Abstract
The primary objective of this study was to probe the involvement of leukotriene B4 (LTB4) in itraconazole (0·1–5 µM)-mediated inhibition of Ca2+ uptake by chemoattractant-activated human neutrophils. Following exposure of the cells to platelet-activating factor (PAF, 200 nM), LTB4 was measured by immunoassay, while neutrophil cytosolic Ca2+ concentrations were determined by a fura-2/AM-based spectrofluorimetric procedure. Activation of neutrophils was accompanied by an abrupt and sustained (for about 1 min) elevation in cytosolic Ca2+ which was associated with increased generation of LTB4, both of which were attenuated significantly by itraconazole at 0·5 µM and higher. The inhibitory effect of the anti-mycotic on Ca2+ uptake by PAF-activated cells was mimicked by an LTB4 antibody, as well as by LY255283 (1 µM) and MK886 (0·5 µM), an antagonist of LTB4 receptors and an inhibitor of 5′-lipoxygenase-activating protein, respectively, while addition of itraconazole to purified 5′-lipoxygenase resulted in inhibition of enzyme activity. A mechanistic relationship between itraconazole-mediated inhibition of LTB4 production and Ca2+ influx was also supported by the observation that pulsed addition of purified LTB4 to PAF-activated neutrophils caused substantial restoration of Ca2+ uptake by cells treated with the anti-mycotic. Taken together, these observations suggest that the potentially beneficial anti-inflammatory interactions of itraconazole with activated neutrophils result from interference with production of LTB4, with consequent attenuation of a secondary LTB4-mediated wave of Ca2+ uptake by the cells.
Keywords: 5′-lipoxygenase, calcium, itraconazole, leukotriene B4, neutrophils, platelet-activating factor
Introduction
Itraconazole, an imidazole anti-mycotic, antagonizes the uptake of Ca2+ by activated human neutrophils, as well as by other types of mammalian cell [1–5]. In the case of neutrophils, itraconazole-mediated inhibition of Ca2+ influx not only interferes with the Ca2+-dependent proinflammatory activities of the cells, but also prevents their reactivation by preventing store-operated refilling of intracellular Ca2+ stores [1,2,5]. These inhibitory effects of the anti-mycotic on Ca2+ influx have been observed not only in chemoattractant-activated neutrophils, but also following the addition of Ca2+ to neutrophils with depleted intracellular Ca2+ stores suspended in Ca2+ free medium [5]. Although potentially beneficial, the molecular/biochemical basis of these secondary, anti-inflammatory interactions of itraconazole with neutrophils have not been established. Possible mechanisms include inhibition of cytochrome P450 (CYP450) enzyme systems, specifically CYP450 epoxygenase, which converts arachidonate to epoxyeicosatrienoic acid, a putative Ca2+ influx factor [1,2,6,7], as well as direct antagonism of Ca2+ channels [8,9].
Interference with the production of leukotriene B4 (LTB4) represents an alternative, albeit unexplored, mechanism of itraconazole-mediated interference with store-operated uptake of Ca2+ by activated neutrophils. In this respect it is noteworthy that itraconazole has been reported to inhibit the production of LTB4 by intact neutrophils activated with the Ca2+ ionophore, A23187 [10], while endogenous-generated LTB4 has been reported to amplify the uptake of Ca2+ following addition of the chemoattractant, platelet-activating factor (PAF), to neutrophils [11].
In the current study we have investigated the effects of itraconazole on the production of LTB4 by PAF-activated neutrophils and its relationship to alterations in Ca2+ influx.
Materials and methods
Chemicals
Itraconazole was kindly provided by Janssen Pharmaceutica (Geel, Belgium) and dissolved in dimethylacetamide (DMA) to give a stock concentration of 10 mM. Subsequent dilutions were made in the same solvent and the final concentrations of itraconazole used in the assays described below ranged from 0·1 to 5 µM, while that of DMA was 0·1%. Unless indicated, all other chemicals and reagents were purchased from Sigma Chemical Co. (St Louis, MO, USA).
Neutrophils
The study was approved by the Faculty of Health Sciences Research Ethics Committee of the University of Pretoria (Protocol 43/2006), and prior informed consent was obtained from all participants.
Purified neutrophils were prepared from heparinized (5 units of preservative-free heparin/ml) venous blood of healthy, non-smoking adult human volunteers and separated from mononuclear leucocytes by centrifugation on Histopaque®-1077 (Sigma Diagnostics) cushions at 400 g for 25 min at room temperature. The resultant pellet was suspended in phosphate-buffered saline (PBS; 0·15 M, pH 7·4) and sedimented with 3% gelatine to remove most of the erythrocytes. After centrifugation, erythrocytes were removed by selective lysis with 0·84% ammonium chloride at 4°C for 10 min. The neutrophils, which were routinely of high purity (> 90%) and viability (> 95%), were resuspended at 1 × 107/ml in PBS and held on ice until used.
Measurement of LTB4
A competitive binding enzyme immunoassay procedure (Correlate-EIA™; Assay Designs Inc., Ann Arbor, MI, USA) was used to measure LTB4 in the supernatants of neutrophils activated with PAF (200 nM) in the absence or presence of itraconazole (0·1–5 µM). Neutrophils (2 × 106/ml, final) in Hank's balanced salt solution (HBSS) were preincubated for 10 min at 37°C with itraconazole after which the chemoattractant, platelet-activating factor (PAF; 200 nM, final) was added to the cells and the reactions stopped after 3 min incubation at 37°C (predetermined in preliminary time–course experiments) by the addition of an equal volume of ice-cold HBSS to the tubes which were then held in an ice-bath prior to pelletting the cells by centrifugation. The cell-free supernatants were then assayed for LTB4 using the enzyme immunoassay (EIA) procedure. Supernatants from cells activated with PAF were diluted 1 : 4 prior to assay. These results are expressed as picograms (pg)/107 cells.
The effects of LY255283 (1 µM) and MK886 (0·5 µM), an LTB4 receptor antagonist [12] and 5′-lipoxygenase-activating protein (FLAP) inhibitor [13], respectively (both from the Cayman Chemical Co., Ann Arbor, MI, USA), on LTB4 production by PAF-activated neutrophils were also investigated. The concentrations of MK886 and LY255283 used for these experiments (0·5 µM and 1 µM, respectively) were based on data derived from preliminary experiments. At a concentration of 0·5 µM, MK886 effectively attenuated the generation of LTB4 by neutrophils activated with the calcium ionophore, A23187 (1 µM), while LTB4 (50 nM)-mediated mobilization of Ca2+ from neutrophil intracellular stores was completely antagonized by 1 µM LY255283 (data not shown).
The effects of itraconazole (5 µM) on the production of LTB4 by PAF-activated neutrophils suspended in Ca2+ replete and Ca2+ free HBSS were also investigated. To minimize loss of Ca2+ from intracellular stores, neutrophils were preincubated in Ca2+ replete HBSS for 10 min at 37°C, pelleted by centrifugation and resuspended in either Ca2+ replete HBSS (control cells) or HBSS containing 50 µM CaCl2. The cells were then preincubated at 37°C for 9 min, after which the Ca2+ chelating agent, ethylene glycol tetraacetic acid (EGTA) (500 µM final) was added to cells suspended in HBSS containing 50 µM CaCl2, followed by transfer to cuvettes and activation 1–2 min later with PAF. With this procedure we were unable to detect influx of Ca2+ into neutrophils treated with the pneumococcal pore-forming toxin, pneumolysin (8·37 ng/ml, final), which causes influx of extracellular Ca2+ without mobilizing the cation from neutrophil intracellular stores [14].
To exclude possible interference of itraconazole, LY255283, and MK886 at concentrations of 5, 1 and 0·5 µM, respectively, with LTB4 in the EIA, we investigated the reactivity of a fixed concentration (3000 pg/ml) of the LTB4 standard in the presence and absence of the LTB4 receptor antagonist.
Activity of purified 5′-lipoxygenase (5-LO)
The effects of itraconazole (5–50 µM, final) or the DMA solvent (1% final in this system) on the reactivity of purified 5-LO were investigated using the Lipoxygenase Inhibitor Screening Assay Kit from the Cayman Chemical Co., which is a colorimetric assay using purified potato 5-LO (5 units/assay) and linoleic acid (100 µM, final) as enzyme and substrate, respectively (as recommended by the manufacturer). The enzyme and DMA or itraconazole in assay buffer were preincubated for 15 min at room temperature, followed by addition of substrate and incubation for an additional period of 5 min. Enzyme activity was monitored spectrophotometrically at 490 nm according to the magnitude of chromogen-reactive hydroxperoxide formation. Substrate and enzyme were omitted from negative control systems. The results of these experiments are expressed as the mean percentage activity of the itraconazole-free control systems.
Spectrofluorimetric measurement of Ca2+ fluxes
Fura-2/AM was used as the fluorescent Ca2+ sensitive indicator for these experiments [15]. Neutrophils (1 × 107/ml) suspended in PBS were prewarmed for 5 min at 37°C followed by addition of fura-2/AM (2 µM, final) and reincubated for 25 min at 37°C. The fura-2-loaded neutrophils were then pelleted by centrifugation and resuspended in indicator-free, Ca2+ replete (1·25 mM CaCl2) HBSS, pH 7·4. The neutrophils (2 × 106/ml) were then preincubated for 10 min at 37°C with one of the following: itraconazole (0·1–5 µM); MK886 (0·5 µM) or LY255283 (1 µM); an equivalent amount of the relevant DMA (itraconazole) or dimethylsulphoxide (DMSO; MK886, LY255283) solvent control systems. The cells were then transferred to disposable reaction cuvettes which were maintained at 37°C in a Hitachi 650 10S fluorescence spectrophotometer with excitation and emission wavelengths set at 340 and 500 nm, respectively. After a stable baseline was obtained (1 min), the neutrophils were activated by addition of PAF (200 nM). Alterations in fluorescence intensity were then monitored over a 5–10-min period. The final volume in each cuvette was 3 ml containing a total of 6 × 106 neutrophils.
In a related series of experiments, the involvement of LTB4 in sustaining cytosolic Ca2+ transients in neutrophils activated with PAF was also probed using an anti-LTB4 antibody produced in rabbit whole anti-serum (Sigma). The antibody (60 µl of a 5-mg/ml solution) was added to the cells 1 min prior to the activators. An equivalent concentration of a control antibody to prostaglandin F2α (PGF2α) (rabbit whole anti-serum; Sigma) was added to control systems. The efficacy of the LTB4 antibody was demonstrated in preliminary experiments in which it was found to antagonize the mobilization of Ca2+ from neutrophil intracellular stores following addition of purified LTB4 (50 nM) to the cells.
In an attempt to clarify the possible association between itraconazole-mediated inhibition of LTB4 production and Ca2+ influx, an additional series of experiments was designed to investigate the potential of added, purified LTB4 to reverse the inhibitory effects of itraconazole (5 µM) on the uptake of Ca2+ by PAF-activated neutrophils. Three strategies were used: (i) LTB4 (75 nM) and PAF (200 nM) were added simultaneously to control or itraconazole-treated cells; (ii) LTB4 (75 nM) was added 10 s after PAF; and (iii) LTB4 (15 nM) and PAF were added simultaneously, followed 10 s later by addition of a second, higher dose of LTB4 (75 nM) only.
Cellular adenosine triphosphate (ATP) levels
The cytotoxic potential of itraconazole (5 µM), LY255283 (1 µM) and MK886 (0·5 µM) was determined by measurement of cellular ATP in the lysates of neutrophils (106/ml) which had been exposed to the test agents for 10 min at 37°C using a luciferin/luciferase chemiluminescence procedure [16]. The results are expressed as picomoles ATP/107 cells.
Expression and statistical analysis of results
The results of each series of experiments are expressed as the mean values ± s.e.m., with the exception of several of the fura-2 experiments for which the traces are shown. Statistical analysis was performed using Student's t-test when comparing two groups or by analysis of variance with subsequent Tukey–Kramer multiple comparisons test for multiple comparisons.
Results
Effects of itraconazole on LTB4 production by activated neutrophils
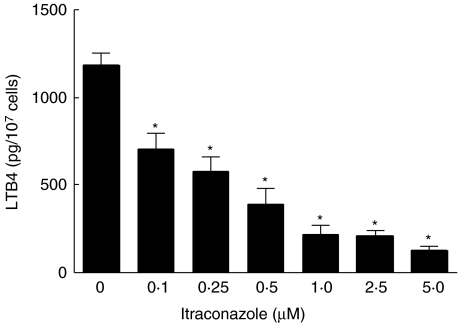
The effects of itraconazole at concentrations of 0·1–5 µM on the production of LTB4 by neutrophils activated with PAF are shown in Fig. 1. Itraconazole caused dose-related inhibition of the production of LTB4 by the chemoattractant, which attained statistical significance at concentrations of 0·1 µM.
Fig. 1.
Effects of itraconazole (0·1–5 µM) on the production of leukotriene B4 (LTB4) by platelet-activating factor (PAF) (200 nM)-activated neutrophils. The results of three experiments with five replicates for each system are expressed as the mean values ± s.e.m. The values for the unstimulated control, and for the PAF-activated control systems were 43 ± 5 and 1190±67 pg LTB4/107 cells, respectively. *P < 0·05 for comparison with the corresponding itraconazole-free control system.
A comparison of the effects of LY255283 and MK886 on PAF-activated production of LTB4 by neutrophils is shown in Table 1. Both agents caused statistically significant inhibition of LTB4 production. Importantly, itraconazole (5 µM), LY255283 (1 µM) and MK886 (0·5 µM) did not affect the reactivity of LTB4 in the EIA (data not shown).
Table 1.
Effects of LY255283 (1 µM) and MK886 (0·5 µM) on the production of leukotreine B4 (LTB4) by platelet-activating factor (PAF)-activated neutrophils.
| PAF-activated neutrophils incubated with | Leukotriene B4 (pg/107 cells) |
|---|---|
| Solvent only (control) | 1054 ± 49a |
| 1·0 µM LY255283 | 396 ± 42* |
| 0·5 µM MK886 | 78 ± 16* |
The results of three different experiments with five replicates for each system are expressed as the mean values ± s.e.m. The basal values for unstimulated cells was 59 ± 10 pg LTB4/107.
P < 0·05.
Comparison of the effects of itraconazole on LTB4 production by PAF-activated neutrophils suspended in Ca2+ replete and Ca2+ free HBSS
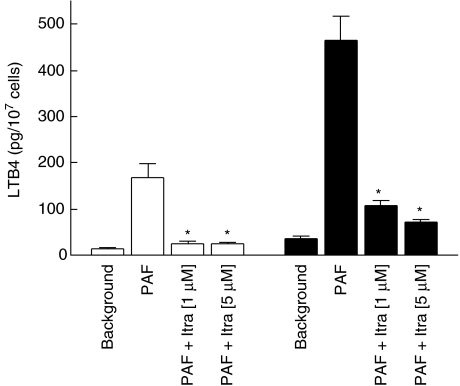
These results are shown in Fig. 2. Although markedly reduced relative to the corresponding responses in Ca2+ replete HBSS, the production of LTB4 by PAF-activated neutrophils was still detectable and increased significantly above basal, unstimulated values. The production of LTB4 by PAF-activated neutrophils was significantly (P < 0·05) inhibited by itraconazole irrespective of the Ca2+ content of the cell-suspending medium (Fig. 2).
Fig. 2.
Effects of itraconazole (Itra) at concentrations of 1 and 5 µM on the production of leukotriene B4 (LTB4) by platelet-activating factor (PAF) (200 nM)-activated, Ca2+ loaded neutrophils (to ensure that intracellular Ca2+ stores are full) suspended in Ca2+ free (□) or Ca2+ replete (▪) medium. The results of three experiments with five replicates for each system in each experiment are expressed as the mean values ± s.e.m. *P < 0·05 for comparison with the corresponding itraconazole-free, PAF-activated system.
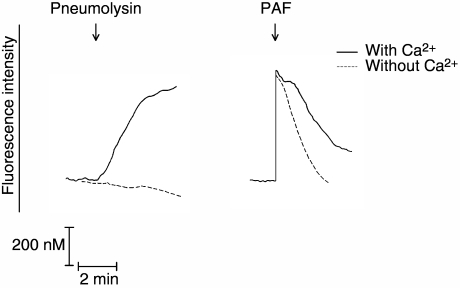
Cytosolic Ca2+ fluxes of neutrophils suspended in either Ca2+ replete or Ca2+ free HBSS and treated with pneumolysin or PAF are shown in Fig. 3. The influx of Ca2+ which accompanies treatment of neutrophils with pneumolysin was abolished completely in the Ca2+ free system, while the abrupt increase in cytosolic Ca2+ remained intact in PAF-activated cells, but was associated with attenuation of the plateau phase and a rapid decline in fura-2 fluorescence intensity due, presumably, to absence of influx of Ca2+ as described in detail in the section on cytosolic Ca2+ fluxes below. These observations validate the experimental design used to eliminate Ca2+ influx during activation of the cells with PAF, in the setting of retention of mobilization of the cation from intracellular stores.
Fig. 3.
Traces showing the fura-2 fluorescence responses of Ca2+ loaded neutrophils (to ensure that intracellular Ca2+ stores are full) suspended in either Ca2+ free or Ca2+ replete Hank's balanced salt solution, followed by addition of either pneumolysin (8·37 ng/ml) or platelet-activating factor (PAF) (200 nM) as denoted by the arrow (↓).
Effects of itraconazole on purified 5-LO
Itraconazole caused dose-related inhibition of the activity of purified 5-LO in a cell-free assay system with linoleic acid as substrate. The mean percentages of the DMA-treated control systems were 83 ± 4, 77 ± 4, 71 ± 6, and 55 ± 5% for systems containing 5, 10, 20 and 50 µM itraconazole, respectively (data from six experiments; P < 0·05 for comparison of each of the itraconazole-treated systems with the control system). These experiments were complicated by the insolubility of itraconazole in aqueous, cell-free systems, and may have under-estimated the inhibitory potency of itraconazole for 5-LO.
Effects of itraconazole, LY255283, MK886 and LTB4-antibody on cytosolic Ca2+ transients in activated neutrophils
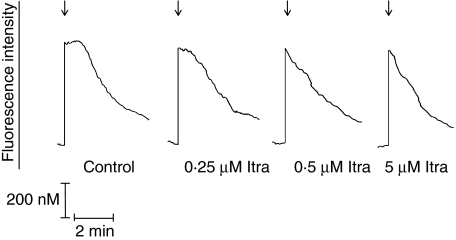
The effects of itraconazole at concentrations of 0·1–5 µM on cytosolic Ca2+ fluxes in PAF-activated neutrophils are shown in Fig. 4 and Table 2, while the comparative effects of itraconazole, LY255283, MK886 and the LTB4-antibody are shown in Fig. 5 and Table 2. Addition of PAF to neutrophils was accompanied by the typical, abrupt increase in fluorescence intensity which accompanies G-protein-coupled receptor activation of phospholipase C and inositol triphosphate-mediated release of Ca2+ from intracellular stores. Peak fluorescence intensity was sustained for about 1–1·5 min, subsiding gradually thereafter, compatible with the previously described early influx of Ca2+ into PAF-activated neutrophils and slow clearance of cytosolic Ca2+ [17]. Pretreatment of neutrophils with itraconazole did not affect the magnitude of the immediate peak cytosolic Ca2+ response of PAF-activated neutrophils (535 ± 41 nM for the control system, rising from a basal value of 116 ± 12 nM). However, the duration of the plateau phase of peak fluorescence intensity was shortened significantly by treatment of the cells with the anti-mycotic, compatible with inhibition of Ca2+ influx. These effects of itraconazole were statistically significant at concentrations of ≥ 0·5 µM (Fig. 4 and Table 2).
Fig. 4.
Traces from a single representative experiment (six in the series) showing the effects of itraconazole (Itra) at concentrations of 0·25, 0·5 and 5 µM on the fura-2 fluorescence responses of platelet-activating factor (PAF) (200 nM)-activated neutrophils added as denoted by the arrow (↓).
Table 2.
Effect of itraconazole, LY255283, MK886 and the leukotreine B4 (LTB4) antibody on duration of the peak cytosolic response of platelet-activating factor (PAF)-activated neutrophils.
| PAF-activated neutrophils incubated with | Duration of the peak cytosolic Ca2+ response (min) |
|---|---|
| Solvent only (control) | 1·20 ± 0·05a |
| 0·1 µM itraconazole | 1·00 ± 0·10 |
| 0·25 µM itraconazole | 0·80 ± 0·13 |
| 0·5 µM itraconazole | 0·23 ± 0·08* |
| 1·0 µM itraconazole | 0·25 ± 0·03* |
| 2·5 µM itraconazole | 0·30 ± 0·03* |
| 5·0 µM itraconazole | 0·33 ± 0·02* |
| 1 µM LY255283 | 0·60 ± 0·03* |
| 0·5 µM MK886 | 0·13 ± 0·02* |
| LTB4-antibody | 0·70 ± 0·03* |
The results of six different experiments are presented as the mean duration (min) ± s.e.m. of the peak cytosolic Ca2+ response. The mean peak cytosolic Ca2+ response of the control system was 535 ± 41 nM, rising from a basal value of 116 ± 41 nM.
P < 0·05 for comparison with the itraconazole-free control system.
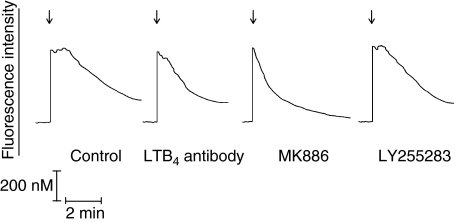
Fig. 5.
Traces from a single representative experiment (six to eight in the series) showing the effects of an anti-leukotriene B4 (LTB4) antibody (60 µg/ml), MK886 (0·5 µM) and LY255283 (1 µM) on the fura-2 fluorescence responses of platelet-activating factor (PAF) (200 nM)-activated neutrophils added as denoted by the arrow (↓).
As shown in Fig. 5 and Table 2, treatment of neutrophils with LY255283, MK886 or the LTB4-antibody also (P < 0·05) shortened significantly the duration of the peak plateau phase of fura-2 fluorescence intensity in PAF-activated cells, without affecting the magnitude of the immediate peak increase in cytosolic Ca2+. The duration of the plateau phase was not affected by treatment of the cells with the control, PGF2α-antibody (not shown).
As shown in Table 3, pulsed addition of purified LTB4 (i.e. PAF + 15 nM LTB4, followed 10 s later by 75 nM LTB4) substantially restored Ca2+ influx to itraconazole (5 µM)-treated neutrophils, without affecting the fura-2 responses of itraconazole-free control cells. On the other hand, simultaneous addition of LTB4 (75 nM) with PAF only, or addition of LTB4 (75 nM) 10 s after PAF, was less effective in counteracting the inhibitory effects of itraconazole on Ca2+ influx (data not shown).
Table 3.
Effects of pulsed addition of leukotriene B4 (LTB4) on itraconazole (5 µM)-mediated attenuation of the sustained, peak cytosolic Ca2+ transient in platelet-activating factor (PAF) (200 nM)-activated neutrophils.
| System | Duration of the peak cytosolic Ca2+ response (min) |
|---|---|
| Neutrophils + PAF | 1·05 ± 0·03a |
| Neutrophils + itraconazole + PAF | 0·25 ± 0·02 |
| Neutrophils + PAF + LTB4 | 1·09 ± 0·03 |
| Neutrophils + itraconazole + PAF + LTB4 | 0·82 ± 0·03* |
Results are expressed as the mean values ± s.e.m. for the duration of the abruptly occurring peak Ca2+ transients in neutrophils activated with PAF only, or with PAF + LTB4 (15 nM) added simultaneously followed 10 s later by 75 nM LTB4 in the absence or presence of 5 µM itraconazole (data from 10 determinations).
P < 0·05 for comparison between the itraconazole-treated systems activated with PAF only or with PAF + LTB4.
Although not shown, we also observed that treatment of neutrophils with itraconazole (5 µM) did not affect the fura-2 fluorescence responses of cells activated with purified LTB4 (50 nM), excluding possible LTB4 receptor blockading effects of the anti-mycotic.
ATP levels
At the maximum concentrations used, ATP was unaffected by treatment with itraconazole (5 µM), LY255283 (1 µM) or MK886 (0·5 µM). In the case of itraconazole the values for the solvent control (0·1% DMA) and drug-treated systems were 29·4 ± 2·7 and 30·0 ± 2·1 pmoles ATP/107 cells, respectively, while the corresponding values for the DMSO (0·1%) and LY255283- and MK886-treated systems were 25·5 ± 2·4, 25·2 ± 1·8 and 27·0 ± 2·1 pmoles ATP/107 cells. At the concentrations used (0·1%), neither of the solvents affected neutrophil ATP levels (not shown).
Discussion
In the current study, we have observed that itraconazole attenuates both LTB4 production and Ca2+ influx following activation of human neutrophils with the chemoattractant, PAF. Assuming a mechanistic relationship between these events, two possibilities were evident. Firstly, the anti-mycotic acts primarily as an antagonist of Ca2+ influx leading to secondary inhibition of LTB4 production; alternatively, the inhibitory effects of the anti-mycotic on Ca2+ influx are indirect, resulting from interference with production of LTB4.
Taken together, the following observations clearly supported a primary inhibitory effect of itraconazole on LTB4 production, with consequent secondary inhibition of Ca2+ influx in PAF-activated neutrophils; (i) although the magnitude of the LTB4 response was dependent upon the Ca2+ content of the cell-suspending medium, the inhibitory effects of the anti-mycotic were evident irrespective of the presence or absence of the cation in the medium; (ii) the inhibitory effects of itraconazole on Ca2+ uptake were mimicked by treatment of the cells with an antibody to LTB4, as well as by non-cytotoxic concentrations of LY255283 and MK886, antagonists of LTB4 receptors and FLAP, respectively; (iii) the inhibitory effects of itraconazole on Ca2+ influx were negated largely by addition of LTB4, while the responses of itraconazole-free control cells were unaffected by the eicosanoid; and (iv) itraconazole antagonized the activity of purified 5′-lipoxygenase (5-LO) in a cell-free system.
In addition to 5-LO, other possible targets of itraconazole which would result in decreased production of LTB4 by activated neutrophils include cytosolic phospholipase A2 and FLAP. Cytosolic phospholipase A2, however, appears to be an improbable target of the anti-mycotic. This contention is based on a previous report that the production of prostaglandin E2 by human neutrophils activated with the calcium ionophore, A23187 is unaffected by itraconazole [10], and our own unpublished observations that the generation of arachidonic acid by A23187 (1 µM)-activated human neutrophils is insensitive to the anti-mycotic. Although involvement of FLAP cannot be excluded, the direct inhibitory effects of itraconazole on purified 5-LO suggest that this is the primary target of the anti-mycotic, which is in keeping with the well-recognized inhibitory interactions of pharmacological agents which possess an imidazole moiety with this enzyme [18,19]. It is noteworthy, however, that the inhibitory effects of itraconazole on purified 5-LO were of lesser magnitude than those observed with LTB4 production by PAF-activated neutrophils. This may simply reflect the strongly lipophilic properties of itraconazole, resulting in intracellular accumulation of the anti-mycotic. Alternatively, other intermediates of arachidonic acid metabolism, such as 5-hydroperoxyeicosatetraenoic acid, may also contribute to Ca2+ uptake by PAF-activated neutrophils.
The inhibitory effects of LY255283 on the production of LTB4 by PAF-activated neutrophils are compatible with a sequence of events which result in a protracted, autocrine activation of the cells. Exposure of neutrophils to the chemoattractant results in mobilization of Ca2+ from both intracellular and extracellular reservoirs and consequent production of LTB4; the eicosanoid in turn interacts with LTB4 receptors and initiates a secondary, autocrine amplification cascade resulting in sustained Ca2+ influx and prolonged activation of the cells.
Unlike the well-recognized involvement of cysteinyl leukotrienes in the pathogenesis of bronchial asthma, the clinical importance of LTB4-mediated autocrine amplification cascades remains to be established. Very recently, however, LTB4 has been reported to be involved critically in both the initiation and perpetuation of neutrophil influx and synovial damage in experimental inflammatory arthritis [20,21], while membrane and nuclear receptors for LTB4 are up-regulated in the lungs of smokers who are susceptible to the development of chronic obstructive pulmonary disease [22]. These reports, together with recent studies which have identified an important role for LTB4 in the pathogenesis of asthma [23,24], clearly underscore the validity of pharmacological anti-inflammatory strategies which target LTB4 and its receptors, although no such agents are currently available for widespread clinical use. In this regard it is noteworthy that the fenamate subgroup of non-steroidal anti-inflammatory agents has been reported to inhibit both leukotriene B4 production and Ca2+ uptake by activated human neutrophils; however, the relationship between these two events was not investigated in these studies [25,26].
Notwithstanding conventional anti-fungal activity, the inhibitory effects of itraconazole on LTB4 production by activated neutrophils described in the current study, together with a possible small contribution from contaminating cells, may also contribute to the usefulness of this and other imidazole anti-mycotics in the prophylaxis and/or therapy of both acute and chronic inflammatory disorders, including septic shock [27], chronic granulomatous disease [5,28] and allergic bronchopulmonary aspergillosis [29]. Moreover, PAF, and by implication LTB4, is considered to be a key proinflammatory mediator of both hyperacute and chronic inflammatory disorders such as septic shock and bronchial asthma [30,31].
In conclusion, itraconazole at therapeutically attainable concentrations of 0·5 µM [32], antagonizes the activity of 5-LO in PAF-activated neutrophils, preventing the initiation of a secondary amplification mechanism with the potential to intensify and prolong harmful inflammatory responses.
References
- 1.Alvarez J, Montero M, Garcia-Sancho J. Cytochrome P-450 may link intracellular Ca2+ stores with plasma membrane Ca2+ influx. Biochem J. 1991;274:193–7. doi: 10.1042/bj2740193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montero M, Alvarez J, Garcia-Sancho J. Control of plasma membrane Ca2+ entry by the intracellular stores. Kinetic evidence for a short-lived mediator. Biochem J. 1992;288:519–25. doi: 10.1042/bj2880519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hornstein EH, Vassilopoulos D, Thomas DE, Friedman FK, Tsokos GC. Modulation of human T-lymphocyte plasma membrane Ca2+ permeability by imidazole antimycotics. Immunopharmacol Immunotoxicol. 1996;18:237–45. doi: 10.3109/08923979609052734. [DOI] [PubMed] [Google Scholar]
- 4.Tran Q-M, Ohashi K, Watanabe H. Calcium signalling in endothelial cells. Cardiovasc Res. 2000;48:13–22. doi: 10.1016/s0008-6363(00)00172-3. [DOI] [PubMed] [Google Scholar]
- 5.Steel HC, Anderson R. Itraconazole antagonizes store-operated influx of calcium into chemoattractrant-activated human neutrophils. Clin Exp Immunol. 2004;136:255–61. doi: 10.1111/j.1365-2249.2004.02443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao YF, Huang L, Morgan JP. Cytochrome P450: a novel system modulating Ca2+ channels and contraction in mammalian heart cells. J Physiol. 1998;508:777–92. doi: 10.1111/j.1469-7793.1998.777bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Zhu P, Freedman BD. Multiple eicosanoid-activated nonselective cation channels regulate B-lymphocyte adhesion to integrin ligands. Am J Physiol Cell Physiol. 2006;290:C873–82. doi: 10.1152/ajpcell.00229.2005. [DOI] [PubMed] [Google Scholar]
- 8.Clementi E, Meldolesi J. Pharmacological and functional properties of voltage-independent Ca2+ channels. Cell Calcium. 1996;19:269–79. doi: 10.1016/s0143-4160(96)90068-8. [DOI] [PubMed] [Google Scholar]
- 9.Elliott AC. Recent developments in non-excitable cell calcium entry. Cell Calcium. 2001;30:73–93. doi: 10.1054/ceca.2001.0215. [DOI] [PubMed] [Google Scholar]
- 10.Steinhilber D, Jaschonik K, Knospe J, Morof O, Roth HJ. Effects of novel antifungal azole derivatives on the 5-lipoxygenase and cyclooxygenase pathway. Arzneimittelforschung. 1990;40:1260–3. [PubMed] [Google Scholar]
- 11.Gaudreault E, Stankova J, Rola-Pleszczynski M. Involvement of leukotriene B4 receptor 1 signaling in platelet-activating factor-mediated neutrophil degranulation and chemotaxis. Prostaglandins Other Lipid Mediat. 2005;75:25–34. doi: 10.1016/j.prostaglandins.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Herron DK, Goodson T, Bollinger NG, et al. Leukotriene B4 receptor antagonists: the LY255283 series of hydroxyacetophenones. J Med Chem. 1992;35:1818–28. doi: 10.1021/jm00088a018. [DOI] [PubMed] [Google Scholar]
- 13.Rouzer CA, Ford-Hutchinson AW, Morton HE, Gillard W. MK886, a potent and specific leukotriene biosynthesis inhibitor blocks and reverses the membrane association of 5-lipoxygenase in ionophore-challenged leukocytes. J Biol Chem. 1990;265:1436–42. [PubMed] [Google Scholar]
- 14.Cockeran R, Theron AJ, Steel HC, et al. Proinflammatory interactions of pneumolysin with human neutrophils. J Infect Dis. 2001;183:604–11. doi: 10.1086/318536. [DOI] [PubMed] [Google Scholar]
- 15.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3340–50. [PubMed] [Google Scholar]
- 16.Holmsen H, Storm E, Day HJ. Determination of ATP and ADP in blood platelets: a modification of the firefly luciferase assay for plasma. Anal Biochem. 1972;46:489–501. doi: 10.1016/0003-2697(72)90323-5. [DOI] [PubMed] [Google Scholar]
- 17.Steel HC, Anderson R. Dissociation of the PAF-receptor from NADPH oxidase and adenylate cyclase in human neutrophils results in accelerated influx and delayed clearance of cytosolic calcium. Br J Pharmacol. 2002;136:81–9. doi: 10.1038/sj.bjp.0704685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mano T, Stevens RW, Ando K, et al. Optimization of imidazole 5-lipoxygenase inhibitors and selection and synthesis of a development candidate. Chem Pharm Bull (Tokyo) 2005;53:965–73. doi: 10.1248/cpb.53.965. [DOI] [PubMed] [Google Scholar]
- 19.De Luca L. Naturally occurring and synthetic imidazoles: their chemistry and biological activities. Curr Med Chem. 2006;13:1–23. [PubMed] [Google Scholar]
- 20.Chen M, Lam BK, Kanaoka Y, et al. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med. 2006;203:837–42. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BTL1 for neutrophil recruitment in inflammatory arthritis. J Exp Med. 2006;203:829–35. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marian E, Baraldo S, Visentin A, et al. Up-regulated membrane and nuclear leukotriene B4 receptors in COPD. Chest. 2006;129:1523–30. doi: 10.1378/chest.129.6.1523. [DOI] [PubMed] [Google Scholar]
- 23.Miyahara N, Takeda K, Miyahara S, et al. Requirement for leukotriene B4 receptor in allergen-induced airway hyperresponsiveness. Am J Respir Crit Care Med. 2005;172:161–7. doi: 10.1164/rccm.200502-205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terawaki K, Yokomizo T, Nagase T, et al. Absence of leukotriene B4 receptor 1 confers resistance to airway hyperresponsiveness and Th2-type immune responses. J Immunol. 2005;175:4217–25. doi: 10.4049/jimmunol.175.7.4217. [DOI] [PubMed] [Google Scholar]
- 25.Kankaanranta H, Moilanen E, Vapaatalo H. Effects of non-steroidal anti-inflammatory drugs on polymorphonuclear leukocyte functions in vitro: focus on fenamates. Naynyn Schmeidebergs Arch Pharmacol. 1994;340:685–91. doi: 10.1007/BF00169375. [DOI] [PubMed] [Google Scholar]
- 26.Kankaanranta H, Moilanen E. Flufenamic acid and tolfenamic acids inhibit calcium influx in human polymorphonuclear leukocytes. Mol Pharmacol. 1995;47:1006–13. [PubMed] [Google Scholar]
- 27.Jacobs S, Price Evans DA, Tariq M, Al Omar NF. Fluconazole improves survival in septic shock: a randomized double-blind prospective study. Crit Care Med. 2003;31:1938–46. doi: 10.1097/01.CCM.0000074724.71242.88. [DOI] [PubMed] [Google Scholar]
- 28.Gallin JI, Alling DW, Malech HL, et al. Itraconazole to prevent fungal infections in chronic granulomatous disease. N Engl J Med. 2003;348:2416–22. doi: 10.1056/NEJMoa021931. [DOI] [PubMed] [Google Scholar]
- 29.Wark PA, Hensley MJ, Saltos N, et al. Anti-inflammatory effect of itraconazole in stable allergic bronchopulmonary aspergillosis: a randomized controlled trial. J Allergy Clin Immunol. 2003;111:952–7. doi: 10.1067/mai.2003.1388. [DOI] [PubMed] [Google Scholar]
- 30.Bulger EM, Arbabi S, Garcia I, Maier RV. The macrophage response to endotoxin requires platelet activating factor. Shock. 2002;17:173–9. doi: 10.1097/00024382-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Prescott SM, McIntyre TM, Zimmerman GA. Platelet-activating factor: a phospholipid mediator of inflammation. In: Gallin JI, Snyderman R, editors. Inflammation: basic principles and clinical correlates. Philadelphia: Lippincott, Williams & Wilkins; 1999. pp. 387–96. [Google Scholar]
- 32.Kageyama S, Masuya M, Tanaka I, et al. Plasma concentration of itraconazole and its antifungal prophylactic efficacy in patients with neutropenia after chemotherapy for acute leukemia. J Infect Chemother. 1999;5:213–16. doi: 10.1007/s101560050038. [DOI] [PubMed] [Google Scholar]