Abstract
In patients with anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis, indirect immunofluorescence (IF) distinguishes between cytoplasmic (C-ANCA) and perinuclear (P-ANCA) neutrophil staining patterns. In patients with primary systemic vasculitis such as Wegener's granulomatosis, microscopic polyangiitis and Churg–Strauss syndrome, these IF staining patterns correspond broadly with antibodies to the two major antigens: the C-ANCA pattern is associated generally with antibodies to serine protease 3 (PR3) and the P-ANCA pattern with antibodies to myeloperoxidase (MPO). However, some sera positive for ANCA by IF are negative for anti-PR3 and anti-MPO antibodies, suggesting the presence of antibodies to minor antigens of PMN granules. We tested sera from a previously well-defined clinical cohort of patients for antibodies to four possible minor antigens: bactericidal permeability increasing protein, elastase, cathepsin G and lactoferrin. IF-positive (+) sera had significantly higher antibody frequencies to the minor antigens than did the IF-negative (−) sera (P < 0·01). Patients with IF− PR3−MPO− sera showed the most varied reactivity to the minor antigens. Among the IF− groups, the IF− PR3−/MPO− sera showed the lowest reactivity to the minor antigens. Patients with well-defined ANCA specificities, e.g. the PR3-ANCA response associated with Wegener's granulomatosis, are less likely than are other patient subsets to have antibodies to minor antigen targets. Autoantibodies to these minor antigens contribute to the overall pattern of ANCA identified by IF and help to explain why the correlation between IF and enzyme immunoassays show discrepancies. While the pathophysiological significance of antibodies to minor target antigens needs further evaluation, they may be markers of inflammation associated with disease processes.
Keywords: ANCA, bactericidal permeability increasing protein, cathepsin G, elastase, lactoferrin, myeloperoxidase, serine protease 3
Introduction
Anti-neutrophil cytoplasmic antibodies (ANCA) are serological markers for a significant subset of patients with primary systemic vasculitis [1,2]. In patients with vasculitis, ANCA targets two major antigens in the primary granules of neutrophils: serine proteinase 3 (PR3) and myeloperoxidase (MPO) [3,4]. However, human neutrophils contain at least three types of granules, each of which contains a variety of constituent proteins: azurophilic granules [PR3, MPO, bactericidal permeability increasing protein (BPI), elastase (Elast), cathepsin G (Cath G)]; secondary granules [lactoferrin (LF), lysozyme]; and tertiary granules (gelatinase) [5–7]. Antigens within any of these granules are potential targets for an ANCA response [8].
Certain types of vasculitis have been shown to correlate well with immunofluorescence (IF) ANCA testing. Most patients with Wegener's granulomatosis (WG), for example, have antibodies that show a cytoplasmic (C-ANCA) pattern on IF testing [9]. In contrast, microscopic polyangiitis (MPA) and Churg–Strauss syndrome are associated more frequently with antibodies that produce a P-ANCA pattern [3,10]. Certainly, serological overlaps between these ‘ANCA-associated’ forms of vasculitis exist, such that WG may be associated with a P-ANCA pattern of IF, and MPA and Churg–Strauss syndrome may be associated with a C-ANCA pattern. Among patients with vasculitis, the C-ANCA pattern is produced most often by antibodies to proteinase 3 (PR3-ANCA) and the P-ANCA pattern by antibodies to myeloperoxidase ANCA-associated conditions [2]. One finding was that WG patients with active disease, regardless of treatment status, were more likely to have ANCA by IF than those with inactive disease (P = 0·02). However, the same comparison did not reach statistical significance for the enzyme-linked immunosorbent assay (ELISA) tests [2]. This finding suggests that antibodies other than MPO and PR3 showing an IF− ANCA may be involved.
Antibodies to other antigens, sometimes termed ‘minor’ antigens, have also been reported in systemic vasculitis, but their clinical significance remains unclear [11–13]. It has been reported that antibodies to these minor antigens are undetectable in normal healthy subjects [14]. Elast has a strong homology to PR3 and sometimes elicits a C-ANCA pattern on IF testing. Wiesner et al. reported that Elast antibodies are frequent in cocaine-induced midline destructive lesions, and that the presence of Elast antibodies may discriminate between this clinical entity and WG, which it may mimic closely [15]. Antibodies to Cath G, associated with a P-ANCA pattern on indirect IF testing, have been reported in Sjögren's syndrome and paediatric WG [16]. LF, which produces a P-ANCA pattern, has been reported in patients with rheumatoid arthritis, vasculitis, ankylosing spondylitis, ulcerative colitis and systemic lupus erythematosus [17–19]. LF and Cath G antibodies can also be induced by propylthiouracil in patients with Graves' disease [20]. Finally, although BPI antibodies could present themselves as a C-ANCA pattern on ethanol-fixed slides, these antibodies have not been found in association with vasculitis, but rather with inflammatory bowel disease (IBD), pulmonary inflammatory disorders, rheumatoid arthritis and cystic fibrosis [21–23]. In an earlier study, we determined the sensitivity, specificity, positive and negative predictive values, and likelihood ratios of both IF and MPO and PR3 ELISA testing on a cohort of well-characterized patients from our institution [2]. In that patient cohort, we recognized that many C- or P-ANCA-positive sera did not contain antibodies to either PR3 or MPO. We suggested that antibodies to other target antigens may display a positive IF pattern even in the absence of PR3- or MPO-ANCA. Because the potential importance of antibodies to the minor antigens has not been evaluated in a systematic fashion, we tested sera from the previously well-characterized group of patients in a cross-sectional study [2], using commercially available ELISAs for the detection of ANCA directed against BPI, Elast, Cath G and LF.
Materials and methods
Patients
This study was approved by the Johns Hopkins Medical Institutions' Joint Committee on Clinical Investigation. Patient samples were obtained from both in-patients and out-patients visiting various clinics at the Johns Hopkins Medical Institutions. The study samples were identified from specimens evaluated for ANCA at the Johns Hopkins Medical Institutions. Samples were sent to the laboratory primarily to diagnose or exclude an ANCA-associated vasculitis. The study group in this report is a subset of a cohort of patients defined and tested previously for ANCA by immunofluorescence and ELISA (MPO and PR3) to determine the relationship of these antibodies with vasculitis [2]. In our previous study, 62 sera showed an unusual correlation pattern between IF and ELISA testing [2]. They were IF− but were MPO− and PR3− by ELISA. These samples were evaluated for our present study and compared to three other groups from the same study. A medical record review determined the diagnosis of each patient. Diagnoses are summarized in Table 1. Disease classifications of patients with WG and MPA were based upon the Chapel Hill Consensus Conference definitions [24]. Of the 216 patients, diagnoses fell into the following categories: WG, MPA, renal-limited vasculitis (RLV), IBD, other vasculitic or renal disorder and other disease processes (Table 1). The other disease processes include many different disorders ranging from infectious disease (HIV, fungal infections, pneumonia, viral conditions), cancer, rheumatic conditions [arthritis, systemic lupus erythematosus (SLE)], coronary artery disease (CAD), autoimmune conditions (myasthenia gravis, alopecia, sarcoid, diabetes) and other miscellaneous conditions such as gout, asthma and cystic fibrosis. The diagnosis of patients has been described more fully previously [2]. The test groups are as follows.
Table 1.
Number of patients in the study and their diagnosis.
| Diagnosis | No. of patients |
|---|---|
| Wegener's granulomatosis (WG) | 32 |
| Microscopic polyangiitis (MPA) | 10 |
| Inflammatory bowel disease (IBD) | 9 |
| Other vascultilis and renal disorders* | 52 |
| Other diagnoses (Dx)** | 113 |
| Total | 216 |
Includes Churg–Strauss syndrome, polyarteritis nodosa, focal segmental glomerulonephritis, renal cell carcinoma, human immunodeficiency virus (HIV) nephropathy.
Includes HIV, gout, sarcoid, acute myeloid leukaemia (AML), arthritis, systemic lupus erythematosus (SLE), diabetes, sinusitis, adenocarcinoma, pericarditis, lymphoma, fungal sepsis, coronary artery disease (CAD), polymyositis.
Group 1
Sixty-two patients who were IF positive (+) for C-ANCA (n = 31) or P-ANCA (n = 31), but were negative (–) by ELISA for PR3 or MPO (IF− PR3−MPO−). Diagnoses for this group are summarized in Table 2. Briefly, the group includes patients with WG, IBD, MPA, other vasculitis disorders and other miscellaneous disorders, as described previously [2]. The other miscellaneous disorders include other types of glomerulonephritis, infections, pulmonary fibrosis, cystic fibrosis, cancer and autoimmune disease.
Table 2.
Frequency of antibodies to minor neutrophil antigens in patients positive for anti-neutrophil cytoplasmic antibodies (ANCA) by immunofluorescence but negative for serine protease 3 (PR3) or myeloperoxidase (MPO) by enzyme-linked immunosorbent assay (ELISA) (group 1).
| Diagnosis | IF-P* | No. | PR3 | MPO | BPI** no. (%) | Elastase no. (%) | Cathepsin G no. (%) | Lactoferrin no. (%) |
|---|---|---|---|---|---|---|---|---|
| WG | C | 3 | – | – | 2 (67) | 1 (33) | 2 (67) | 1 (33) |
| IBD | C | 2 | – | – | 1 (50) | 0 (0) | 0 (0) | 0 (0) |
| MPA | C | 1 | – | – | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other vasculitis | C | 8 | – | – | 3 (38) | 2 (25) | 6 (75) | 2 (25) |
| Other Dx† | C | 17 | – | – | 8 (50) | 2 (13) | 13 (76) | 3 (19) |
| Total | 31 | – | 14 (45) | 5 (16) | 21 (68) | 6 (19) | ||
| WG | P | 4 | – | – | 1 (25) | 0 (0) | 3 (75) | 1 (25) |
| IBD | P | 7 | – | – | 3 (43) | 0 (0) | 2 (29) | 3 (43) |
| MPA | P | 2 | – | – | 0 (0) | 0 (0) | 0 (0) | 1 (50) |
| Other vasculitis | P | 4 | – | – | 0 (0) | 1 (25) | 1 (25) | 2 (50) |
| Other Dx‡ | P | 14 | – | – | 4 (28) | 4 (28) | 9 (64) | 7 (50) |
| Total | 31 | 8 (26) | 5 (16) | 15 (32) | 14 (23) |
Immunofluorescence pattern: C = cytoplasmic; P = perinuclear.
BPI = bacterial-permeability increasing protein.
Includes idiopathic pulmonary fibrosis, viral infection, dilated cardiomyopathy, proteinuria, cyctic fibrosis, AIDS, haemosiderosis.
Includes Chronic lymphocytic leukemia, renal cell carcinoma, Alzheimer's, myasthenia gravis, arthritis, pneumonia, chronic sinusitis, systemic lupus erythematosus (SLE).
Group 2
This group comprised 15 patients who were IF ANCA− (four C-ANCA and 11 P-ANCA), and by ELISA were PR3− but MPO− (IF− PR3−MPO−). Diagnoses included WG (n = 3), MPA (n = 9), non-crescentic glomerulonephritis (n = 1), Churg–Strauss syndrome (n = 1) and renal insufficiency (n = 1).
Group 3
This group comprised 25 patients who were IF ANCA− (24 C-ANCA and one P-ANCA) and by ELISA were MPO− but PR3− (IF− PR3− MPO−). Diagnoses included primarily WG (n = 21), but also included patients with MPA (n = 1), infectious disease (n = 1) and autoimmune disease (n = 2), as described previously [2].
Group 4
This group comprised 114 patients who were IF− and by ELISA were PR3− and MPO−. Diagnoses here include other vasculitis disorders [central nervous system (CNS) vasculitis n = 4, polyarteritis nodosa (PAN) n = 8, Takayasu's arteritis n = 3, WG n = 2 and miscellaneous n = 6], other renal conditions (membranous glomerulonephritis n = 4, end-stage renal disease and chronic renal insufficiency n = 7, IgA nephropathy n = 2, other glomerulonephritis disorders n = 6), infections n = 16, cancer (haematological n = 3, non-haematological n = 6, CAD n = 5, immunological/rheumatological (autoimmune, sarcoid, asthma, arthritis, gout, etc.) n = 21, neurological (aseptic meningitis, stroke, gliomatosis, Bell's palsy, vascular neuropathy, uveitis) n = 13, and other miscellaneous disorders (n = 8) as mentioned above and described previously [2]. This group included all IF−PR3−MPO− samples from the previous study [2].
Indirect immunofluorescence (IF)
IF was performed on both ethanol and formalin-fixed normal human neutrophils, as described previously [2]. The neutrophil substrate was incubated with patient serum starting at a dilution of 1 : 10 for 30 min. The slides were then washed for 30 min in phosphate-buffered saline (PBS) to remove excess serum. The slides were incubated with fluorescein-labelled anti-human IgG immunoglobulin antibodies (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) for 30 min. Excess conjugate was removed by washing as above. Slides were mounted with a solution of polyvinyl alcohol (PVA) and examined by fluorescence microscopy, using a Zeiss microscope, for ANCA staining patterns (C-ANCA and P-ANCA). Positive sera were titrated by testing further twofold serial dilutions until a negative reaction was reached. The titre is considered to be the reciprocal of the last dilution to give a positive reactivity. A positive reaction is considered to be a titre equal to or greater than 20.
Anti-PR3 and -MPO enzyme immunoassays (EIAs)
PR3- and MPO-ANCA EIAs were performed according to the manufacturer's instructions (INOVA Diagnostics, San Diego, CA, USA). All EIAs involved the semiquantitative determination of PR3 and MPO antibodies. The microtitre wells were coated with human PR3 or MPO antigens. Upon addition of human serum at a 1 : 100 dilution, theantigen–antibody complex was reacted with an enzyme-labelled secondary antibody. The assay was evaluated spectrophotometrically using an MRX Revelation microplate reader (Dynex Technologies Inc., Chantilly, VA, USA). The reactivity of each sample was calculated by dividing the average optical density (OD) of the sample by the average OD of a known standard low positive sample, and then multiplied by a number of units that have been assigned by the manufacturer to that standard. Because the rate of colour development is not proportional to the concentration of antibody in the patient serum, this procedure is only semiquantitative. A positive PR3- or MPO-ANCA assay was defined as a titre greater than 20 units. After testing all sera using both IF and EIA methods, patients with four different patterns of reactivity were evaluated. These four groups were: group 1, IF−/PR3−/MPO− (n = 62); group 2, IF−/MPO−/PR3− (n = 15); group 3, IF−/PR3−/MPO− (n = 25); and group 4, IF−/PR3−/MPO− (n = 114).
EIAs for antibodies to minor antigens
The anti-BPI, anti-Elast, anti-Cath G and anti-LF EIAs were performed according to the manufacturer's instructions (ORGEnTec, Mainz, Germany). The principle of these semiquantitative assays is the same as for the PR3− and MPO−ANCA EIAs, as described above. The assays detect ANCA directed against BPI, Elast, Cath G and LF in a semiquantitative manner. As with assays for PR3- and MPO-ANCA, all assays were designed to recognize IgG class antibodies. The microtitre plates were coated with one of the ANCA antigens (BPI, Elast, Cath G or LF), followed by the addition of patients' sera at dilutions of 1 : 100. If antibodies to the target antigen(s) were present, they would bind to the plate. The plate was then washed and an enzyme-labelled secondary antibody was added to recognize the complex. Finally, 3,3′,5,5′-tetramethyl-benzidine (TMB) enzyme substrate was added, and the colour development stopped with 1 M hydrochloric acid. Consistent with the manufacturer's instructions, an antibody to a minor antigen was considered present if the value was equal to or greater than 10 units.
Statistical analysis
For between-group comparison, Fisher's exact test and the χ2 test were used, as appropriate (Sigma Stat, San Jose, CA, USA). A difference was considered to be significant if P < 0·05.
Results
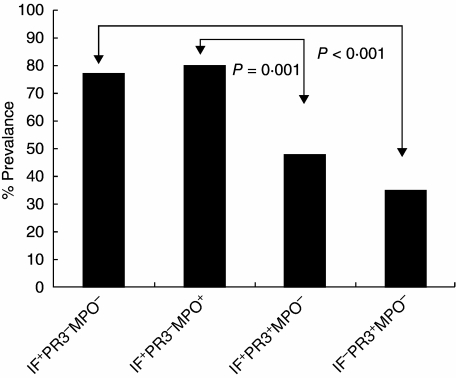
Overall, all IF− patient sera (groups 1–3) had significantly higher frequencies of reactivity to the minor antigens than did the IF− patient sera (group 4) (P < 0·01 for each group). Among the IF− sera, those that were PR3− (groups 1 and 2) showed the greatest overall reactivity to all minor antigens (P = 0·0001). In contrast, those sera that were both IF− and PR3− showed the least reactivity among the IF− groups with the four antigens tested (P < 0·03). The minor antigen reactivity for each group is detailed below.
Group 1: IF−/PR3−/MPO− (n = 62)
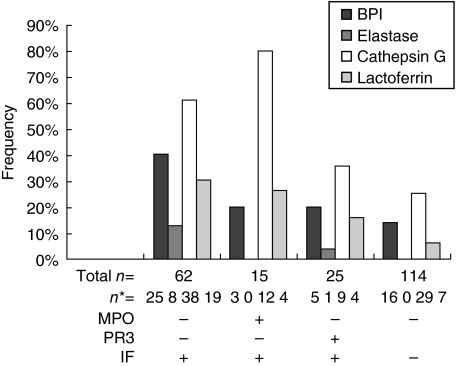
Almost 80% of patients in the IF−/PR3−/MPO− group reacted with at least one of the minor target antigens (Fig. 1). This group contained patients with many diagnoses, including WG, microscopic polyangiitis, IBD, other vasculitic diseases and other diagnoses as described in the Materials and methods section (Table 2). In this group, patients who showed a C-ANCA pattern of IF displayed antibodies to Cath G (68%), BPI (45%), LF (19%) and Elast (16%) in decreasing frequency (Table 2). Patients with a P-ANCA pattern of IF showed antibodies to Cath G (32%), BPI (26%), LF (23%) and Elast (16%) in decreasing frequency (Table 2). Considering the entire group regardless of IF pattern, antibodies to all four minor antigens, including 40% reactivity to BPI and 61% reactivity to Cath G, were detected (Fig. 2). This group showed the greatest ‘multiple’ reactivities in patient sera. Only 35% showed only a single antibody, while over 24% had two reactivities, 16% had three antibodies and 3% even showed antibodies to all four minor antigens.
Fig. 1.
Antibody frequency in patients among the four experimental groups reactive to at least one minor anti-neutrophil cytoplasmic antibodies (ANCA) antigen. The greatest prevalence was found in patients with positive immunofluorescence (IF). Patients with a positive IF and positive serine protease 3 (PR3) had a greater prevalence than patients with a negative IF (P = 0·001). Similar results were found in patients with positive IF and positive myeloperoxidase (MPO). Minor antigens were least prevalent in patients with IF−, PR3− and MPO−.
Fig. 2.
Antibody reactivity to the individual anti-neutrophil cytoplasmic antibodies (ANCA) minor antigens. Patients with immunofluorescence (IF)-positive/myeloperoxidase (MPO)/serine protease 3 (PR3)-negative showed the highest reactivity to all four minor antigens. Antibodies to cathepsin G were most frequently present in all groups. n = number of individuals in a group with antibodies to each minor antigen.
The diagnosis of the 12 patients who were negative for antibodies to all minor antigens included WG (n = 2), IBD (n = 4), other types of glomerulonephritis (n = 3), infection (n = 1), idiopathic pulmonary fibrosis (n = 1) and Alport's syndrome (n = 1). These ANCA titres ranged from 20 to 640, with a median of 40. Both C-ANCA and P-ANCA patterns were observed. These results suggest that there may be additional minor antigens to which antibodies develop that have not yet been identified.
Group 2: IF−/PR3−/MPO− (n =15)
MPO-ANCA are regarded as highly specific for pauci-immune vasculitis, The patients had either microscopic polyangiitis (n = 9) or WG (n = 3), Churg–Strauss syndrome (n = 1), non-crescentic glomerulonephritis (n = 1) or renal insufficiency (n = 1).
Over 80% of patients in this group exhibited antibody reactivities to at least one minor target antigen (Fig. 2). Sixty per cent showed a single antibody but only 13% had two, and only a further 13% had three antibody reactivities. No patient reacted to all four minor antigens. Only two patients, one with microscopic polyangiitis and one with WG, were negative for antibodies to all four minor antigens tested.
The MPO-ANCA positive sera reacted frequently with Cath G (80%) and less frequently with the other target antigens (LF 27% and BPI 20%). None of the patients in this group reacted to Elast (Fig. 2). It has been suggested by other investigators that the antibodies to some of the minor antigens may cross-react with MPO-ANCA, thus creating multiple reactivities in EIA testing [21].
Group 3: IF−/PR3−/MPO− (n = 25)
This group consisted mainly of patients with WG (n = 21). The diagnosis of the other patients included microscopic polyangiitis, poliomyelitis, multiple sclerosis or Sjögren's syndrome. As a whole, group 3 showed significantly fewer reactions with the minor antigens than did PR3 negative sera (P = 0·026). The highest antibody reactivity within this group − only 36% − was to Cath G. Of the patient population in this group only 36% showed antibody to one, 8% to two and 8% to three minor antigens. Only one of the WG patients had antibodies to Elast (Fig. 2). More than half the WG patients (n = 11) failed to develop antibodies reactive to the minor antigens. The serum of the single patient with microscopic polyangiitis also did not react to any of the minor antigens tested.
Group 4: IF−/PR3−/MPO− (n = 114)
The IF−/PR3−/MPO− consisted of patients diagnosed with forms of vasculitis other than traditional ‘ANCA-associated vasculitis’ (n = 42) and miscellaneous non-rheumatological diagnoses, as described fully in the Methods section (n = 72). As anticipated, because of the negative ANCA, these sera showed the lowest degree of reactivity to the minor antigens. The most common reactivities were to Cath G (25%). No sample in the group had reacted with Elast. Other reactivities to minor neutrophil antigens included 14% to BPI and 6% to LF (Fig. 2). Antibodies to BPI and LF antigens are often found in patients with negative ANCA by IF [21–23]. This group showed the least reactivity among the groups to multiple antigens. Twenty-two per cent showed reactivity to a single minor antigen but only 12% reacted to two minor antigens and no patients reacted to further antigens.
The diagnoses of the patients negative for ANCA by IF and negative for MPO and PR3 by ELISA but positive for antibodies reactive to the minor antigens include the following: other types of vasculitis (polyarteritis nodosa n = 3, Takayasu's arteritis n = 1, hypersensitivity vasculitis n = 1, leucocytoclastic vasculitis, n = 1, miscellaneous vasculitis disorders n = 2), coronary artery disease n = 4, infections n = 5 [human immunodeficiency virus (HIV), hepatitis c virus (HCV), acute respiratory disease syndrome (ARDS)], sarcoidosis n = 2, glomerulonephritis n = 2, various chronic inflammatory disorders n = 3, diabetes n = 2, Behçet's disease n = 2, stroke n = 1, chronic granulomatosis n = 1, pulmonary haemosiderosis n = 1, acute myelogenous leukaemia n = 1 and a few patients with miscellaneous diagnoses n = 7.
Discussion
In WG and microscopic polyangiitis, ANCA are directed primarily against PR3 and MPO, respectively. As seen in this study and others [25], some patients WG or with microscopic polyangiitis − up to 40% in some reports, particularly patients with ‘limited’ WG [26] − do not have antibodies to either PR3 or MPO. In IF− sera that do not contain PR3- or MPO-ANCA, there is a greater frequency of antibodies to minor antigens than in sera that contain PR3- or MPO-ANCA. Multiple combinations of minor target antigen reactivities are common. Thus, a variety of antibody specificities could account for a positive ANCA even in the absence of antibodies to PR3 and MPO.
The fact that ANCA is positive in PR3 and MPO sera explains the imperfect correlation between IF and EIA testing. Antibodies to minor antigens may be found in patients with conditions not associated with primary systemic vasculitis. This underscores the importance of confirming positive IF studies with PR3- or MPO-specific EIAs, and also indicates that testing for minor antigens may be helpful in explaining IF− sera that are PR3- and MPO-ANCA negative.
Antigens to minor antigens such as BPI, potentially important in pulmonary inflammatory disorders such as cystic fibrosis, have also been detected in patients with prolonged co-existent infections [21]. Due to the small number of patients in this study, we were unable to establish a correlation between the other minor antigens tested and specific clinical scenarios. We detected very little anti-Elast antibody in any group. Anti-Elast was reported to present itself as a C-ANCA pattern on IF [12]. In combination with PR3, Elast is known to stimulate endothelial tissue factor, which in turn could be relevant to the development of vascular inflammation [27]. In contrast to the study by Wiesner et al. [15], we did not note any connection between the finding of anti-Elast antibodies and a history of cocaine abuse. The number of patients with anti-Elast antibodies in our cohort was small, however, and it is conceivable that not all patients were forthcoming about their histories of cocaine use. Only one WG patient (who did not have a history of cocaine use) showed Elast antibodies. All other patients (a total of six) with anti-Elast antibodies were in the ‘other diagnosis’ group.
An atypical P-ANCA pattern of IF is associated with the presence of Cath G antibodies in patients with acute malaria [28]. Even though our study did not look specifically at atypical P-ANCA patterns, we did observe high levels of anti-Cath G antibodies in all groups. Thus, it is conceivable that antibodies to minor antigens reflect high levels of non-specific inflammation in some patients. In conclusion, the presence of antibody to these minor antigens appear to explain the ‘false positive’ IF results detected in patients who are negative for PR3- and MPO-ANCA. The clinical and pathophysiological significance of multiple antibodies to leucocytic antigens in patients' sera is not currently clear. However, Manolova and Dantcheva reoprted that rheumatoid arthritis patients with P-ANCA by IF showing reactivities to major or minor neutrophil antigens exhibited significantly higher inflammatory activity [14]. Furthermore, a recent report by Gao et al. suggests that patients with multiple ‘ANCA-associated antigens’ may be at increased risk of developing overt clinical vasculitis [29]. Clearly, while these antibodies may have no pathological role determined as yet, they may at least be markers of an inflammatory process.
The principal value of testing for antibodies to minor antigens at this time would be to explain IF reactivity in the absence of PR3- and MPO-ANCA negativity. We were unable to establish specific correlations between the presence of ANCA-associated minor antigens and a particular disease pattern, nor do antibodies to these four antigens appear to distinguish among different forms of vasculitis. These results do suggest, however, that many conditions associated with inflammation may develop antibodies to components of the neutrophil granules. In most cases, these antibodies are benign and are not associated with disease but may be markers of inflammation. We may speculate that, in a few cases, the antibody response may spread to additional minor granular components of neutrophils, such as we see in the ANCA-positive but MPO− and PR3− patients. Whether or not these antibodies are associated with pathology is as yet unknown. In certain cases, the immunological response to the minor antigens may lead to further epitope/antigen spread to the major antigens, MPO or PR3; these patients may be those who develop what we call ‘ANCA-associated vasculitis’. Additional studies will focus on defining the relationship between changes in titres of minor antigen antibodies and the level of disease activity in inflammatory disorders.
Acknowledgments
This work was supported in part by the General Clinical Research Center at Johns Hopkins Hospital (NIH/NCRR/GCRC grant no. RR00052) and by NIH/NIAMS grant no. K24 AR049185-01. Dr Stone is a Hugh and Renna Cosner Scholar in the Center for Innovative Medicine at the Johns Hopkins Bayview Medical Center.
References
- 1.Van Der Woude FJ, Rassmussen N, Lobatto S, et al. Autoantibodies to neutrophils and monocytes: a new tool for diagnosis and a marker for disease activity in Wegener's granulomatosis. Lancet. 1985;1:425–9. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- 2.Stone JH, Talor M, Stebbing J, et al. Test characteristics of immunofluorescence and ELISA tests in 856 consecutive patients with possible ANCA-associated conditions. Arthritis Care Res. 2000;13:424–34. doi: 10.1002/1529-0131(200012)13:6<424::aid-art14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–7. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman GS, Specks U. Antineutrophil cytoplasmic antibodies. Arthritis Rheum. 1998;41:1521–37. doi: 10.1002/1529-0131(199809)41:9<1521::AID-ART2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 5.Savige JA, Davies DJ, Gatenby PA. Anti-neutrophil cytoplasmic antibodies (ANCA). their detection and significance: report from workshops. Pathology. 1994;26:186–93. doi: 10.1080/00313029400169451. [DOI] [PubMed] [Google Scholar]
- 6.Hohn PA, Popescu NC, Hanson RD, Salvesen G, Ley TJ. Genomic organization and chromosomal localization of the human cathepsin G gene. J Biol Chem. 1989;264:13412–19. [PubMed] [Google Scholar]
- 7.Calafat J, Janssen H, Knol EF, Malm J, Egesten A. The bactericidal/permeability-increasing protein (BPI) is membrane-associated in azurophil granules of human neutrophils, and relocation occurs upon cellular activation. APMIS. 2000;108:201–8. doi: 10.1034/j.1600-0463.2000.d01-45.x. [DOI] [PubMed] [Google Scholar]
- 8.Roos D, Dolman KM. Neutrophil involvement in inflammatory tissue damage. Neth J Med. 1990;36:89–94. [PubMed] [Google Scholar]
- 9.Gaskin G, Savage CO, Ryan JJ, et al. Anti-neutrophil cytoplasmic antibodies and disease activity during long-term follow-up of 70 patients with systemic vasculitis. Nephrol Dial Transplant. 1991;6:689–94. doi: 10.1093/ndt/6.10.689. [DOI] [PubMed] [Google Scholar]
- 10.Tervaert JW, Kallenberg CG. Anti-myeloperoxidase antibodies in Churg–Strauss syndrome. J Neurol. 1993;240:449–50. doi: 10.1007/BF00867361. [DOI] [PubMed] [Google Scholar]
- 11.Goldschmeding R, van der Schoot CE, ten Bokkel HD, et al. Wegener's granulomatosis autoantibodies identify a novel diisopropylfluorophosphate-binding protein in the lysosomes of normal human neutrophils. J Clin Invest. 1989;84:1577–87. doi: 10.1172/JCI114335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nassberger L, Jonsson H, Sjoholm AG, Sturfelt G, Heubner A. Circulating anti-elastase in systemic lupus erythematosus. Lancet. 1989;1:509. doi: 10.1016/s0140-6736(89)91420-7. [DOI] [PubMed] [Google Scholar]
- 13.Kuwana T, Sato Y, Saka M, et al. Anti-cathepsin G antibodies in the sera of patients with ulcerative colitis. J Gastroenterol. 2000;35:682–9. doi: 10.1007/s005350070047. [DOI] [PubMed] [Google Scholar]
- 14.Manolova I, Dantcheva M. Antineutrophil cytoplasmic antibodies in Bulgarian patients with rheumatoid arthritis: characterization and clinical associations. Rheumatol Int. 2005;26:107–14. doi: 10.1007/s00296-004-0517-2. [DOI] [PubMed] [Google Scholar]
- 15.Wiesner O, Russell KA, Lee AS, et al. Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum. 2004;50:2954–65. doi: 10.1002/art.20479. [DOI] [PubMed] [Google Scholar]
- 16.Nishiya K, Chikazawa H, Hashimoto K, Miyawaki S. Antineutrophil cytoplasmic antibody in patients with primary Sjogren's syndrome. Clin Rheumatol. 1999;18:268–71. doi: 10.1007/s100670050100. [DOI] [PubMed] [Google Scholar]
- 17.Coremans IE, Hagen EC, Daha MR, et al. Antilactoferrin antibodies in patients with rheumatoid arthritis are associated with vasculitis. Arthritis Rheum. 1992;35:1466–75. doi: 10.1002/art.1780351210. [DOI] [PubMed] [Google Scholar]
- 18.Vecchi M, Sinico A, Bianchi MB, et al. Recognition of bactericidal/permeability-increasing protein by perinuclear anti-neutrophil cytoplasmic antibody-positive sera from ulcerative colitis patients: prevalence and clinical significance. Scand J Gastroenterol. 1998;33:1284–8. doi: 10.1080/00365529850172377. [DOI] [PubMed] [Google Scholar]
- 19.Caccavo D, Rigon A, Picardi A, et al. Anti-lactoferrin antibodies in systemic lupus erythematosus: isotypes and clinical correlates. Clin Rheumatol. 2005;24:381–7. doi: 10.1007/s10067-004-1040-2. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Zhao M, Zhang Y, Guo X, Wang H. [Clinicopathological characteristics of propylthiouracil-induced antineutrophil cytoplasmic antibodies-positive vasculitis and their target antigens: a report of 4 cases and literature review] Zhonghua Nei Ke Za Zhi. 2002;41:404–7. [PubMed] [Google Scholar]
- 21.Zhao MH, Jayne DR, Ardiles LG, Culley F, Hodson ME, Lockwood CM. Autoantibodies against bactericidal/permeability-increasing protein in patients with cystic fibrosis. Q J Med. 1996;89:259–65. doi: 10.1093/qjmed/89.4.259. [DOI] [PubMed] [Google Scholar]
- 22.Schultz H, Csernok E, Schuster A, Schmitz TS, Ernst M, Gross WL. Anti-neutrophil cytoplasmic antibodies directed against the bactericidal/permeability-increasing protein (BPI) in pediatric cystic fibrosis patients do not recognize N-terminal regions important for the anti-microbial and lipopolysaccharide-binding activity of BPI. Pediatr Allergy Immunol. 2000;11:64–70. doi: 10.1034/j.1399-3038.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- 23.Savige JA, Chang L, Wilson D, Buchanan RR. Autoantibodies and target antigens in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides. Rheumatol Int. 1996;16:109–14. doi: 10.1007/BF01409982. [DOI] [PubMed] [Google Scholar]
- 24.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 25.Radice A, Sinico RA. Antineutrophil cytoplasmic antibodies (ANCA) Autoimmunity. 2005;38:93–103. doi: 10.1080/08916930400022673. [DOI] [PubMed] [Google Scholar]
- 26.Savige J, Davies D, Falk RJ, Jennette JC, Wiik A. Antineutrophil cytoplasmic antibodies and associated diseases. a review of the clinical and laboratory features. Kidney Int. 2000;57:846–62. doi: 10.1046/j.1523-1755.2000.057003846.x. [DOI] [PubMed] [Google Scholar]
- 27.Haubitz M, Gerlach M, Kruse HJ, Brunkhorst R. Endothelial tissue factor stimulation by proteinase 3 and elastase. Clin Exp Immunol. 2001;126:584–8. doi: 10.1046/j.1365-2249.2001.01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yahya TM, Benedict S, Shalabi A, Bayoumi R. Anti-neutrophil cytoplasmic antibody (ANCA) in malaria is directed against cathepsin G. Clin Exp Immunol. 1997;110:41–4. doi: 10.1046/j.1365-2249.1997.4981395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Y, Chen MYeH, Guo XH, Zhao MH, Wang HY. The target antigens of antineutrophil cytoplasmic antibodies (ANCA) induced by propylthiouracil. Int Immunopharmacol. 2007;7:55–60. doi: 10.1016/j.intimp.2006.07.033. [DOI] [PubMed] [Google Scholar]