels of circulating plasma matrix metalloproteinase 9 in non-small cell lung cancer patients. Clin Cancer Res. 1999;5:149–53. [PubMed] [Google Scholar]
- 20.Vuoristo MS, Laine S, Huhtala H, et al. Serum adhesion molecules and interleukin-2 receptor as markers of tumour load and prognosis in advanced cutaneous melanoma. Eur J Cancer. 2001;37:1629–34. doi: 10.1016/s0959-8049(01)00192-7. [DOI] [PubMed] [Google Scholar]
- 21.Boyano MD, Garcia-Vazquez MD, Lopez-Michelena T, et al. Soluble interleukin-2 receptor, intercellular adhesion molecule-1 and interleukin-10 serum levels in patients with melanoma. Br J Cancer. 2000;83:847–52. doi: 10.1054/bjoc.2000.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma S, Saha K, Shinghal RN, Malik GB. Serum soluble interleukin-2 (IL-2) receptor levels in women with breast carcinoma and its correlation with IL-2 receptor expression on blood lymphocytes and lymphocytic infiltration within the tumour. Cancer Immunol Immunother. 1991;33:198–202. doi: 10.1007/BF01756142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murakami S, Satomi A, Ishida K, Murai H, Okamura Y. Serum soluble interleukin-2 receptor in colorectal cancer. Acta Oncol. 1994;33:19–21. doi: 10.3109/02841869409098369. [DOI] [PubMed] [Google Scholar]
- 24.Berghella AM, Pellegrini P, Piancatelli D, et al. Progression mechanisms in colon cancer: soluble interleukin-2 (IL-2) receptor, IL-2 plus anti-CD3 proliferative response and tumour stage correlations. Cancer Immunol Immunother. 1994;38:160–6. doi: 10.1007/BF01525636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang A, Quinn H, Glover C, Henderson DC, Allen-Mersh TG. The presence of interleukin-2 receptor alpha in the serum of colorectal cancer patients is unlikely to result only from T cell up-regulation. Cancer Immunol Immunother. 2002;51:53–7. doi: 10.1007/s00262-001-0250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zucker S, Lysik RM, Zarrabi MH, Moll U. M(r) 92,000 type IV collagenase is increased in plasma of patients with colon cancer and breast cancer. Cancer Res. 1993;53:140–6. [PubMed] [Google Scholar]
- 27.Nikkola J, Vihinen P, Vuoristo MS, Kellokumpu-Lehtinen P, Kahari VM, Pyrhonen S. High serum levels of matrix metalloproteinase-9 and matrix metalloproteinase-1 are associated with rapid progression in patients with metastatic melanoma. Clin Cancer Res. 2005;11:5158–66. doi: 10.1158/1078-0432.CCR-04-2478. [DOI] [PubMed] [Google Scholar]
- 28.Riedel F, Gotte K, Schwalb J, Hormann K. Serum levels of matrix metalloproteinase-2 and -9 in patients with head and neck squamous cell carcinoma. Anticancer Res. 2000;20:3045–9. [PubMed] [Google Scholar]
- 29.Kuropkat C, Plehn S, Herz U, Dunne AA, Renz H, Werner JA. Tumor marker potential of serum matrix metalloproteinases in patients with head and neck cancer. Anticancer Res. 2002;22:2221–7. [PubMed] [Google Scholar]
- 30.Ranuncolo SM, Matos E, Loria D, et al. Circulating 92-kilodalton matrix metalloproteinase (MMP-9) activity is enhanced in the euglobulin plasma fraction of head and neck squamous cell carcinoma. Cancer. 2002;94:1483–91. doi: 10.1002/cncr.10356. [DOI] [PubMed] [Google Scholar]
- 31.Gottschlich S, Gorogh T, Lippert BM, Niemann AM, Folz BJ, Werner JA. The soluble interleukin-2 receptor − a marker for squamous cell carcinoma of the upper aerodigestive tract. Anticancer Res. 1997;17:2921–2. [PubMed] [Google Scholar]
- 32.Tartour E, Deneux L, Mosseri V, et al. Soluble interleukin-2 receptor serum level as a predictor of locoregional control and survival for patients with head and neck carcinoma: results of a multivariate prospective study. Cancer. 1997;79:1401–8. [PubMed] [Google Scholar]
- 33.Tartour E, Mosseri V, Jouffroy T, et al. Serum soluble interleukin-2 receptor concentrations as an independent prognostic marker in head and neck cancer. Lancet. 2001;357:1263–4. doi: 10.1016/s0140-6736(00)04420-2. [DOI] [PubMed] [Google Scholar]
- 34.Franchi A, Santucci M, Masini E, Sardi I, Paglierani M, Gallo O. Expression of matrix metalloproteinase 1, matrix metalloproteinase 2, and matrix metalloproteinase 9 in carcinoma of the head and neck. Cancer. 2002;95:1902–10. doi: 10.1002/cncr.10916. [DOI] [PubMed] [Google Scholar]
- 35.Riedel F, Gotte K, Schwalb J, Bergler W, Hormann K. Expression of 92-kDa type IV collagenase correlates with angiogenic markers and poor survival in head and neck squamous cell carcinoma. Int J Oncol. 2000;17:1099–105. doi: 10.3892/ijo.17.6.1099. [DOI] [PubMed] [Google Scholar]
- 36.de Vicente JC, Fresno MF, Villalain L, Vega JA, Hernandez Vallejo G. Expression and clinical significance of matrix metalloproteinase-2 and matrix metalloproteinase-9 in oral squamous cell carcinoma. Oral Oncol. 2005;41:283–93. doi: 10.1016/j.oraloncology.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Katayama A, Bandoh N, Kishibe K, et al. Expressions of matrix metalloproteinases in early-stage oral squamous cell carcinoma as predictive indicators for tumor metastases and prognosis. Clin Cancer Res. 2004;10:634–40. doi: 10.1158/1078-0432.ccr-0864-02. [DOI] [PubMed] [Google Scholar]
- 38.Yorioka CW, Coletta RD, Alves F, Nishimoto IN, Kowalski LP, Graner E. Matrix metalloproteinase-2 and -9 activities correlate with the disease-free survival of oral squamous cell carcinoma patients. Int J Oncol. 2002;20:189–94. [PubMed] [Google Scholar]
- 39.Sheu BC, Hsu SM, Ho HN, Lien HC, Huang SC, Lin RH. A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res. 2001;61:237–42. [PubMed] [Google Scholar]
- 40.American Joint Committee on Cancer (AJCC) AJCC cancer staging manual. 5. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 41.Hori T, Uchiyama T, Tsudo M, et al. Establishment of an interleukin 2-dependent human T cell line from a patient with T cell chronic lymphocytic leukemia who is not infected with human T cell leukemia/lymphoma virus. Blood. 1987;70:1069–72. [PubMed] [Google Scholar]
- 42.Lelongt B, Bengatta S, Delauche M, Lund LR, Werb Z, Ronco PM. Matrix metalloproteinase 9 protects mice from anti-glomerular basement membrane nephritis through its fibrinolytic activity. J Exp Med. 2001;193:793–802. doi: 10.1084/jem.193.7.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Z, Shipley JM, Vu TH, et al. Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J Exp Med. 1998;188:475–82. doi: 10.1084/jem.188.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loughnan MS, Sanderson CJ, Nossal GJ. Soluble interleukin 2 receptors are released from the cell surface of normal murine B lymphocytes stimulated with interleukin 5. Proc Natl Acad Sci USA. 1988;85:3115–19. doi: 10.1073/pnas.85.9.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner DK, Wong HL, Gately MK, Nelson DL. Cellular source of soluble interleukin 2 receptors in serum of mice after recombinant interleukin 2 administration. Cytokine. 1990;2:337–43. doi: 10.1016/1043-4666(90)90063-y. [DOI] [PubMed] [Google Scholar]
- 46.Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–72. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 47.Belleguic C, Corbel M, Germain N, et al. Increased release of matrix metalloproteinase-9 in the plasma of acute severe asthmatic patients. Clin Exp Allergy. 2002;32:217–23. doi: 10.1046/j.1365-2222.2002.01219.x. [DOI] [PubMed] [Google Scholar]
- 48.Ikebe T, Shinohara M, Takeuchi H, et al. Gelatinolytic activity of matrix metalloproteinase in tumor tissues correlates with the invasiveness of oral cancer. Clin Exp Metastasis. 1999;17:315–23. doi: 10.1023/a:1006642428826. [DOI] [PubMed] [Google Scholar]
- 49.Giannelli G, Erriquez R, Fransvea E, et al. Proteolytic imbalance is reversed after therapeutic surgery in breast cancer patients. Int J Cancer. 2004;109:782–5. doi: 10.1002/ijc.20009. [DOI] [PubMed] [Google Scholar]
- 50.Hornebeck W, Lambert E, Petitfrere E, Bernard P. Beneficial and detrimental influences of tissue inhibitor of metalloproteinase-1 (TIMP-1) in tumor progression. Biochimie. 2005;87:377–83. doi: 10.1016/j.biochi.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 51.Noda M, Oh J, Takahashi R, Kondo S, Kitayama H, Takahashi C. RECK: a novel suppressor of malignancy linking oncogenic signaling to extracellular matrix remodeling. Cancer Metastasis Rev. 2003;22:167–75. doi: 10.1023/a:1023043315031. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi C, Sheng Z, Horan TP, et al. Regulation of matrix metalloproteinase-9 and inhibition of tumor invasion by the membrane-anchored glycoprotein RECK. Proc Natl Acad Sci USA. 1998;95:13221–6. doi: 10.1073/pnas.95.22.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–83. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez CA, Yan L, Louis G, Yang J, Kutok JL, Moses MA. The matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin Cancer Res. 2005;11:5390–5. doi: 10.1158/1078-0432.CCR-04-2391. [DOI] [PubMed] [Google Scholar]
- 55.Bosse M, Chakir J, Rouabhia M, Boulet LP, Audette M, Laviolette M. Serum matrix metalloproteinase-9: tissue inhibitor of metalloproteinase-1 ratio correlates with steroid responsiveness in moderate to severe asthma. Am J Respir Crit Care Med. 1999;159:596–602. doi: 10.1164/ajrccm.159.2.9802045. [DOI] [PubMed] [Google Scholar]
- 56.Zucker S, Cao J. Measurement of matrix metalloproteinases in serum of patients with melanoma: snarled in technical pitfalls. Clin Cancer Res. 2005;11:5069–70. doi: 10.1158/1078-0432.CCR-05-0774. [DOI] [PubMed] [Google Scholar]
- 57.Jung K, Meisser A, Bischof P. Blood sampling as critical preanalytical determinant to use circulating MMP and TIMP as surrogate markers for pathological processes. Int J Cancer. 2005;116:1000–1. doi: 10.1002/ijc.21129. author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 58.Alby C, Ben Abdesselam O, Foglietti MJ, Beaudeux JL. Preanalytical aspects regarding the measurement of metalloproteinase-9 and tissue inhibitor or metalloproteinase-1 in blood. Clin Chim Acta. 2002;325:183–6. doi: 10.1016/s0009-8981(02)00247-4. [DOI] [PubMed] [Google Scholar]
- 59.Rouy D, Ernens I, Jeanty C, Wagner DR. Plasma storage at −80 degrees C does not protect matrix metalloproteinase-9 from degradation. Anal Biochem. 2005;338:294–8. doi: 10.1016/j.ab.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 60.Kleiner DE, Stetler-Stevenson WG., Jr Structural biochemistry and activation of matrix metalloproteases. Curr Opin Cell Biol. 1993;5:891–7. doi: 10.1016/0955-0674(93)90040-w. [DOI] [PubMed] [Google Scholar]
- 61.Junghans RP, Waldmann TA. Metabolism of Tac (IL2Ralpha): physiology of cell surface shedding and renal catabolism, and suppression of catabolism by antibody binding. J Exp Med. 1996;183:1587–602. doi: 10.1084/jem.183.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van den Steen PE, Husson SJ, Proost P, Van Damme J, Opdenakker G. Carboxyterminal cleavage of the chemokines MIG and IP-10 by gelatinase B and neutrophil collagenase. Biochem Biophys Res Commun. 2003;310:889–96. doi: 10.1016/j.bbrc.2003.09.098. [DOI] [PubMed] [Google Scholar]
- 63.Van Den Steen PE, Wuyts A, Husson SJ, Proost P, Van Damme J, Opdenakker G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur J Biochem. 2003;270:3739–49. doi: 10.1046/j.1432-1033.2003.03760.x. [DOI] [PubMed] [Google Scholar]