Abstract
Ulcerative colitis (UC) is a chronic relapsing–remitting inflammatory bowel disease (IBD) that affects the colon and the rectum producing debilitating symptoms, which impair ability to function and quality of life. The aetiology of IBD is incompletely understood, but within the lymphocyte population, specific T cell subsets are known to be major factors in the development of intestinal immune pathology while different subsets are essential regulators, controlling IBD. Hence, IBD is thought to reflect dysregulated T cell behaviour. This study was to investigate if the normal molecular configuration of the T cell receptor (TCR) repertoire is compromised in patients with UC. The percentage of T cell-bearing β-chain 4 (TCRBV4) was high in patients with UC, and T cells showed polyclonal expansion in the presence of bacterial superantigens (SA) such as streptococcal mitogenic exotoxin Z-2 (SMEZ-2), indicating that bacterial SA promote specific TCRBV family expansion. Further, in patients with UC, the duration of UC was significantly longer in patients with skewed TCRBV4 compared with patients without TCRBV4 skewing, suggesting that long-term exposure to bacterial SA such as SMEZ-2 might promote systemic immune disorders like the remission-relapsing cycles seen in patients with UC. In conclusion, our observations in this study support the perception that the systemic activation of T cells by enteric bacterial SA might lead to a dysregulated, but exuberant immune activity causing the remission and flare-up cycle of mucosal inflammation in patients with UC. Future studies should strengthen our findings and increase understanding on the aetiology of IBD.
Keywords: bacterial superantigens, inflammatory bowel disease, T lymphocytes, TCR repertoire, ulcerative colitis
Introduction
Ulcerative colitis (UC) is a chronic relapsing–remitting inflammatory bowel disease (IBD) that affects the colon and the rectum and produces debilitating symptoms, which impair both ability to function and quality of life [1]. At present, factors which exacerbate and perpetuate UC are not well understood. Multiple factors, including environmental influences [2–4], enteric flora [1,5–7] and genetic susceptibility [8], are thought to contribute to the dysregulated immune function [9,10] seen in patients with IBD. However, active UC is associated frequently with infiltration of a vast number of leucocytes, mainly activated T cells, monocytes/macrophages and polymorphonuclear cells, into the intestinal mucosa [1,11–15]. In line with these observations, several studies have suggested that T cells are major players in the immunopathogenesis of UC [9,12–18]. Accordingly, certain immunosuppressants such as cyclosporin A, a T cell-specific immunosuppressive agent, has been used to induce remission of active UC [19]. Given that certain T cell subsets such as the CD4−CD25− phenotype (known popularly as regulatory T cells) are involved intimately in the control of intestinal immune pathology [20,21], it is logical to look for factors which are associated with dysregulated T cell features in the peripheral blood and within the intestinal mucosa of patients with UC.
T cells are known to recognize the antigen presented by antigen-presenting cells through the T cell receptor (TCR) in the context of major histocompatibility complex (MHC) class I and class II molecules [22]. The fine specificity of T cells is determined by the TCR displayed on the cell surface, a heterodimer composed of an α-chain and a β-chain or a γ-chain and a δ-chain. The variable regions of these chains are responsible for antigen recognition and are encoded with variable (V), joining (J) and diversity (D) (for the β-chain) gene segments. Random insertion of non-germinal element (N) nucleotides or deletion of nucleotides has been observed in the VN(D)NJ junction region called CDR3, and is thought to be responsible for an antigenic peptide content [23,24]. Thus, any specific recognition of antigens by CDR3 can lead to the clonal expansion of T cells. Further, it has been known that superantigens (SA) such as staphylococcal enterotoxins and streptococcal pyrogenic exotoxins produced by bacteria bind to the outside of the MHC class II α-chain and V region of TCR β-chain (TCRBV) to form a cross-linking; a given SA can stimulate all T cells that bear the appropriate TCRBV in polyclonal settings [22]. As CDR3 has different sequences and lengths, it is possible to analyse the diversity of the TCRs by using the CDR3 size spectratyping method that provides a rapid scan of all TCRBV transcripts, grouped according to the utilized Vβ gene and the lengths of the chains [25,26]. Using this technique with TCR repertoire analysis, it is possible to investigate the diversity of TCRs [27–29]. By using TCR repertoire analysis and CDR3 spectratyping, this study aimed to gain further understanding on T cell profiles associated with UC, to investigate if the normal molecular configuration of the TCR repertoire of T cells is compromised in patients with UC.
Materials and methods
Test samples
Peripheral blood samples were obtained from 22 patients with active UC and 20 healthy controls after obtaining informed consent at the Sakura Medical Center of Toho University (Japan). The study protocol was reviewed and approved by the local Committee on Ethics of experiments involving humans. Peripheral blood mononuclear cells (PBMC) were separated from heparinized blood using lymphocyte separation medium (H-SMF; Jimro, Gunma, Japan) gradient centrifugation. PBMC were washed with RPMI-1640 (Invitrogen, Carlsbad, CA, USA) and used in several experiments. The plasma samples were kept frozen until assay. In an additional investigation, colonic biopsies from five patients were processed. In each case, two or three small macroscopically inflamed mucosal biopsy specimens were obtained at colonoscopy. The biopsy samples were soaked in RNAlater™ (Qiagen, Hilden, Germany) and kept frozen until use. Peripheral blood specimens were also obtained from these five patients.
Stimulation of PBMC with recombinant streptococcal mitogenic exotoxin Z-2 (rSMEZ-2) and recombinant toxic shock syndrome toxin 1 (rTSST-1)
PBMC were incubated with 1 ng/ml of rTSST-1 (Toxin Technology, Sarasota, FL, USA) or with 20 pg/ml of rSEMZ-2 (a kind gift from Dr John D. Fraser, University of Auckland, New Zealand) at 1 × 106 cells/ml for 3 days. After 3 days, 20 ng/ml of interleukin (IL)-2 (Shionogi, Osaka, Japan) was added to each well and the cells were incubated for a further 24 h (for rSMEZ-2 stimulation only). The cells were harvested and used for analysis of the TCR repertoire.
Analysis of TCR repertoire
Crude cellular RNA from PBMC, stimulated PBMC or biopsy samples was extracted by using an RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. Adaptor-ligation polymerase chain reaction (PCR) and microplate hybridization assay were performed as previously described [30]. Briefly, 1 µg of total RNA was converted to a double-stranded cDNA with the SuperScript II cDNA synthesis kit (Invitrogen) according to the manufacturer's instructions, except for priming with BSL-18B primer adaptor containing the Not I site. The P20EA/P10EA universal adaptors were ligated at the 5′ end of BSL-18B primer cDNA. Three rounds of Cα- and Cβ-specific PCR were performed by using Cα and Cβ sequence-specific oligonucleotide probes (SSOP) to prepare amplified and biotinylated TCR cDNA pools. Hybridization was between biotinylated PCR products and Vα or Vβ SSOP, which were immobilized on a carboxylate-modified enzyme-linked immunosorbent assay (ELISA) plate (Sumitomo Bakelite, Tokyo, Japan). The hybridization was visualized by p-nitrophenylphosphate (Nacalai Tesque, Osaka, Japan). The visualized signals were estimated at 405 nm using Multiskan JX (Thermo Labsystems, Helsinki, Finland). The relative expansion of the TCRAV or TCRBV region repertoire was calculated by the following formula: frequency (%) = 100 (the corresponding SSOP signal)/(ΣTCRV SSOP signals).
CDR3 size analysis of TCRBV
By knowing the size of the CDR3 region in TCRBV, it is possible to estimate the polyclonal expansion of T cells [31]. We used this technique to determine the polyclonality of T cells from patients with active UC who had skewed TCRBV4 and PBMC from healthy controls before and after in vitro stimulation with SA. The second PCR products described above were labelled by the 20-cycle PCR amplification with fluorescent dye-labelled Cβ–SSOP [31]. After the labelled PCR products were mixed with size marker (CEQ™ DNA Size Standard Kit-600; Beckman Coulter, Fullerton, CA, USA), they were loaded onto a polyacrylamide sequencing gel (CEQ™ Separation Gel-LPA I; Beckman Coulter) to determine the size and fluorescence intensity by using an automated capillary DNA sequencer (CEQ™ 8000; Beckman Coulter). Data were analysed by using Genetic Analysis System software (Beckman Coulter).
Human leucocyte antigen D-related (HLA-DRB1) genotyping
HLA-DRB1 genotyping was performed using the Genoresearch HLA-DRB1 kit (Medical Biological Laboratories, Tokyo, Japan) according to the manufacturer's instructions.
Detection of antibodies to SMEZ-2 and TSST-1 in plasma samples
Levels of immunoglobulin antibodies against SMEZ-2 and TSST-1 in plasma samples were assayed by an ELISA method using rTSST-1 or rSMEZ-2 as antigens. Recombinant proteins were diluted to 1 µg/ml in 10 mM phosphate-buffered saline (PBS, pH 7·4), and a 100 µl diluted toxin was added to each well of a 96-well microplate (Nalge Nunc International, Rochester, NY, USA). The plates were incubated overnight at 4°C to allow binding of antigens to the wells. Unbounded antigens were removed by aspiration, and the wells were washed four times with washing buffer. After blocking with 1% bovine serum albumin (BSA)–PBS, the wells were washed four times with washing buffer and filled with dilution buffer (PBS containing 0·1% BSA). The toxin-coated plates were stored at 4°C until assay.
Plasma samples from 27 patients with active UC and seven healthy controls were diluted to 1 : 200 with dilution buffer and 100 µl diluted plasma was added to the toxin-coated wells. The plates were then incubated overnight at 4°C. At the end of the incubation time, the wells were washed four times with washing buffer. One hundred µl peroxidase-conjugated anti-human IgG antibody (Southern Biotechnology Associates, Birmingham, AL, USA) (diluted to 1 : 10 000 with dilution buffer) was added to each well; the plates were then incubated at 30°C for 2 h. The wells were again rinsed four times with washing buffer. The product was visualized by subsequent reaction with 100 µl 3,3′,5,5′-tetramethlbenzidine (TMB) solution (Wako, Osaka, Japan) for 5 min at room temperature. The reaction was terminated by addition of 50 µl of 1 M sulphuric acid, and the absorbance of each well was read at 450 nm with a plate spectrophotometer (Multiskan JX; Thermo Labsystems). The antibodies to SMEZ-2 and TSST-1 in plasma samples were corrected with the antibodies to BSA.
Detection of plasma anti-streptolysin-O antibody
Detection of plasma anti-streptolysin-O (ASO) antibody, a marker for group A streptococcal infections, was performed by SRL Inc. (Hachioji, Tokyo, Japan), a clinical diagnosis laboratory. Determination of anti-SMEZ-2, anti-TSST-1 (above) and ASO titres was to investigate the nature of the background SA and contribution to TCRBV4 skewing (see Results section).
Statistical analysis
For statistical analysis, a software package StatView 5·0 for Windows (SAS Institute, Cary, NC, USA) was used. For all comparisons except in vitro studies, non-parametric tests (Mann–Whitney U-test, Wilcoxon's signed-rank test, Kruskal–Wallis test and Spearman's rank correlation test) were applied. For three independent samples, it was confirmed that there were significant differences by Kruskal–Wallis test before the comparison between each two groups was performed. Paired t-test was used for comparisons in vitro studies. Accordingly, results of comparisons are given as mean or median values. P < 0·05 was considered significant.
Results
Demography of patients with UC
Twenty-seven patients (19 males and eight females) with active UC, clinical activity index (CAI) ≥ 5 [32], were enrolled into the study (Table 1). The mean age at entry was 33 years (range 16–64 years); the mean disease duration was 5·5 years (range 1 month−32 years) and the mean CAI was 9·4, range 5–17. HLA-DRB1 typing revealed the presence of the allele (*1502) in 11 of 23 patients (48%); *1502 is known to be associated with UC in Asians [33,34]. HLA-DRB1 typing was not performed in four patients because DNA could not be collected.
Table 1.
Baseline demography of the 27 patients who were included in this study.
| Patient ID | Age (years) | Gender | UC duration (years) | CAIa | Location | Severity | Medicationb |
|---|---|---|---|---|---|---|---|
| UC-01 | 41 | Male | 12 | 14 | Left | Severe | 5-ASA |
| UC-02 | 16 | Male | 2 | 11 | Total | Severe | PSL, SASP |
| UC-03 | 21 | Female | 2 | 14 | Total | Severe | PSL, SASP |
| UC-04 | 52 | Male | 12 | 6 | Left | Moderate | 5-ASA, SASP |
| UC-05 | 17 | Female | 2 | 9 | Total | Severe | PSL, SASP |
| UC-06 | 45 | Male | 5 | 9 | Left | Moderate | PSL, 5-ASA |
| UC-07 | 30 | Male | 2 | 9 | Left | Moderate | PSL, SASP |
| UC-08 | 33 | Male | 16 | 8 | Total | Severe | 5-ASA |
| UC-09 | 37 | Male | 11 | 11 | Total | Severe | PSL |
| UC-10 | 29 | Male | 10 | 7 | Left | Moderate | PSL, 5-ASA |
| UC-11 | 19 | Male | 4 | 5 | Total | Moderate | 5-ASA |
| UC-12 | 19 | Male | 1·75 | 11 | Total | Moderate | SASP |
| UC-13 | 42 | Female | 19 | 5 | Total | Severe | 5-ASA |
| UC-14 | 34 | Female | 2·7 | 9 | Total | Severe | PSL, 5-ASA |
| UC-15 | 25 | Male | 6 | 5 | Total | Moderate | PSL, 5-ASA |
| UC-16 | 20 | Male | 0·08 | 8 | Total | Severe | SASP, 5-ASA |
| UC-17 | 47 | Male | 0·25 | 10 | Total | Moderate | 5-ASA, betamethasone |
| UC-18 | 20 | Male | 0·25 | 9 | Total | Severe | PSL, 5-ASA, SASP |
| UC-19 | 18 | Female | 2 | 10 | Total | Severe | PSL, SASP |
| UC-20 | 29 | Male | 4 | 9 | Left | Moderate | PSL, 5-ASA |
| UC-21 | 64 | Female | 0·08 | 5 | Total | Moderate | 5-ASA |
| UC-22 | 38 | Male | 0·16 | 17 | Left | Severe | PSL, 5-ASA |
| UC-101 | 57 | Male | 32 | 9 | Total | Moderate | PSL, SASP |
| UC-102 | 48 | Male | 0·67 | 10 | Total | Moderate | 5-ASA |
| UC-103 | 32 | Male | 0·16 | 9 | Total | Moderate | PSL, 5-ASA |
| UC-104 | 35 | Male | 0·08 | 12 | Total | Severe | SASP |
| UC-105 | 24 | Female | 0·16 | 12 | Total | Severe | PSL, 5-ASA |
CAI indicates the disease activity. The final score is the total of several score for symptoms and signs, and the maximum value is 21 [29].
5-ASA; 5-aminosalicylic acid, SASP; salazosulfapyridine, PSL; prednisolone, UC: ulcerative colitis.
Selective expansion of TCRBV subfamilies in PBMC from patients with UC
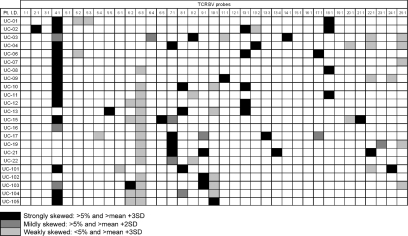
Initially, we performed TCR repertoire analysis on PBMC from 23 of 27 patients with active UC to determine whether T cell features were associated with UC. The expansion of TCR-bearing T cells in patients with UC was defined as significant when the percentage frequency of the relevant T cell subset was greater than 5%, and exceeded the mean percentage plus 3 standard deviations (s.d.) of the corresponding T cells bearing the relevant TCR in 20 healthy controls. Four patients, UC-05, UC-14, UC-18 and UC-20, were excluded from the analysis due to unsuccessful collection of RNA. Twenty-one of 23 (91%) patients with UC had skewed a TCR repertoire in any TCRBV subfamily. In particular, 14 of 23 (61%) patients had skewed TCR in the TCRBV4 (Fig. 1). There was no selective expansion of TCRAV subfamilies in patients with UC (data not presented).
Fig. 1.
T cell receptor β-chain (TCRBV) gene expression profile in peripheral blood mononuclear cells (PBMC) of patients with active ulcerative colitis (UC). Total RNA was extracted from PBMC and reverse-transcribed into cDNA and the adaptor was ligated. This adaptor-ligated cDNA was then used as a template for individual polymerase chain reaction (PCR) amplifications. The primer sets were then applied to the adaptor sequence and TCRBC gene elements. The PCR products were determined by semiquantitative PCR–enzyme-linked immunosorbent assay. Twenty-one of 23 (91%) patients had strongly skewed TCR repertoire in any TCRBV subfamilies and intense skew was observed in TCRBV4.
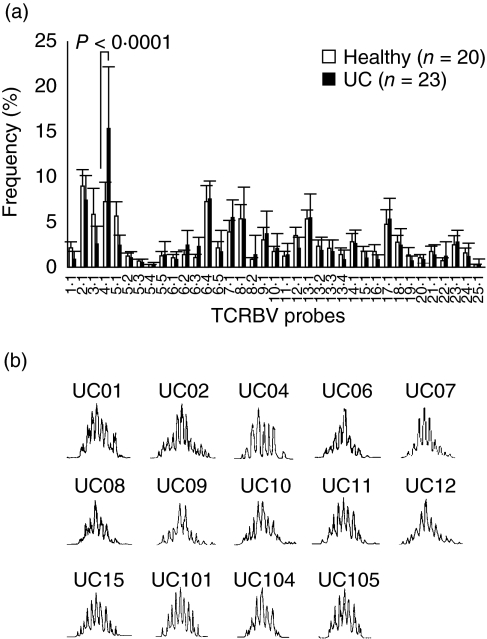
The percentage frequency of TCRBV4-bearing T cells was increased markedly (P < 0·0001) in patients with UC compared with healthy controls (Fig. 2a), but was not associated with HLA-DRB1 types. CDR3 size spectratyping was undertaken to determine whether the TCRBV4 T cells were expanded in a monoclonal or polyclonal manner. Figure 2b shows that there were multiple Gaussian-like patterns with three nucleotide intervals, indicating polyclonal expansion in all CDR3 size distributions [35].
Fig. 2.
The polyclonal expansion of T cell receptor β-chain 4 (TCRBV4)-bearing T cells in peripheral blood mononuclear cells (PBMC) of patients with active ulcerative colitis (UC). (a) The percentage of TCRBV4 in PBMC specimens of patients with UC was strikingly high compared with healthy individuals (P < 0·0001). Mean ± s.d. values are presented; the P-value is by the Mann–Whitney U-test. (b) The CDR3 size spectratype profiles of the TCRBV4 gene rearrangement. The TCRBV4 gene was selected among the PBMC of 14 patients with skewed TCRBV4. Each of the 14 subjects had 7–10 peaks showing a Gaussian-like distribution.
TCRBV4-bearing T cells expansion by rSMEZ-2
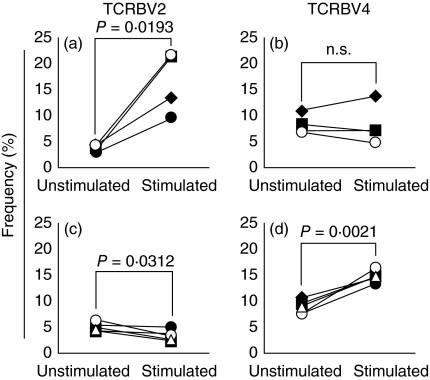
It has been suggested that TSST-1 or SMEZ-2 might induce selective expansion of TCRBV4-bearing T cells [36,37]. We were interested to identify which SA stimulates the TCRBV4-bearing T cells in vitro. The percentage frequency of TCRBV2-bearing T cells increased in the PBMC from all four donors when stimulated with rTSST-1. In contrast, the percentage frequency of TCRBV4-bearing T cells did not increase by TSST-1 stimulation, but increased significantly in PBMC from all five donors when stimulated by rSMEZ-2. Similarly, the percentage frequency of TCRBV2-bearing T cells did not increase by rSMEZ-2 (Fig. 3). Further, rSMEZ-2 also stimulated TCRBV8-bearing T cells (data not presented).
Fig. 3.
Changes in the percentage frequency of T cell receptor β-chain (TCRBV) families following stimulation by two different bacterial superantigens (SA), toxic shock syndrome toxin 1 (TSST-1) and streptococcal mitogenic exotoxin Z-2 (SMEZ-2). TSST-1 stimulated TCRBV2-bearing T cells (a) and did not stimulate TCRBV4-bearing T cells (b). SMEZ-2 stimulated TCRBV4-bearing T cells (d) without the expansion of TCRBV2-bearing T cells (c). The P-values are by paired t-test.
Association of SMEZ-2 titre with TCRBV4 skewing and ASO level
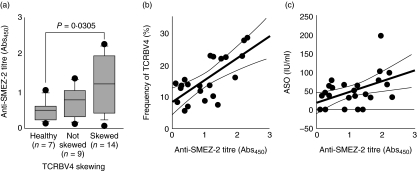
We were convinced that our experimental method could detect the SMEZ-2-induced polyclonal expansion of TCRBV4- and TCRBV8-bearing T cells (without TCRBV2-bearing T cells) both qualitatively and quantitatively. We then examined antibody levels to SMEZ-2 in the plasma samples from 27 patients with UC and from seven healthy donors to investigate exposure of patients to SMEZ-2 (infection with Streptococcus pyogenes). The level of antibodies against SMEZ-2 in patients with skewed TCRBV4 was significantly higher compared with the level in healthy volunteers (P = 0·0305, Fig. 4a). Additionally, there was a significant correlation between the percentage of TCRVB4 and the level of anti-SMEZ-2 titre (ρ = 0·606, P = 0·0045, Fig. 4b), and the levels of ASO antibody and anti-SMEZ-2 titre (ρ = 0·456, P = 0·0227) (Fig. 4c). In contrast, there was no significant correlation between the percentage TCRVB4 and the level of antibodies against TSST-1 (data not presented).
Fig. 4.
Elevated anti-streptococcal mitogenic exotoxin Z-2 (SMEZ-2) antibody in patients with active ulcerative colitis (UC) who had skewed T cell receptor β-chain 4 (TCRBV4). (a) The anti-SMEZ-2 titre was significantly higher in patients with skewed TCRBV4 compared with healthy volunteers (P = 0·0305, by Mann–Whitney U-test). (b) The correlation of the percentage of TCRBV4 with anti-SMEZ-2 titre (n = 23, ρ= 0·606, P = 0·0045, by Spearman's rank correlation). The anti-streptolysin-O (ASO) level (c) also showed significant correlation with the anti-SMEZ-2 titre (n = 27, ρ= 0·352, P = 0·0149, by Spearman's rank correlation test), but no correlation with anti-toxic shock syndrome toxin 1 (TSST-1) titre was seen.
Expansion of intestinal TCRBV in patients with UC
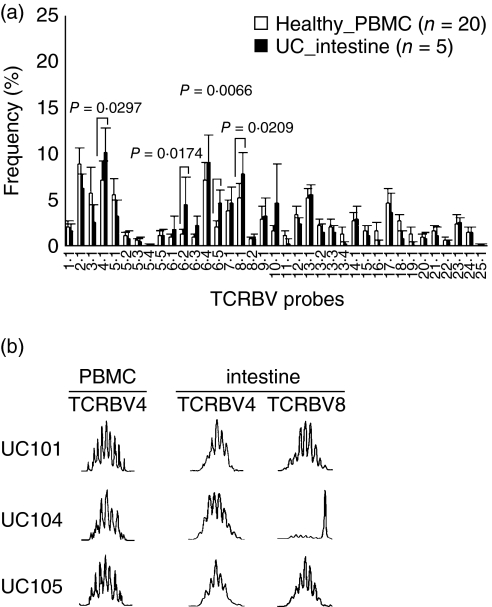
The expansion of TCRBV in pairs of PBMC and intestinal mucosa from an additional five patients was investigated for the polyclonal expansion of TCRBV4-bearing T cells, as the phenomenon observed in PBMC. The percentage frequencies of TCRBV4, BV6·2, BV6·5 and BV8 in the intestinal biopsy samples from UC patients were significantly higher than the level in PBMC specimens from healthy donors (Fig. 5a). The mean percentage frequencies were 10·3%, 4·6%, 4·7% and 7·9%, respectively. There was no significant increase in any other subfamily.
Fig. 5.
Polyclonal expansion of T cell receptor β-chain 4 (TCRBV4) and TCRBV8-bearing T cells within the intestinal mucosa of patients with active ulcerative colitis (UC). (a) The percentage of TCRBV4, BV6·2, BV6·5 and BV8 in intestinal samples from patients with UC were significantly higher than the level in the peripheral blood mononuclear cells (PBMC) of healthy individuals (P = 0·0297, P = 0·0174, P = 0·0066 and P = 0·0209, respectively). Mean ± s.d. values are presented; the P-values are by Mann–Whitney U-test. (b), CDR3 size spectratype profiles of TCRBV gene rearrangements. Three patients who had skewed TCRBV4 within the intestinal mucosa were selected for spectratyping. On the TCRBV4 and TCRBV8 genes, these three subjects had 6–8 peaks with Gaussian-like distribution and spectratype profile except on TCRBV8 in UC104, showing polyclonal expansion.
CDR3 size spectratyping was performed in three patients, UC-101, UC-104 and UC-105, who showed skewed TCRBV4 in PBMC. Similar to the results in PBMC, TCRBV4 and BV8-bearing T cells within local mucosal lesions had multiple Gaussian-like patterns with three nucleotide intervals indicating polyclonal expansion in these patients, except on TCRBV8 within intestinal T cells in UC104 (Fig. 5b). These observations implied that intestinal T cells might also have been exposed to SMEZ-2. In contrast, a few peaks, indicating oligoclonal expansion of T cells, were detected in TCRBV6·2- and TCRBV6·5-bearing T cells within the intestinal mucosa from these three patients (data not presented).
Association of TCRBV4 skewing with UC duration
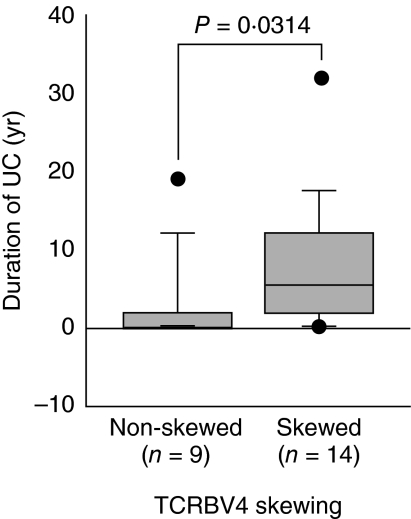
We wished to investigate any probable relationship between TCRBV4 skewing and the UC disease parameters. There was no association between CAI and the percentage TCRBV4. However, there was a significant (P = 0·0314) association between UC disease duration and the TCRBV4 skewing (Fig. 6).
Fig. 6.
Significant (P = 0·0314, by Mann–Whitney U-test) association between duration of ulcerative colitis and T cell receptor β-chain 4 (TCRBV4) skewing. Twenty-three patients were classified into two subgroups based on the skewing of TCRBV4. The skewing was defined to be significant if the percentage frequency of the relevant T cells was greater than 5%, and exceeded the mean percentage plus 3 s.d. of the T cells bearing the corresponding TCR in 20 healthy controls.
Discussion
T cells are believed to be involved intimately in the regulation of the immune function in patients with IBD [20,21,38,39], while injury to the mucosal tissue is caused mainly by granulocytes and monocytes/macrophages [1,40,41]. Hence dysregulated immune activity seen in patients with UC might reflect abnormal T cell behaviour and bacterial SA appear to be potential factors for dysregulated T cells. Accordingly, this study was to further understanding on T cell features associated with UC, whether the TCR repertoire of T cells is biased in patients with UC, and to what extent bacterial SA are involved. Certainly, a better understanding of T cell involvement in the immunopathogenesis of UC is desirable and should be valuable for designing effective therapeutic interventions.
Initially, we performed TCR repertoire analysis on PBMC from patients with active UC because this condition is characterized by multiple systemic clinical manifestations and activated T cells are known to increase not only in the inflamed intestinal mucosa but also in the peripheral blood [42], albeit the total peripheral blood lymphocyte count appears to be compromised in patients with UC [39,43,44]. We found an increase in the percentage of T cells expressing TCRBV4, which appears to be a polyclonal expansion of PBMC in many patients with UC. This specific TCRBV and polyclonal expansion of T cells suggests involvement of bacterial SA in the immunopathogenesis of UC. In line with this thinking, it is widely known that bacterial SA can activate TCRBV-bearing T cells without any other specific antigen due to the cross-linking of HLA class II molecules and the specific interaction of SA with TCRBV. This can lead to systemic immune disorders [45,46]. It is also known that systemic immune disorders might trigger other immune-related episodes such as toxic shock syndrome, Kawasaki disease, psoriasis vulgaris and atopic dermatitis [29,47–49]. Accordingly, interaction of bacterial SA with TCRBV-bearing T cells should serve as an appropriate model to investigate mechanisms of immune disorders.
Staphylococci and Streptococci are common bacterial flora in the pharynx, the larynx, the paranasal sinus and the colon. SMEZ-2 produced by S. pyogenes and TSST-1 produced by Staph. aureus are known as SA, which activate preferentially the TCRBV4-bearing T cells [36,37]. Yang et al. reported that the clinical symptom score improved after the sinus was ablated in patients with both chronic rhinosinusitis and UC; these patients had been infected with Staph. aureus [50]. However, TSST-1 strongly stimulates TCRBV2-bearing T cells. By contrast, SMEZ-2 could strongly stimulate TCRBV4 and BV8-bearing T cells without the expansion of TCRBV2-bearing T cells [36]. Our in vitro study also showed an increase in the percentage frequency of TCRBV4 and BV8-bearing T cells without the expansion of TCRBV2-bearing T cells (by SMEZ-2 stimulation).
The level of antibody against SMEZ-2 in patients with the skewed TCRBV4 was significantly higher than the level in healthy volunteers. There was significant correlation between the ASO levels, the marker for the infection with S. pyogenes, and the level of antibodies against SMEZ-2 (although the ASO levels were mainly within the normal range). Moreover, the percentage frequency of TCRBV4-bearing T cells correlated with the level of antibodies against SMEZ-2, but did not correlate with the antibodies against TSST-1. These observations support a perception that many patients with UC are infected with S. pyogenes, which can specifically promote the expansion of TCRBV4-bearing T cells by its SA.
Interestingly, we found an increase in the percentage of TCTBV4-bearing T cells at the sites of inflamed intestinal mucosa, which were expanded polyclonally similar to the results in PBMC. Moreover, the expansion of TCRBV8-bearing T cells was also observed in the same patients. Previous reports have indicated that antigen-specific T cells exist within the intestinal mucosa not only in patients with IBD, but also in healthy adults [51–55]. The TCRBV4 repertoire is monoclonal within the intestinal mucosa even during infancy, although other TCRBV subfamilies show polyclonal behaviour [56]. Indeed, with our semiquantitative TCR repertoire and sequence analyses, we found that TCRBV6·2- and TCRBV6·5-bearing T cells had expanded within the intestinal mucosa and that these T cells had some common TCRBV-BJ motifs like those reported previously [51] (data not shown). Small amounts of SA might specifically stimulate TCRBV subfamilies and this could lead to the production of inflammatory cytokines, causing disordered host immune function. Such immune disorders might, in turn, cause exaggerated reaction to food antigens and/or self-antigens. In fact, we detected monoclonal or oligoclonal expansions of TCRBV6·5-bearing T cells in the inflamed intestinal tissue, suggesting that selective antigenic pressures are prevalent among activated intestinal T cells. Chott et al. [51] have shown that there is a common TCRBV-BJ motif within CD8− mucosal T cells and these may recognize common foreign antigens. Essentially, the findings reported by Chott and colleagues are in line with the perception that T cell abnormality is a feature in immune pathology. We have also detected some common TCRBV-BJ motifs within the mucosal T cells, such as TCRBV6S7-BJ2S7, in addition to polyclonal expansion of TCRBV4-bearing T cells not only within the mucosa but also in PBMC.
Based on the knowledge that within the lymphocyte population certain T cell subsets are major factors in the immune pathology of intestinal mucosa, while other subsets are essential regulators, controlling IBD [57], we were looking for any relationship between factors associated with skewed TCR repertoire and the UC disease, together with a special interest in the role of bacterial SA in any prevailing T cell behaviour. There was no significant correlation between the percentage frequency of TCRBV4 and CAI. However, when patients with skewed TCRBV4 and non-skewed TCRBV4 were compared with respect to the duration of UC, there was a very significant difference between the two subgroups. It was assumed that long-term exposure (even at a low dose) to bacterial SA such as SMEZ-2 promotes exacerbation, hypersensitivity reaction or exaggerated reaction to food antigens (or self-antigens) within the intestinal immune system and this might give rise to systemic immune disorders. Such exuberant immune activation might cause the remission–relapsing cycles seen in patients with UC (reflecting dysregulated T cell function). The long duration of disease, together with chronic immunosuppressive medication, might provide increased opportunity for infection. Future studies should strengthen our findings and increase understanding of the aetiology of IBD.
Acknowledgments
We thank our colleague Dr A. R. Saniabadi for editing the manuscript and Mr J. Sato for assisting with the sample processing work.
References
- 1.Allison M, Dhillon A, Lewis W, Pounder R. Pathogenesis of inflammatory bowel disease. In: Allison M, Dhillon A, Lewis W, Pounder R, editors. Inflammatory bowel disease. London: Mosby; 1998. pp. 15–95. [Google Scholar]
- 2.Ogunbi SO, Ransom JA, Sullivan K, Schoen BT, Gold BD. Inflammatory bowel disease in African-American children living in Georgia. J Pediatr. 1998;133:103–7. doi: 10.1016/s0022-3476(98)70187-8. [DOI] [PubMed] [Google Scholar]
- 3.Reddy SI, Burakoff R. Inflammatory bowel disease in African Americans. Inflamm Bowel Dis. 2003;9:380–5. doi: 10.1097/00054725-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Robison WW, Bentlif PS, Kelsey JR., Jr Observations on 261 consecutive patients with inflammatory bowel disease seen in the Southwest United States. Dig Dis Sci. 1980;25:198–204. doi: 10.1007/BF01308139. [DOI] [PubMed] [Google Scholar]
- 5.Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92:S5–11. [PubMed] [Google Scholar]
- 6.Conte MP, Schippa S, Zamboni I, et al. Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut. 2006;55:1760–7. doi: 10.1136/gut.2005.078824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obermeier F, Dunger N, Deml L, Herfarth H, Scholmerich J, Falk W. CpG motifs of bacterial DNA exacerbate colitis of dextran sulfate sodium-treated mice. Eur J Immunol. 2002;32:2084–92. doi: 10.1002/1521-4141(200207)32:7<2084::AID-IMMU2084>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 8.Achkar JP, Dassopoulos T, Silverberg MS, et al. Phenotype-stratified genetic linkage study demonstrates that IBD2 is an extensive ulcerative colitis locus. Am J Gastroenterol. 2006;101:572–80. doi: 10.1111/j.1572-0241.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 9.Bregenholt S, Reimann J, Claesson MH. Proliferation and apoptosis of lamina propria CD4+ T cells from scid mice with inflammatory bowel disease. Eur J Immunol. 1998;28:3655–63. doi: 10.1002/(SICI)1521-4141(199811)28:11<3655::AID-IMMU3655>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Reuter BK, Pizarro TT. Commentary: the role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe? Eur J Immunol. 2004;34:2347–55. doi: 10.1002/eji.200425351. [DOI] [PubMed] [Google Scholar]
- 11.Schreiber S, MacDermott RP, Raedler A, Pinnau R, Bertovich MJ, Nash GS. Increased activation of isolated intestinal lamina propria mononuclear cells in inflammatory bowel disease. Gastroenterology. 1991;101:1020–30. doi: 10.1016/0016-5085(91)90729-5. [DOI] [PubMed] [Google Scholar]
- 12.Choy MY, Walker-Smith JA, Williams CB, MacDonald TT. Differential expression of CD25 (interleukin-2 receptor) on lamina propria T cells and macrophages in the intestinal lesions in Crohn's disease and ulcerative colitis. Gut. 1990;31:1365–70. doi: 10.1136/gut.31.12.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi K, Asakura H, Hamada Y, et al. T lymphocyte subpopulations and immunoglobulin-containing cells in the colonic mucosa of ulcerative colitis; a morphometric and immunohistochemical study. J Clin Lab Immunol. 1988;25:63–8. [PubMed] [Google Scholar]
- 14.Rudolphi A, Bonhagen K, Reimann J. Polyclonal expansion of adoptively transferred CD4+ alpha beta T cells in the colonic lamina propria of scid mice with colitis. Eur J Immunol. 1996;26:1156–63. doi: 10.1002/eji.1830260529. [DOI] [PubMed] [Google Scholar]
- 15.Simpson SJ, Hollander GA, Mizoguchi E, et al. Expression of pro-inflammatory cytokines by TCR alpha beta+ and TCR gamma delta+ T cells in an experimental model of colitis. Eur J Immunol. 1997;27:17–25. doi: 10.1002/eji.1830270104. [DOI] [PubMed] [Google Scholar]
- 16.Brandtzaeg P. Inflammatory bowel disease: clinics and pathology. Do inflammatory bowel disease and periodontal disease have similar immunopathogeneses? Acta Odontol Scand. 2001;59:235–43. doi: 10.1080/00016350152509265. [DOI] [PubMed] [Google Scholar]
- 17.Saubermann LJ, Probert CS, Christ AD, et al. Evidence of T cell receptor beta-chain patterns in inflammatory and noninflammatory bowel disease states. Am J Physiol. 1999;276:G613–21. doi: 10.1152/ajpgi.1999.276.3.G613. [DOI] [PubMed] [Google Scholar]
- 18.Shanahan F. Physiological basis for novel drug therapies used to treat the inflammatory bowel diseases I. Pathophysiological basis and prospects for probiotic therapy in inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2005;288:G417–21. doi: 10.1152/ajpgi.00421.2004. [DOI] [PubMed] [Google Scholar]
- 19.Hanauer SB. Can cyclosporine go it alone in severe ulcerative colitis? Curr Gastroenterol Rep. 2001;3:455–6. [PubMed] [Google Scholar]
- 20.Powrie F, Read S, Mottet C, Uhlig H, Maloy K. Control of immune pathology by regulatory T cells. Novartis Found Symp. 2003;252:92–8. discussion 6–14, 8–105. [PubMed] [Google Scholar]
- 21.Kanai T, Watanabe M. Clinical application of human CD4+ CD25+ regulatory T cells for the treatment of inflammatory bowel diseases. Exp Opin Biol Ther. 2005;5:451–62. doi: 10.1517/14712598.5.4.451. [DOI] [PubMed] [Google Scholar]
- 22.Ehrich EW, Devaux B, Rock EP, Jorgensen JL, Davis MM, Chien YH. T cell receptor interaction with peptide/major histocompatibility complex (MHC) and superantigen/MHC ligands is dominated by antigen. J Exp Med. 1993;178:713–22. doi: 10.1084/jem.178.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 24.Engel I, Hedrick SM. Site-directed mutations in the VDJ junctional region of a T cell receptor beta chain cause changes in antigenic peptide recognition. Cell. 1988;54:473–84. doi: 10.1016/0092-8674(88)90068-2. [DOI] [PubMed] [Google Scholar]
- 25.Gorski J, Yassai M, Zhu X, et al. Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDR3 size spectratyping. Correlation with immune status. J Immunol. 1994;152:5109–19. [PubMed] [Google Scholar]
- 26.Puisieux I, Even J, Pannetier C, Jotereau F, Favrot M, Kourilsky P. Oligoclonality of tumor-infiltrating lymphocytes from human melanomas. J Immunol. 1994;153:2807–18. [PubMed] [Google Scholar]
- 27.Matsutani T, Shiiba K, Yoshioka T, et al. Evidence for existence of oligoclonal tumor-infiltrating lymphocytes and predominant production of T helper 1/T cytotoxic 1 type cytokines in gastric and colorectal tumors. Int J Oncol. 2004;25:133–41. [PubMed] [Google Scholar]
- 28.Wagner U, Pierer M, Kaltenhauser S, et al. Clonally expanded CD4+CD28null T cells in rheumatoid arthritis use distinct combinations of T cell receptor BV and BJ elements. Eur J Immunol. 2003;33:79–84. doi: 10.1002/immu.200390010. [DOI] [PubMed] [Google Scholar]
- 29.Yoshioka T, Matsutani T, Toyosaki-Maeda T, et al. Relation of streptococcal pyrogenic exotoxin C as a causative superantigen for Kawasaki disease. Pediatr Res. 2003;53:403–10. doi: 10.1203/01.PDR.0000049668.54870.50. [DOI] [PubMed] [Google Scholar]
- 30.Matsutani T, Yoshioka T, Tsuruta Y, Iwagami S, Suzuki R. Analysis of TCRAV and TCRBV repertoires in healthy individuals by microplate hybridization assay. Hum Immunol. 1997;56:57–69. doi: 10.1016/s0198-8859(97)00102-x. [DOI] [PubMed] [Google Scholar]
- 31.Horiuchi T, Hirokawa M, Kawabata Y, et al. Identification of the T cell clones expanding within both CD8(+) CD28(+) and CD8(+) CD28(–) T cell subsets in recipients of allogeneic hematopoietic cell grafts and its implication in post-transplant skewing of T cell receptor repertoire. Bone Marrow Transplant. 2001;27:731–9. doi: 10.1038/sj.bmt.1702859. [DOI] [PubMed] [Google Scholar]
- 32.Lichtiger S, Present DH. Preliminary report: cyclosporin in treatment of severe active ulcerative colitis. Lancet. 1990;336:16–9. doi: 10.1016/0140-6736(90)91521-b. [DOI] [PubMed] [Google Scholar]
- 33.Futami S, Aoyama N, Honsako Y, et al. HLA-DRB1*1502 allele, subtype of DR15, is associated with susceptibility to ulcerative colitis and its progression. Dig Dis Sci. 1995;40:814–8. doi: 10.1007/BF02064985. [DOI] [PubMed] [Google Scholar]
- 34.Myung SJ, Yang SK, Jung HY, et al. HLA-DRB1*1502 confers susceptibility to ulcerative colitis, but is negatively associated with its intractability: a Korean study. Int J Colorect Dis. 2002;17:233–7. doi: 10.1007/s00384-001-0381-4. [DOI] [PubMed] [Google Scholar]
- 35.Hirokawa M, Horiuchi T, Kitabayashi A, et al. Delayed recovery of CDR3 complexity of the T-cell receptor-beta chain in recipients of allogeneic bone marrow transplants who had virus-associated interstitial pneumonia: monitor of T-cell function by CDR3 spectratyping. J Allergy Clin Immunol. 2000;106:S32–9. doi: 10.1067/mai.2000.106638. [DOI] [PubMed] [Google Scholar]
- 36.Proft T, Moffatt SL, Berkahn CJ, Fraser JD. Identification and characterization of novel superantigens from Streptococcus pyogenes. J Exp Med. 1999;189:89–102. doi: 10.1084/jem.189.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi N. Superantigen in pediatrics. J Jpn Pediatr Soc. 2003;107:1597–607. [Google Scholar]
- 38.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–77. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 39.Heimann TM, Aufses AH., Jr The role of peripheral lymphocytes in the prediction of recurrence in Crohn's disease. Surg Gynecol Obstet. 1985;160:295–8. [PubMed] [Google Scholar]
- 40.Mahida YR. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2000;6:21–33. doi: 10.1097/00054725-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Saniabadi AR, Hanai H, Suzuki Y, et al. Adacolumn for selective leukocytapheresis as a non-pharmacological treatment for patients with disorders of the immune system: an adjunct or an alternative to drug therapy? J Clin Apher. 2005;20:171–84. doi: 10.1002/jca.20046. [DOI] [PubMed] [Google Scholar]
- 42.Kirman I, Nielsen OH, Kjaersgaard E, Brynskov J. Interleukin-2 receptor alpha and beta chain expression by circulating alpha beta and gamma delta T cells in inflammatory bowel disease. Dig Dis Sci. 1995;40:291–5. doi: 10.1007/BF02065412. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki Y, Yoshimura N, Saniabadi AR, Saito Y. Selective granulocyte and monocyte adsorptive apheresis as a first-line treatment for steroid naive patients with active ulcerative colitis: a prospective uncontrolled study. Dig Dis Sci. 2004;49:565–71. doi: 10.1023/b:ddas.0000026299.43792.ae. [DOI] [PubMed] [Google Scholar]
- 44.Aoki A, Nakamura K, Yoshimatsu Y, Shirai K, Suzuki Y. Adacolumn selective leukocyte adsorption apheresis in patients with active ulcerative colitis: clinical efficacy, effects on plasma IL-8 and the expression of toll like receptor 2 on granulocytes. Dig Dis Sci. 2007;52:1427–33. doi: 10.1007/s10620-006-9406-8. [DOI] [PubMed] [Google Scholar]
- 45.Baker MD, Acharya KR. Superantigens: structure–function relationships. Int J Med Microbiol. 2004;293:529–37. doi: 10.1078/1438-4221-00298. [DOI] [PubMed] [Google Scholar]
- 46.Fraser J, Arcus V, Kong P, Baker E, Proft T. Superantigens − powerful modifiers of the immune system. Mol Med Today. 2000;6:125–32. doi: 10.1016/s1357-4310(99)01657-3. [DOI] [PubMed] [Google Scholar]
- 47.Bergdoll MS, Reiser RF, Crass BA, Robbins RN, Thompson NE. Toxic shock syndrome − the role of the toxin. Postgrad Med J. 1985;61(Suppl. 1):35–8. [PubMed] [Google Scholar]
- 48.Diluvio L, Vollmer S, Besgen P, Ellwart JW, Chimenti S, Prinz JC. Identical TCR beta-chain rearrangements in streptococcal angina and skin lesions of patients with psoriasis vulgaris. J Immunol. 2006;176:7104–11. doi: 10.4049/jimmunol.176.11.7104. [DOI] [PubMed] [Google Scholar]
- 49.Leung DY, Hauk P, Strickland I, Travers JB, Norris DA. The role of superantigens in human diseases: therapeutic implications for the treatment of skin diseases. Br J Dermatol. 1998;139(Suppl. 53):17–29. doi: 10.1046/j.1365-2133.1998.1390s3017.x. [DOI] [PubMed] [Google Scholar]
- 50.Yang PC, Liu T, Wang BQ, et al. Rhinosinusitis derived Staphylococcal enterotoxin B possibly associates with pathogenesis of ulcerative colitis. BMC Gastroenterol. 2005;5:28. doi: 10.1186/1471-230X-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balk SP, Ebert EC, Blumenthal RL, et al. Oligoclonal expansion and CD1 recognition by human intestinal intraepithelial lymphocytes. Science. 1991;253:1411–5. doi: 10.1126/science.1716785. [DOI] [PubMed] [Google Scholar]
- 52.Chott A, Probert CS, Gross GG, Blumberg RS, Balk SP. A common TCR beta-chain expressed by CD8+ intestinal mucosa T cells in ulcerative colitis. J Immunol. 1996;156:3024–35. [PubMed] [Google Scholar]
- 53.Gulwani-Akolkar B, Akolkar PN, McKinley M, Fisher SE, Silver J. Crohn's disease is accompanied by changes in the CD4+, but not CD8+, T cell receptor BV repertoire of lamina propria lymphocytes. Clin Immunol Immunopathol. 1995;77:95–106. doi: 10.1016/0090-1229(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 54.Gulwani-Akolkar B, Akolkar PN, Minassian A, McKinley M, Fisher S, Silver J. CD4+ cell oligoclonality in Crohn's disease: evidence for an antigen-specific response. Hum Immunol. 1996;48:114–24. doi: 10.1016/0198-8859(96)00079-1. [DOI] [PubMed] [Google Scholar]
- 55.Van Kerckhove C, Russell GJ, Deusch K, et al. Oligoclonality of human intestinal intraepithelial T cells. J Exp Med. 1992;175:57–63. doi: 10.1084/jem.175.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams AM, Bland PW, Phillips AC, et al. Intestinal alpha beta T cells differentiate and rearrange antigen receptor genes in situ in the human infant. J Immunol. 2004;173:7190–9. doi: 10.4049/jimmunol.173.12.7190. [DOI] [PubMed] [Google Scholar]
- 57.Yokoyama Y, Fukunaga K, Fukuda Y, et al. Demonstration of low-regulatory CD25(high+) CD4(+) and high-pro-inflammatory CD28(–) CD4(+) T-cell subsets in patients with ulcerative colitis: modified by selective granulocyte and monocyte adsorption apheresis. Dig Dis Sci. 2007. in press. [DOI] [PubMed]