Abstract
Natrium aurothiomaleate (GSTM) is a useful disease-modifying anti-rheumatic drug, but causes a variety of immune-mediated adverse effects in many patients. A murine model was used to study further the interaction of GSTM with the immune system, including induction of systemic autoimmunity. Mice were given weekly intramuscular injections of GSTM and controls equimolar amounts of sodium thiomaleate. The effects of gold on lymphocyte subpopulations were determined by flow cytometry. Humoral autoimmunity was measured by indirect immunofluorescence and immunoblotting, and deposition of immunoglobulin and C3 used to assess immunopathology. Gold, in the form of GSTM, stimulated the murine immune system causing strain-dependent lymphoproliferation and autoimmunity, including a major histocompatibility complex (MHC)-restricted autoantibody response against the nucleolar protein fibrillarin. GSTM did not cause glomerular or vessel wall IgG deposits. However, it did elicit a strong B cell-stimulating effect, including both T helper 1 (Th1)- and Th2-dependent isotypes. All these effects on the immune system were dependent on the MHC genotype, emphasizing the clinical observations of a strong genetic linkage for the major adverse immune reactions seen with GSTM treatment.
Keywords: ANoA, autoimmunity, mice, natriumaurothiomaleate
Introduction
Autoimmune diseases are controlled both by genetic predisposition and environmental factors which may influence the immune system as well as self-epitopes. Epidemiological and experimental studies have lent support for the role of environmental factors, including microbial agents and xenobiotics in human autoimmune diseases [1]. A good example of the former is coxsackie-virus-induced myocarditis [2]. A recent example underscoring the importance of the latter is primary biliary cirrhosis, characterized by antibodies to E2 subunits of the mitochondrial 2-oxo-acid dehydrogenase complexes (2-OADC-E2). The anti-mitochondrial antibodies in these patients react strongly with peptide antigens of 2-OADC-E2 modified by 2-nonyoic acid, a component in cosmetics [3], which has been suggested to be able to break T cell tolerance to the native autoantigen [4]. Other well-known examples of xenobiotic-associated autoimmune diseases are scleroderma with the organic solvent vinyl chloride, silica, and the drug bleomycin [1], as well as the connective tissue type of disease in the ‘toxic oil syndrome’ [5,6].
Another class of xenobiotics with autoimmune effects is metals, with mercury as a prime example [7,8]. Gold (Au) is another metal with well-known and diverse interactions with the immune system, resulting in stimulatory and suppressive responses. Gold is one of the most prevalent (15%) skin sensitizers in humans, although clinical relevance is observed in less than 1% of cases [9]. In contrast to this immunostimulatory effect, gold has been successfully used for more than 70 years as an immunosuppressive in patients with rheumatoid arthritis [10,11].
The immunosuppressive effect of gold(I) compounds, such as natrium aurothiomaleate (GSTM) and auranofin, has been explained by inhibition of the release of proinflammatory cytokines such as interleukin (IL)-1β, IL-6 and tumour necrosis factor (TNF)-α [12,13]. However, recent studies have revealed a much more complex effect, resulting in up- or down-regulation of cytokine production, especially when gold is present in conjunction with cell activators such as TNF-α or lipopolysaccharide (LPS) [14–16]. B cells are more susceptible than T cells to the direct suppressive effect of gold(I) [17]. Gold may inhibit T cell activation efficiently in vitro by interfering with IL-2-mediated proliferation [18,19], but also indirectly by binding to cysteine residues in the target antigen prohibiting its presentation to T cells [20,21].
The use of gold as a therapeutic agent is often complicated by the development of adverse immune reactions including glomerulonephritis, cytopenias and hypoglobulinaemia with relative sparing of Th2 isotypes [22], hepatitis and pneumonitis, as well as systemic reactions with skin rash, fever and lymphadenopathy [23]. The thrombocytopenia is caused by anti-platelet antibodies [24], and membranous glomerulonephritis is immune-complex-mediated [25]. Susceptibility to many of these adverse effects is linked to genes within the major histocompatibility complex (MHC) [26–28], and these effects necessitate discontinuation of gold therapy in up to one-third of the patients [23].
These clinical observations have stimulated studies with gold(I) compounds in rodents as a model of human immune responses. Brown Norway rats develop, during gold treatment, an autoimmune syndrome with Th2-restricted hyperimmunoglobulinaemia, anti-laminin and anti-DNA antibodies, vessel wall immune deposits and a biphasic immune-mediated glomerulonephritis [29], features identical to those induced by mercury in the same strain. In contrast, the Lewis strain is resistant to the effect of both gold and mercury [30]. Recently, quantitative trait factors for the gold-induced increase in IgE, development of autoantibodies and renal immune deposits were localized to chromosomes 9, 10 and 20 of the rat [31,32].
Gold-induced antinuclear antibodies were first described in mice [33], and considerable effort was focused on the T cell mechanisms involved [34,35]. While autoimmune manifestations in gold-treated mice have often been alluded to in review articles, the numbers of original publications are few. This study aimed at assessing the autoimmune syndrome induced by gold in mice in order to make a comparison with the syndrome induced by mercury and silver, and to increase the understanding of adverse immune-mediated effects observed after anti-rheumatic treatment with gold.
Materials and methods
Mice
Female mice, aged 8–12 weeks at the beginning of the experiment, were used throughout the study. SJL/N and A.SW (H-2s) mice were obtained from Bomholtgaard Breeding and Research Centre (Ry, Denmark), A.TH (H-2t2) and A.TL (H-2t1) mice from Harlan Ltd (Bicester, Oxon, UK). Mice were kept in steel-wire cages in a high barrier unit under 12-h dark−12-h light cycles and given sterilized food pellets (type R36; Lactamin, Vadstena, Sweden) and water ad libitum.
Treatment protocol
Sodium aurothiomaleate (GSTM) was a gift from Rhone-Poulenc Rorer (Dagenham, Essex, UK), and sodium thiomaleate (TM) was obtained from Sigma Chemical Co. (St Louis, MO, USA). The substances were diluted in phosphate-buffered saline (PBS), and each mouse received weekly intramuscular injections of 0·1 ml containing 0·45 mg GSTM/mouse [about 22·5 mg/kg body weight (bw)], or an equimolar dose of TM corresponding 0·21 mg TM/mouse (about 10·6 mg/kg bw) for 12 weeks.
Blood sampling
Blood was obtained from the retro-orbital plexus during the week before onset of treatment, after 5 weeks treatment and at sacrifice after 12 weeks' treatment. The collected blood was allowed to clot, centrifuged at 500 g for 10 min, and the serum stored at −70°C.
Analysis of anti-nuclear antibodies by IF
The presence, pattern and titre of serum anti-nuclear antibodies of the IgG class were determined by indirect immunofluorescence using HEp-2 cells as a substrate [36].
Analysis of anti-nuclear antibodies by immunoblotting
The specificity of the anti-nuclear antibodies in the serum was assessed by immunoblotting as described previously [37], with minor modifications. Briefly, mouse liver nuclei were isolated [38], and aliquots of boiled nuclei were sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) separated using a 12·5% gel. The electrophoretic transfer to 0·45-µm nitrocellulose membranes (Bio-Rad Laboratory, Hercules, CA, USA) was performed for 1 h at 0·8 mA/cm2 under water cooling (Criterion Blotter; Bio-Rad Laboratory). Nitrocellulose strips were blocked in a Tris-buffered solution (TBS)-5% non-fat dry milk (blotting grade; Bio-Rad Laboratory)-0·05% Tween 20 overnight at 4°C before being incubated with sera diluted 200-fold in TBS-Tween. Bound murine IgG antibody was detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Southern Biotechnology, Birmingham, AL, USA) diluted 1 : 5000, followed by enhanced chemiluminescence (ECL Western blotting detection reagents; Amersham, Stockholm, Sweden). For human sera bound IgG was detected with appropriately diluted HRP-conjugated goat anti-mouse IgG, followed by ECL as above. Human reference sera specific for RNP/Sm, Sm or fibrillarin were used (Binding Site, Birmingham, UK).
Protocol for cell preparation
The spleen was removed at sacrifice after 12 weeks' treatment in A.SW and A.TL mice, and single-cell suspensions prepared in RPMI-1640 (Gibco, Paisley, UK) by teasing the spleen through a mesh wire followed by repeated pipetting and centrifugation at 300 g for 10 min. All preparation steps were performed on ice using media containing 0·01% NaN3.
Preparation of cells for flow cytometry
The single-cell suspensions of splenocytes from A.SW and A.TL mice were subjected to lysis of erythrocytes by resuspending the cells in 1 ml 0·84% NH4Cl for 2 min, followed by the immediate addition of 10 volumes of RPMI-1640, two washings in RPMI-1640 and finally resuspension in RPMI-1640 containing 10% fetal calf serum (FCS) (Myoclone; Gibco). The cells were then counted in a haematocytometer, and the cell count adjusted to 10–20 × 106 mononuclear cells/ml 100 µl of the cell suspension was incubated with 100 µl of appropriately diluted monoclonal antibodies (mAb) conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) or biotin targeting pan-T cells CD3 (clone 145–2C11), T helper cells CD4 (RM4-5), cytotoxic T cells CD8a (53–6·7), or pan-B cell marker B220 (RA3–6B2) or isotype controls for 30 min. When biotin-conjugated mAbs were used, streptavidin-FITC or streptavidin-PE was added for 30 min after a single wash in RPMI-1640 FCS. After a final wash in RPMI-1640 FCS, the cells were resuspended in 1 ml PBS and analysed using a FACScan (BD, Mountain View, CA, USA) equipped with a 488-nm argon laser. Only living cells were acquired, dead cells gated away using propidium iodide. After acquisition in list mode, analysis was performed with the lysys ii program.
Cytospin preparation and immunofluorescence
A fraction of the single-cell splenocyte suspensions from A.SW and A.TL mice was subjected to separation by density centrifugation using Lympholyte-M (Cedarlane, Ontario, Canada), and the mononuclear cell population was centrifuged onto glass slides at 80 g for 7 min (Cytospin; Shandon, Cheshire, UK) and allowed to dry at room temperature. The slides were then fixed in absolute ethanol containing 5% glacial acetic acid at −20°C for 20 min and stored in PBS at 4° until further analysed. The cells were incubated subsequently with biotin-conjugated rat mAb anti-mouse IgG1, IgG2a, IgG2b, IgE, IgM (Pharmingen) or FITC-conjugated goat anti-mouse IgG3 Ab (Southern Biotechnology, Birmingham, AL, USA) diluted with Hank's balanced salt solution containing 2% FCS for 20 min, washed in PBS and viewed in a fluorescence microscope. The number of brightly stained cells among the first 200 counted lymphocytes was noted for each slide.
Tissue immune deposits
Pieces of the left kidney and the spleen were examined by immunofluorescence as described before [39], using FITC-conjugated goat anti-mouse IgG and IgM antibodies (Southern Biotechnology) and anti-C3c antibodies (Organon-Technica, West Chester, PA, USA). The titre was determined by serial dilution of the antibodies to 1 : 5120. The end-point titre of the deposits was defined as the highest dilution of antibody at which a specific fluorescence could be detected. When no specific fluorescence was detected at a dilution of 1 : 40, the result was recorded as ‘0’.
Statistics
Statistical analyses were performed using GraphPad software (GraphPad Software Inc.; San Diego, CA, USA). The Mann–Whitney U-test was used to study differences in parameters between GSTM- and TM-treated mice. The difference between the numbers of ANoA-positive mice in the different strains was tested by Fisher's exact test.
Results
Anti-nuclear serum autoantibodies of the IgG isotype
Before onset of treatment with TM or GSTM none of the mice showed ANoA. After 5 weeks' treatment with GSTM, 55% of SJL, 91% of A.SW and 83% of A.TH mice (Table 1) showed a modest titre of IgG antibodies fulfilling the criteria for a clumpy nucleolar pattern (Fig. 1), which often included a weak homogeneous staining of the nucleoplasm and weak staining of the condensed chromosomes [40]. GSTM-treated A.TL mice did not develop any ANoA.
Table 1.
Serum IgG anti-nucleolar antibodies (ANoA).
| ANoA | |||||
|---|---|---|---|---|---|
| Strain | H-2 | Treatment | Treatment time (weeks) | Fraction (pos./total) | Titrea (mean ± s.e.m.) |
| SJL | s | TM | 0 | 0/6 | 0 |
| 5b | 0/5 | 0 | |||
| 12 | 0/5 | 0 | |||
| GSTM | 0 | 0/11 | 0 | ||
| 5 | 6/11c | 310 ± 119 | |||
| 12 | 6/11c | 187 ± 92 | |||
| A.SW | s | TM | 0 | 0/8 | 0 |
| 5 | 0/8 | 0 | |||
| 12 | 0/8 | 0 | |||
| GSTM | 0 | 0/11 | 0 | ||
| 5 | 10/11d | 184 ± 32e | |||
| 12 | 9/11d | 604 ± 273e | |||
| A.TH | t2 | TM | 0 | 0/7 | 0 |
| 5 | 0/7 | 0 | |||
| 12 | 0/7 | 0 | |||
| GSTM | 0 | 0/6 | 0 | ||
| 5 | 5/6c | 192 ± 54f | |||
| 12 | 5/6c | 272 ± 100f | |||
| A.TL | t1 | TM | 0 | 0/7 | 0 |
| 5 | 0/7 | 0 | |||
| 12 | 0/7 | 0 | |||
| GSTM | 0 | 0/7 | 0 | ||
| 5 | 0/7 | 0 | |||
| 12 | 0/7 | 0 | |||
Titre in positive mice.
One mouse died after 3 weeks.
P < 0·05 significantly different from pretreated (0) mice (Fisher's exact test).
P < 0·001 significantly different from pretreated (0) mice (Fisher's exact test).
P < 0·005 significantly different from pretreated (0) mice (Mann–Whitney U-test).
P < 0·05 significantly different from pretreated (0) mice (Mann–Whitney U-test). TM, treatment with a weekly intramuscular (i.m.) injection of 10·6 mg sodium thiomaleate/kg body weight (bw). Natrium aurothiomaleate (GSTM), treatment with a weekly i.m. injection of 22·5 mg sodium aurothiomaleate/kg bw.
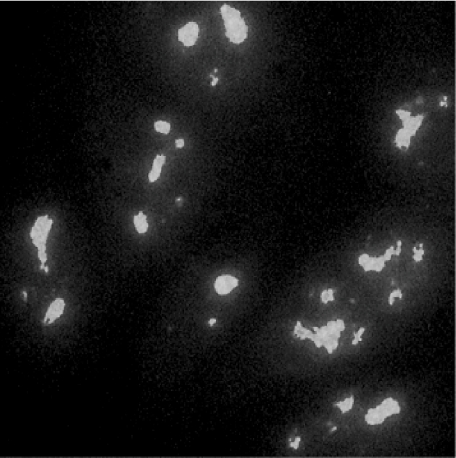
Fig. 1.
Serum from an A.SW mouse treated with natrium aurothiomaleate (GSTM) for 12 weeks incubated on HEp-2 cells, followed by incubation with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG antibodies. Strong nucleolar staining with nuclear dots.
After 12 weeks' GSTM treatment the fraction of ANoA-positive mice was 82% in the A.SW strain and unchanged in the SJL and A.TH strains compared with the situation after 5 weeks (Table 1). A.TL mice remained negative. Nine per cent of A.TH mice treated with GSTM had a combined speckled, nucleolar and nucleoplasmic staining (Fig. 2).
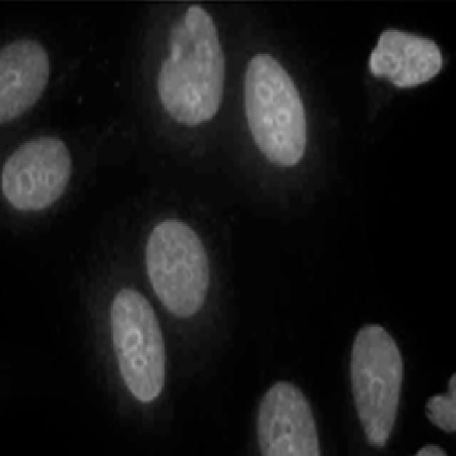
Fig. 2.
Serum from an A.TH mouse treated with natrium aurothiomaleate (GSTM) for 12 weeks incubated on HEp-2 cells, followed by incubation with fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG antibodies. Combined speckled, nucleolar and nucleoplasmic staining.
Immunoblotting of serum autoantibodies
Sera from mice with the H-2As haplotype (SJL, A.SW, A.TH), treated with GSTM for 12 weeks and showing ANoA, reacted with a 34-kDa nuclear protein (Fig. 3, lanes 7–17), corresponding to the reactivity of a human AFA reference serum (Fig. 2, lane 2). Serum from a single SJL mouse treated with GSTM reacted with the 34-kDa and additional proteins of approximately 45 and 16 kDa (Fig. 3, lane 7). The single GSTM-treated A.TH mouse with a combined nucleolar, nucleoplasmic and speckled anti-nuclear antibody (ANA) pattern reacted with proteins of an apparent molecular weight of 28–30 kDa (double band), 16 kDa and 34 kDa (Fig. 3, lane 16). TM-treated H-2As mice lacked ANoA and did not react with any protein in the immunoblot (Fig. 3, lanes 3–6, 18).
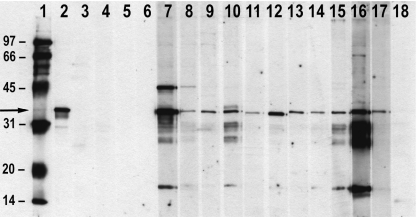
Fig. 3.
Immunoblotting of sera using sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) separated mouse liver nuclei. Lane 1: molecular weight markers (kDa). Lane 2: human reference serum blotting the 34-kDa protein fibrillarin (arrow). Lanes 3–4 and 5–6: anti-nuclear antibody (ANA)-negative SJL and A.SW mice treated with sodium thiomaleate (TM) for 12 weeks, respectively. No blotting. Lanes 7–12 and 13–15: anti-nucleolar antibodies (ANoA)-positive SJL and A.SW mice, respectively, treated with natrium aurothiomaleate (GSTM) for 12 weeks, respectively. Strong blotting of a 34-kDa protein. Lane 16: A.TH mouse treated with GSTM for 12 weeks showing a combined speckled, nucleoplasmic and nucleolar ANA pattern. Multiple staining bands (see Results). Lane 17: A.TH mouse treated with GSTM for 12 weeks showing a strong nucleolar staining. Blotting of a 34-kDa protein. Lane 18: A.TH mouse treated with TM for 12 weeks, ANA-negative. No blotting.
Tissue immune deposits
Only marginal titres of IgG were seen in the renal glomerular mesangium of mice from the three different strains, whereas mesangial IgM and C3 deposits were seen in mice treated both with GSTM and TM (Table 2). The only significant increase in titre was seen for mesangial IgM and C3 deposits in GSTM-treated A.SW mice. Neither GSTM- nor TM-treated mice showed immune deposits in the vessel walls of the kidney or spleen (data not shown).
Table 2.
Renal mesangial immune deposits in gold-treated mice and controls after 12 weeks.
| TM | GSTM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | H-2 | No.a | IgGb | IgMb | C3cb | No.a | IgGb | IgMb | C3cb |
| A.SW | s | 6 | 27 ± 8 | 640 ± 0 | 160 ± 0 | 6 | 20 ± 14 | 1280 ± 0c | 747 ± 107c |
| A.TH | t2 | 6 | 13 ± 8 | 400 ± 80 | 240 ± 36 | 6 | 13 ± 8 | 667 ± 204 | 320 ± 72 |
| A.TL | t1 | 7 | 11 ± 7 | 640 ± 171 | 320 ± 60 | 9 | 49 ± 23 | 604 ± 99 | 204 ± 30 |
Number of animals examined.
Reciprocal titre ± s.e.m.
P < 0·05 significantly different compared with animals given TM (Mann–Whitney U-test). TM, treatment with a weekly intramuscular (i.m.) injection of 10·6 mg sodium thiomaleate/kg bw. GSTM, treatment with a weekly i.m. injection of 22·5 mg sodium aurothiomaleate/kg bw. GSTM: natrium aurothiomaleate.
Number and phenotype of splenocytes
A.SW mice showed a significant, 50% increase in the number of splenocytes after 12 weeks GSTM treatment compared with TM-treated mice (Table 3). The fraction of B cells remained unchanged, while the fraction of CD3− and CD4− cells declined. Due to the increase in the total number of splenocytes, the number of B cells in the spleen increased by approximately 50% in the GSTM-treated mice, while the number of T cells (CD3−, CD4−, CD8−) did not differ significantly between mice treated with GSTM and TM (Table 3). There was no significant increase in the total number of splenocytes in A.TL mice after 12 weeks' GSTM treatment, or an increase of T or B cells (Table 3).
Table 3.
The fraction and total number of splenocytes with B and T cell markers in gold-treated mice and controls after 12 weeks.
| Strain | Treatment | No. | No. spleen cellsa | B220 (%) | B220a | CD3 (%) | CD3a | CD4 (%) | CD4a | CD8 (%) | CD8a |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A.SW | TM | 4 | 68 ± 0·82 | 27 ± 2·8 | 14·9 ± 1·4 | 32 ± 1·5 | 16·8 ± 0·8 | 23 ± 1·7 | 12·1 ± 0·9 | 9 ± 0·5 | 4·9 ± 0·2 |
| GSTM | 4 | 102 ± 8·2b | 27 ± 2·3 | 22·1 ± 2·9 | 22 ± 2·4b | 17·3 ± 1·8 | 16 ± 1·3b | 12·9 ± 1·4 | 8·0 ± 0·9 | 5·9 ± 0·8 | |
| A.TL | TM | 4 | 58 ± 5·7 | 25 ± 0·6 | 11·4 ± 1·5 | 23 ± 3·0 | 10·1 ± 1·8 | 15 ± 2·0 | 6·7 ± 1·3 | 8·0 ± 0·9 | 3·4 ± 0·6 |
| GSTM | 4 | 69 ± 9·9 | 24 ± 1·1 | 13·3 ± 2·3 | 17 ± 1·5 | 9·4 ± 2·0 | 11 ± 1·8 | 6·1 ± 1·8 | 7·0 ± 0·8 | 3·9 ± 0·9 |
Numbers are mean ± SEM (× 106).
P < 0·05 significantly different from mice treated with TM (Mann–Whitney U-test). TM, treatment with a weekly intramuscular (i.m.) injection of 10·6 mg sodium thiomaleate/kg bw. Natrium aurothiomaleate (GSTM), treatment with a weekly i.m. injection of 22·5 mg sodium aurothiomaleate/kg bw.
Immunoglobulin-producing splenocytes
The fraction of splenocytes producing IgG1, IgG2a, IgG3 or IgM was increased significantly, and in the case of IgG2a and IgG3, markedly so in GSTM-treated A.SW mice compared with mice given TM (Table 4). In contrast, A.TL mice treated with GSTM showed no increase in Ig-producing splenocytes of any isotype compared with TM-treated controls. Age- and strain-matched A.SW and A.TL mice treated with NaCl did not differ significantly from the TM-treated mice with regard to the number of Ig-producing cells (data not shown), indicating no effect of treatment with TM.
Table 4.
Fraction of cytoplasmic Ig-positive splenocytes in mice treated with gold and controls after 12 weeks.
| No. of pos. cIg/2 × 105 splenocytes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Strain | Treatment | No. | IgG1a | IgG2a | IgG2b | IgG3 | IgM | IgE |
| A.SW | TM | 4 | 3·5 ± 1·0 | 3·0 ± 0·7 | 3·5 ± 0·6 | 9·5 ± 1·0 | 132 ± 5·6 | 0·3 ± 0·3 |
| GSTM | 4 | 14 ± 2·4b | 46 ± 5·5b | 7·3 ± 1·5 | 48 ± 5·2b | 328 ± 17b | 0·5 ± 0·3 | |
| A.TL | TM | 4 | 0·8 ± 0·3 | 0·5 ± 0·3 | 0·8 ± 0·3 | 11 ± 1·3 | 100 ± 2·5 | 0·5 ± 0·3 |
| GSTM | 4 | 1·5 ± 0·3 | 0·3 ± 0·3 | 1·8 ± 0·3 | 6·5 ± 1·0 | 100 ± 4·1 | 0·3 ± 0·3 | |
Numbers are mean ± s.e.m.
P < 0·05 significantly different from mice treated with TM (Mann-Whiney U-test). TM, treatment with a weekly intramuscular (i.m.) injection of 10·6 mg sodium thiomaleate/kg bw. Natrium aurothiomaleate (GSTM), treatment with a weekly i.m. injection of 22·5 mg sodium aurothiomaleate/kg bw.
Discussion
In 1986 Goter-Robinson reported that mice of the H-2s haplotype developed ANoA during treatment with mercury or gold [33]. The target of the mercury-induced ANoA was later identified as the 34-kDa nucleolar protein fibrillarin [41,42], while the specificity of the gold-induced ANoA has remained unidentified. We show in this study that the gold-induced ANoA in the H-2As strains SJL, A.SW and A.TH target fibrillarin. The susceptibility to develop gold-induced ANoA/anti-fibrillarin antibodies (AFA) is linked to H-2A in mice with the A background, as the lack of AFA response in A.TL mice localizes the susceptibility to the right of the H-2K locus, while the positive AFA response in the A.TH mice restricts susceptibility to the left of the H-2D locus, i.e. in either the H-2A or H-2E loci. Because the A.TH strain does not express H-2E, the genetic susceptibility must reside in the H-2 A locus. The genetic susceptibility for induction of AFA with gold is therefore identical with that of mercury [39,43] and silver [44] in mice on the A background.
While mercury, silver and gold share a genetically restricted autoantibody response, other features of the metal-induced autoimmune syndrome in H-2As mice differ between the three metals. Induction of AFA with gold requires a longer induction phase, with substantially lower titres of ANoA after 5 weeks' treatment with GSTM (present study) [35,45] already compared with the high titre after 4–5 weeks' treatment with mercury [46] or silver [47]. As the titre of mercury-induced AFA is dose-dependent [48], we examined if this might also be the case for the gold-induced response. However, doubling the dose of GSTM did not shorten the induction time significantly or increase the AFA titre (Hultman et al., unpublished observation). Schumann et al., who also noted the prolonged induction time [35], showed that the anamnestic T cell response in mice treated with gold(I) substances for 12 weeks is not directed against gold(I), but against gold(III), which is a metabolite thought to be generated in vivo by oxidation of gold(I) [49]. At present, the mechanism for induction of AFA by gold has not been established, but requires T cells because SJL-nu/− but not SJL-nu/nu mice treated with GSTM develop AFA (Hultman et al., unpublished observations), which is analogous to findings for mercury- [50] and silver [44]-induced AFA. Studies with mercury have indicated that alteration of the autoantigen by binding the metal, possibly causing an altered cleavage pattern (revealing cryptic peptides) [51] will activate T cells not tolerant to these neoantigens [52]. The time needed for metabolizing Au(I) ions to Au(III) ions might partly explain the prolonged induction time when compared with the immediate formation of Hg2+ and Ag− ions in the body after exposure. Furthermore, the gold concentration in the liver and spleen of mice given the same dose of intramuscular (i.m.) GSTM injections increases slowly [53] compared with treatment using silver and mercury [54], which may also explain the delayed development of high AFA titres.
The autoimmune murine syndrome induced by mercury, silver and gold in H-2As mice also differed with regard to immunopathology. While mercury treatment of H-2As strains induces extensive immune-complex deposits dominated by IgG and C3c in the glomerular mesangium, as well as systemically in vessel walls [39,55], gold-treated H-2As strains showed only a slight increase of IgM and C3c deposits in the mesangium. The lack of increased mesangial and vessel wall IgG deposits in gold-treated H-2As mice is in agreement with findings in silver-treated H-2As mice [44,55]. Schuhmann et al. [35] reported focal mesangial IgG deposits and occasional deposits of IgG in the capillaries and blood vessels after 8 weeks' gold treatment in ANoA resistant H-2b and H-2d mice (C57BL/6 J and DBA/2, respectively). This does not constitute the type of immune deposits seen in H-2As mice after mercury treatment [39,55], and we are not aware of any other study which has examined gold-treated H-2As mice in this respect.
Another feature of the mercury- and silver-induced autoimmune syndrome in H-2As mice is lymphoproliferation including T as well as B cells, accompanied by a substantial increase in the number of Ig-producing cells and serum immunoglobulin levels [46,47,50]. In gold-treated A.SW mice the number of splenocytes was increased substantially after 12 weeks, which resulted in an increased number of B cells, although the fraction of B cells remained constant. Due to the increase in total number of splenocytes the total number of the different T cell subsets was preserved, although the fraction of CD3− and CD4− cells declined. As we examined the lymphocyte status after 12 weeks' treatment only, it cannot be excluded that an increase in T cells took place at an earlier point in time, although 12 weeks' treatment resulted in the strongest AFA response. There may be multiple explanations for the large increase in splenocytes compared with the modest increase of the lymphocytes assessed, mainly B220− cells. As shown by the increase in cIg− cells (present study), and the large increase of serum Igs described in GSTM-treated A.SW mice [45], there must be an increased number of plasma cells, which are B220– [56]. Other B cell subpopulations may also be B220–, for example preplasma memory cells [57]. However, we cannot rule out that increase in other, mononuclear non-lymphoid cell populations make up for part of the large increase in the total number of splenocytes.
The increased number of B cells in GSTM-treated A.SW mice was also reflected in the increased number of Ig-producing splenocytes of the IgM, IgG1, IgG2a and IgG3 isotypes, but not the IgG2b or IgE isotype. Therefore, the increase of the different IgG isotypes included Th1- (IgG2a) as well as Th2 (IgG1)-associated Ig isotypes, although the increase in IgG2a-producing cells was 15-fold but only fourfold for IgG1. Previous studies have shown an increase of serum Ig in gold-treated brown Norway rats [29] and A.SW mice [45]. The A.SW (H-2s) mice showed a six- and sevenfold increase in IgM and IgG, respectively, in C57BL/6 J (H-2b) mice a fourfold increase of IgM only, while there was no increase of either IgG or IgM in the DBA/2 (H-2d) strain [45]. Pietsch et al. observed a substantial increase of serum IgE in A. SW mice after 6–8 weeks of GSTM treatment [45], but we found no increase of IgE-producing splenocytes after 12 weeks' treatment. While the serum Ig concentration is dependent upon other factors in addition to the number of Ig-producing cells such as catabolism of Igs, a rough correlation is expected. Because splenocytes from mercury-treated A.SW mice showed the previously reported [47] increase in IgE-producing splenocytes (data not shown), we are confident that the difference observed is not due to technical problems in identifying IgE-producing cells. However, we studied the IgE-production after 12 weeks and Pietsch et al. after 8 weeks, and as the IgE response after treatment with mercury is strikingly transient, reaching a maximum with a ‘window’ of only a single week and then returning to a level close to the controls [45,46], a probable explanation is that the increase in IgE observed by Pietsch et al. after 8 weeks' gold treatment had disappeared 4 weeks later.
The lack of any significant splenic lymphoproliferation including B cells and Ig-producing cells in A.TL mice compared with A.SW mice indicates that this trait is localized to the H-2 region, which is in accordance with the findings after mercury treatment [46].
What are the implications of these observations with regard to the use of GSTM as a disease-modifying anti-rheumatic drug in humans? With regard to lymphoproliferation, most in vitro studies have shown a direct immunosuppressive effect of GSTM on T [18,19] and B [17] cells. However, recent in vitro studies indicated that GSTM may stimulate human peripheral blood lymphocytes (PBMC) [14], and an in vivo study using PBMC from patients treated with GSTM for 3 months showed an increased spontaneous production not only of IL-10 but also IL-6 and interferon (IFN)-γ [58]. Taken together, these reports indicate a large individual variability in the immunological response to GSTM, similar to the variations observed in therapeutic and adverse effects [59]. The adverse immunological effects of GSTM are linked to certain MHC haplotypes [26–28,60], but also to non-MHC genes causing slow sulphoxidizer status [61]. The importance of MHC for adverse effects to develop in humans treated with GSTM concurs with the present observation that a mouse strain with a certain H-2 haplotype (A.TL) showed neither lymphoproliferation nor autoantibodies (AFA), while a non-H-2 congenic strain with another H-2 haplotype (A.SW) showed both (present study). The present study therefore confirms the observation in humans of GSTM as an immunologically potent substance, and reinforces the importance of genetic factors, especially MHC, in regulating susceptibility.
The dose used in the present study was 22·5 mg/kg bw/week, allowing a direct comparison with the doses used in previous experimental studies on gold-induced autoimmunity in mice: 22·5 mg/kg bw/week [35] and 10 mg/kg bw/week [33]. While the efficient dose of GSTM used in anti-rheumatic therapy may vary, weekly doses up to 200 mg (approximately 2·8 mg/kg bw) have been advocated [59]. The currently recommended weekly maintenance dose is 50 mg, which is approximately 0·7 mg/kg bw. The several-fold higher dose given to mice (present study) should have caused a substantially higher concentration in the murine organs compared with humans under treatment with GSTM. The kinetics and accumulation of gold in organs during GSTM treatment is highly dependent on genetic factors, as shown by the differences between inbred strains of mice [53], while there is no systematic study on the distribution of gold in the tissues of humans under chronic treatment with GSTM, let alone any correlation with the genotype. This makes it difficult to compare the organ concentrations attained in humans and mice after a certain dose. Furthermore, while adverse effects of GSTM have been reported to increased with a higher dose (150 mg/kg bw/week) compared with a lower dose (50 mg/kg bw/week) [62], the influence of different genotypes has not been studied.
In conclusion, we have shown that treatment with gold in the form of GSTM, a disease-modifying anti-rheumatic drug, induces autoantibodies reacting with the nucleolar protein fibrillarin in genetically susceptible mice. This underlines the potential of GSTM to cause adverse immunological effects, and its dependency on genotype, as observed in the use of GSTM in humans. The MHC-dependent lymphoproliferation observed in the mice concurs with recent studies indicating that GSTM may have an immunostimulating effect in patients during the first 3 months of GSTM treatment [58], although the genetic dependency of these reactions has not been studied in humans.
Finally, our study underscores that gold is a potent interactor with the immune system, which is also interesting in view of the ability of gold ions to be liberated in substantial quantities from metallic gold implanted in the body [63].
Acknowledgments
This study was supported by a grant from the Swedish Research Council, Branch of Medicine (project no. 9453), the Research Funds of Linköping University Hospital and NIH grant ES07511. Christer Bergman and Marie-Louise Eskilsson provided excellent technical assistance.
References
- 1.Hultman P. Environmental factors in autoimmunity. In: Pollard KM, editor. Autoantibodies and autoimmunity: molecular mechanisms in health and disease. Berlin: Wiley-VCH; 2006. pp. 519–41. [Google Scholar]
- 2.Fairweather D, Rose NR. Coxsackievirus-induced myocarditis in mice: a model of autoimmune disease for studying immunotoxicity. Methods. 2007;41:118–22. doi: 10.1016/j.ymeth.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rieger R, Leung PS, Jeddeloh MR, et al. Identification of 2-nonynoic acid, a cosmetic component, as a potential trigger of primary biliary cirrhosis. J Autoimmun. 2006;27:7–16. doi: 10.1016/j.jaut.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Rieger R, Gershwin ME. The X and why of xenobiotics in primary biliary cirrhosis. J Autoimmun. 2007;28:76–84. doi: 10.1016/j.jaut.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koller LD, Stang BV, Hall JA, Posada de la Paz M, Ruiz Mendez MV. Immunoglobulin and autoantibody responses in MRL/lpr mice treated with ‘toxic oils’. Toxicology. 2002;178:119–33. doi: 10.1016/s0300-483x(02)00232-9. [DOI] [PubMed] [Google Scholar]
- 6.Lahoz C, del Pozo V, Gallardo S, et al. Immunological aspects of the toxic oil syndrome. Arch Toxicol Suppl. 1997;19:65–73. doi: 10.1007/978-3-642-60682-3_6. [DOI] [PubMed] [Google Scholar]
- 7.Havarinasab S, Hultman P. Organic mercury compounds and autoimmunity. Autoimmun Rev. 2005;4:270–5. doi: 10.1016/j.autrev.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Rowley B, Monestier M. Mechanisms of heavy metal-induced autoimmunity. Mol Immunol. 2005;42:833–8. doi: 10.1016/j.molimm.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 9.Bruze M, Edman B, Bjorkner B, Moller H. Clinical relevance of contact allergy to gold sodium thiosulfate. J Am Acad Dermatol. 1994;31:579–83. doi: 10.1016/s0190-9622(94)70219-5. [DOI] [PubMed] [Google Scholar]
- 10.Forestier J. Rheumatoid arthritis and its treatment by gold salts. Lancet. 1934;2:646–8. [Google Scholar]
- 11.Rau R. Have traditional DMARDs had their day? Effectiveness of parenteral gold compared to biologic agents. Clin Rheumatol. 2005;24:189–202. doi: 10.1007/s10067-004-0869-8. [DOI] [PubMed] [Google Scholar]
- 12.Barrera P, Boerbooms AM, van de Putte LB, van der Meer JW. Effects of antirheumatic agents on cytokines. Semin Arthritis Rheum. 1996;25:234–53. doi: 10.1016/s0049-0172(96)80035-7. [DOI] [PubMed] [Google Scholar]
- 13.Bondeson J. The mechanisms of action of disease-modifying antirheumatic drugs: a review with emphasis on macrophage signal transduction and the induction of proinflammatory cytokines. Gen Pharmacol. 1997;29:127–50. doi: 10.1016/s0306-3623(96)00419-3. [DOI] [PubMed] [Google Scholar]
- 14.Lampa J, Klareskog L, Ronnelid J. Effects of gold on cytokine production in vitro; increase of monocyte dependent interleukin 10 production and decrease of interferon-gamma levels. J Rheumatol. 2002;29:21–8. [PubMed] [Google Scholar]
- 15.Stern I, Wataha JC, Lewis JB, Messer RL, Lockwood PE, Tseng WY. Anti-rheumatic gold compounds as sublethal modulators of monocytic LPS-induced cytokine secretion. Toxicol In Vitro. 2005;19:365–71. doi: 10.1016/j.tiv.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Wataha JC, Lewis JB, Volkmann KR, Lockwood PE, Messer RL, Bouillaguet S. Sublethal concentrations of Au(III), Pd(II), and Ni(II) differentially alter inflammatory cytokine secretion from activated monocytes. J Biomed Mater Res B Appl Biomater. 2004;69:11–17. doi: 10.1002/jbm.b.20029. [DOI] [PubMed] [Google Scholar]
- 17.Hirohata S, Nakanishi K, Yanagida T, Kawai M, Kikuchi H, Isshi K. Synergistic inhibition of human B cell activation by gold sodium thiomalate and auranofin. Clin Immunol. 1999;91:226–33. doi: 10.1006/clim.1999.4686. [DOI] [PubMed] [Google Scholar]
- 18.Harth M, Cousin K, McCain GA. Sodium aurothiomalate inhibits T cell responses to interleukin-2. Immunopharmacol Immunotoxicol. 1988;10:141–56. doi: 10.3109/08923978809014329. [DOI] [PubMed] [Google Scholar]
- 19.Wolf RE, Hall VC. Inhibition of in vitro proliferative response of cultured T lymphocytes to interleukin-2 by gold sodium thiomalate. Arthritis Rheum. 1988;31:176–81. doi: 10.1002/art.1780310204. [DOI] [PubMed] [Google Scholar]
- 20.Griem P, Gleichmann E. Gold antirheumatic drug: desired and adverse effects of Au(I) and Au(III) [corrected] on the immune system. Z Rheumatol. 1996;55:348–58. [PubMed] [Google Scholar]
- 21.Takahashi K, Kropshofer H, Vogt AB, Gleichmann E, Griem P. Drug-induced inhibition of insulin recognition by T-cells: the antirheumatic drug aurothiomalate inhibits MHC binding of insulin peptide. Mol Immunol. 1998;35:1081–7. doi: 10.1016/s0161-5890(98)00106-0. [DOI] [PubMed] [Google Scholar]
- 22.Kiely PD, Helbert MR, Miles J, Oliveira DB. Immunosuppressant effect of gold on IgG subclasses and IgE; evidence for sparing of Th2 responses. Clin Exp Immunol. 2000;120:369–74. doi: 10.1046/j.1365-2249.2000.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lockie LM, Smith DM. Forty-seven years experience with gold therapy in 1,019 rheumatoid arthritis patients. Semin Arthritis Rheum. 1985;14:238–46. doi: 10.1016/0049-0172(85)90043-5. [DOI] [PubMed] [Google Scholar]
- 24.Greinacher A, Eichler P, Lubenow N, Kiefel V. Drug-induced and drug-dependent immune thrombocytopenias. Rev Clin Exp Hematol. 2001;5:166–200. doi: 10.1046/j.1468-0734.2001.00041.x. discussion 311–12. [DOI] [PubMed] [Google Scholar]
- 25.Hall CL. Gold nephropathy. Nephron. 1988;50:265–72. doi: 10.1159/000185185. [DOI] [PubMed] [Google Scholar]
- 26.Evans DT, Knapp LA, Jing P, et al. Rapid and slow progressors differ by a single MHC class I haplotype in a family of MHC-defined rhesus macaques infected with SIV. Immunol Lett. 1999;66:53–9. doi: 10.1016/s0165-2478(98)00151-5. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Perez M, Gonzalez-Dominguez J, Mataran L, Garcia-Perez S, Salvatierra D. Association of HLA-DR5 with mucocutaneous lesions in patients with rheumatoid arthritis receiving gold sodium thiomalate. J Rheumatol. 1994;21:41–3. [PubMed] [Google Scholar]
- 28.Sakkas LI, Chikanza IC, Vaughan RW, Welsh KI, Panayi GS. Gold induced nephropathy in rheumatoid arthritis and HLA class II genes. Ann Rheum Dis. 1993;52:300–1. doi: 10.1136/ard.52.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tournade H, Guery J-C, Pasquier R, et al. Experimental gold-induced autoimmunity. Nephrol Dial Transplant. 1991;6:621–30. doi: 10.1093/ndt/6.9.621. [DOI] [PubMed] [Google Scholar]
- 30.Fournie GJ, Saoudi A, Druet P, Pelletier L. Th2-type immunopathological manifestations induced by mercury chloride or gold salts in the rat: signal transduction pathways, cellular mechanisms and genetic control. Autoimmun Rev. 2002;1:205–12. doi: 10.1016/s1568-9972(02)00052-6. [DOI] [PubMed] [Google Scholar]
- 31.Mas M, Cavailles P, Colacios C, et al. Studies of congenic lines in the Brown Norway rat model of Th2-mediated immunopathological disorders show that the aurothiopropanol sulfonate-induced immunological disorder (Aiid3) locus on chromosome 9 plays a major role compared to Aiid2 on chromosome 10. J Immunol. 2004;172:6354–61. doi: 10.4049/jimmunol.172.10.6354. [DOI] [PubMed] [Google Scholar]
- 32.Mas M, Subra JF, Lagrange D, et al. Rat chromosome 9 bears a major susceptibility locus for IgE response. Eur J Immunol. 2000;30:1698–705. doi: 10.1002/1521-4141(200006)30:6<1698::AID-IMMU1698>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 33.Robinson CJ, Balazs T, Egorov IK. Mercuric chloride-, gold sodium thiomalate-, and d-penicillamine-induced antinuclear antibodies in mice. Toxicol Appl Pharmacol. 1986;86:159–69. doi: 10.1016/0041-008x(86)90046-3. [DOI] [PubMed] [Google Scholar]
- 34.Layland LE, Wulferink M, Dierkes S, Gleichmann E. Drug-induced autoantibody formation in mice: triggering by primed CD4+CD25- T cells, prevention by primed CD4+CD25+ T cells. Eur J Immunol. 2004;34:36–46. doi: 10.1002/eji.200324406. [DOI] [PubMed] [Google Scholar]
- 35.Schuhmann D, Kubicka-Muranyi M, Mirtschewa J, Gunther J, Kind P, Gleichmann E. Adverse immune reactions to gold. I. Chronic treatment with an Au(I) drug sensitizes mouse T cells not to Au(I), but to Au(III) and induces autoantibody formation. J Immunol. 1990;145:2132–9. [PubMed] [Google Scholar]
- 36.Hultman P, Enestrom S. Mercury induced antinuclear antibodies in mice: characterization and correlation with renal immune complex deposits. Clin Exp Immunol. 1988;71:269–74. [PMC free article] [PubMed] [Google Scholar]
- 37.Warfvinge K, Hansson H, Hultman P. Systemic autoimmunity due to mercury vapor exposure in genetically susceptible mice: dose–response studies. Toxicol Appl Pharmacol. 1995;132:299–309. doi: 10.1006/taap.1995.1111. [DOI] [PubMed] [Google Scholar]
- 38.Chan EKL, Pollard KM. Autoantibodies to ribonucleoprotein particles by immunoblotting. In: Rose N, Conway de Macario E, Fahey JL, Penn H, editors. Manual of clinical laboratory immunology. Washington, DC: American Society for Microbiology; 1992. pp. 755–61. [Google Scholar]
- 39.Hultman P, Bell LJ, Enestrom S, Pollard KM. Murine susceptibility to mercury. I. Autoantibody profiles and systemic immune deposits in inbred, congenic, and intra-H-2 recombinant strains. Clin Immunol Immunopathol. 1992;65:98–109. doi: 10.1016/0090-1229(92)90212-7. [DOI] [PubMed] [Google Scholar]
- 40.Pollard KM, Hultman P. Fibrillarin autoantibodies. In: Shoenfeld Y, Gershwin ME, Meroni PL, editors. Autoantibodies. Amsterdam: Elsevier; 2006. pp. 317–23. [Google Scholar]
- 41.Hultman P, Enestrom S, Pollard KM, Tan EM. Anti-fibrillarin autoantibodies in mercury-treated mice. Clin Exp Immunol. 1989;78:470–7. [PMC free article] [PubMed] [Google Scholar]
- 42.Reuter R, Tessars G, Vohr HW, Gleichmann E, Luhrmann R. Mercuric chloride induces autoantibodies against U3 small nuclear ribonucleoprotein in susceptible mice. Proc Natl Acad Sci USA. 1989;86:237–41. doi: 10.1073/pnas.86.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mirtcheva J, Pfeiffer C, De Bruijn JA, Jacquesmart F, Gleichmann E. Immunological alterations inducible by mercury compounds. III. H-2A acts as an immune response and H-2E as an immune ‘suppression’ locus for HgCl2-induced antinucleolar autoantibodies. Eur J Immunol. 1989;19:2257–61. doi: 10.1002/eji.1830191212. [DOI] [PubMed] [Google Scholar]
- 44.Hultman P, Ganowiak K, Turley SJ, Pollard KM. Genetic susceptibility to silver-induced anti-fibrillarin autoantibodies in mice. Clin Immunol Immunopathol. 1995;77:291–7. doi: 10.1006/clin.1995.1155. [DOI] [PubMed] [Google Scholar]
- 45.Pietsch P, Vohr HW, Degitz K, Gleichmann E. Immunological alterations inducible by mercury compounds. II. HgCl2 and gold sodium thiomalate enhance serum IgE and IgG concentrations in susceptible mouse strains. Int Arch Allergy Appl Immunol. 1989;90:47–53. [PubMed] [Google Scholar]
- 46.Johansson U, Hansson-Georgiadis H, Hultman P. The genotype determines the B cell response in mercury-treated mice. Int Arch Allergy Immunol. 1998;116:295–305. doi: 10.1159/000023959. [DOI] [PubMed] [Google Scholar]
- 47.Johansson U, Hansson-Georgiadis H, Hultman P. Murine silver-induced autoimmunity: silver shares induction of antinucleolar antibodies with mercury, but causes less activation of the immune system. Int Arch Allergy Immunol. 1997;113:432–43. doi: 10.1159/000237619. [DOI] [PubMed] [Google Scholar]
- 48.Nielsen JB, Hultman P. Mercury-induced autoimmunity in mice. Environ Health Perspect. 2002;110(Suppl. 5):877–81. doi: 10.1289/ehp.02110s5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goebel C, Kubicka-Muranyi M, Tonn T, Gonzalez J, Gleichmann E. Phagocytes render chemicals immunogenic: oxidation of gold(I) to the T cell-sensitizing gold(III) metabolite generated by mononuclear phagocytes. Arch Toxicol. 1995;69:450–9. doi: 10.1007/s002040050198. [DOI] [PubMed] [Google Scholar]
- 50.Hultman P, Johansson U, Dagnaes-Hansen F. Murine mercury-induced autoimmunity. the role of T-helper cells. J Autoimmun. 1995;8:809–23. doi: 10.1016/s0896-8411(95)80019-0. [DOI] [PubMed] [Google Scholar]
- 51.Pollard KM, Lee DK, Casiano CA, Bluthner M, Johnston MM, Tan EM. The autoimmunity-inducing xenobiotic mercury interacts with the autoantigen fibrillarin and modifies its molecular and antigenic properties. J Immunol. 1997;158:3521–8. [PubMed] [Google Scholar]
- 52.Kubicka-Muranyi M, Griem P, Lubben B, Rottmann N, Luhrmann R, Gleichmann E. Mercuric-chloride-induced autoimmunity in mice involves up-regulated presentation by spleen cells of altered and unaltered nucleolar self antigen. Int Arch Allergy Immunol. 1995;108:1–10. doi: 10.1159/000237110. [DOI] [PubMed] [Google Scholar]
- 53.Tonn T, Goebel C, Wilhelm M, Gleichmann E. Gold kinetics under long-term treatment with gold(I) disodium thiomalate: a comparison in three different mouse strains. Br J Rheumatol. 1994;33:724–30. doi: 10.1093/rheumatology/33.8.724. [DOI] [PubMed] [Google Scholar]
- 54.Havarinasab S, Bjorn E, Ekstrand J, Hultman P. Dose and Hg species determine the T-helper cell activation in murine autoimmunity. Toxicology. 2007;229:23–32. doi: 10.1016/j.tox.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Hultman P, Turley SJ, Enestrom S, Lindh U, Pollard KM. Murine genotype influences the specificity, magnitude and persistence of murine mercury-induced autoimmunity. J Autoimmun. 1996;9:139–49. doi: 10.1006/jaut.1996.0017. [DOI] [PubMed] [Google Scholar]
- 56.Kumazaki K, Tirosh B, Maehr R, Boes M, Honjo T, Ploegh HL. AID-/-mus-/- mice are agammaglobulinemic and fail to maintain B220-CD138+ plasma cells. J Immunol. 2007;178:2192–203. doi: 10.4049/jimmunol.178.4.2192. [DOI] [PubMed] [Google Scholar]
- 57.Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–20. doi: 10.1016/s1074-7613(03)00267-x. [DOI] [PubMed] [Google Scholar]
- 58.Ernestam S, Lampa J, Rogberg S, Ronnelid J, Klareskog L, Hafstrom I. Evidence for immunostimulatory effects of intramuscular gold in patients with rheumatoid arthritis: correlation with skin reactions. J Rheumatol. 2003;30:1748–55. [PubMed] [Google Scholar]
- 59.Sigler JW. Parenteral gold in the treatment of rheumatoid arthritis. Am J Med. 1983;75:59–62. doi: 10.1016/0002-9343(83)90475-8. [DOI] [PubMed] [Google Scholar]
- 60.Shah P, Griffith SM, Shadforth MF, et al. Can gold therapy be used more safely in rheumatoid arthritis? Adverse drug reactions are more likely in patients with nodular disease, independent of HLA-DR3 status. J Rheumatol. 2004;31:1903–5. [PubMed] [Google Scholar]
- 61.Madhok R, Capell HA, Waring R. Does sulphoxidation state predict gold toxicity in rheumatoid arthritis? BMJ (Clin Res Ed) 1987;294:483. doi: 10.1136/bmj.294.6570.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Furst DE, Levine S, Srinivasan R, Metzger AL, Bangert R, Paulus HE. A double-blind trial of high versus conventional dosages of gold salts for rheumatoid arthritis. Arthritis Rheum. 1977;20:1473–80. doi: 10.1002/art.1780200805. [DOI] [PubMed] [Google Scholar]
- 63.Danscher G. In vivo liberation of gold ions from gold implants. Autometallographic tracing of gold in cells adjacent to metallic gold. Histochem Cell Biol. 2002;117:447–52. doi: 10.1007/s00418-002-0400-8. [DOI] [PubMed] [Google Scholar]