Abstract
IA-2 is a major autoantigen in type 1 diabetes and autoantibodies to it have become important diagnostic and predictive markers. IA-2 also is an intrinsic transmembrane component of dense core secretory vesicles and knock-out studies showed that IA-2 is a regulator of insulin secretion. Here we show that overexpression of IA-2 puts mouse insulinoma MIN-6 beta cells into a pre-apoptotic state and that exposure to high glucose results in G2/M arrest and apoptosis. Molecular study revealed a decrease in phosphoinositide-dependent kinase (PDK)-1 and Akt/protein kinase B (PKB) phosphorylation. Treatment of IA-2-transfected cells with IA-2 siRNA prevented both G2/M arrest and apoptosis and increased Akt/PKB phosphorylation. A search for IA-2 interacting proteins revealed that IA-2 interacts with sorting nexin (SNX)19 and that SNX19, but not IA-2, inhibits the conversion of PtdIns(4,5)P2 to PtdIns(3,4,5)P3 and thereby suppresses the phosphorylation of proteins in the Akt signalling pathway resulting in apoptosis. We conclude that IA-2 acts through SNX19 to initiate the pre-apoptotic state. Our findings point to the possibility that in autoimmune diseases, tissue destruction may be autoantigen-induced, but not necessarily immunologically mediated.
Keywords: Akt, apoptosis, autoimmunity, IA-2, sorting nexin 19
Introduction
Type 1 diabetes in humans is due to destruction of the insulin-producing beta cells in the pancreas [1]. The disease is autoimmune in nature, or at least has a strong immunological component. Three major autoantigens have been identified in human type 1 diabetes: IA-2, glutamic acid decarboxylase (GAD) and insulin. Autoantibodies to IA-2 and GAD are found in 70–80% of newly diagnosed patients and autoantibodies to insulin in 30–50% of newly diagnosed patients [2]. These autoantibodies appear years before the onset of clinical disease and population screening has shown that individuals with autoantibodies to both IA-2 and GAD have about a 50% likelihood of developing type 1 diabetes within 5 years. As a result, autoantibodies are being used widely as predictive markers to identify individuals at high disease risk for entry into therapeutic intervention trials.
IA-2 is a 979 amino acid (a.a.) protein that belongs to the transmembrane protein tyrosine phosphatase family [3,4]. However, because of the lack of key conserved residues at a.a. 877 and a.a. 911, it is enzymatically inactive with known substrates. Enzymatic activity can be restored by site-directed mutagenesis [5]. The protein consists of a signal peptide (a.a. 1–24), extracellular (a.a. 25–576), transmembrane (a.a. 577–600) and intracellular (a.a. 601–979) domain. Autoantibodies to IA-2 are largely conformational in nature and are directed exclusively to epitopes within the intracellular domain [6]. The gene coding IA-2 is located on chromosome 2q35. Homologues showing 46–58% identity, respectively, are found in Caenorhabditis elegans and Drosophila, indicating that IA-2 belongs to an ancient gene family [7]. A closely related protein, IA-2β, also known as phogrin, is located on chromosome 7q36 and has 74% identity to the intracellular and 26% identity to the extracellular domains of IA-2 [8,9].
IA-2 is expressed in neuroendocrine cells throughout the body, including pancreatic beta cells. The protein is an integral component of dense core vesicles (DCV) and knock-out of IA-2 in mice resulted in impaired insulin release and abnormal glucose tolerance tests [10]. Recent overexpression and siRNA knockdown experiments in cell culture showed that IA-2 is an important regulator of glucose-induced and basal insulin secretion [11].
Despite the substantial amount of information that has accumulated about IA-2, it is not known what actually triggers the autoimmune response or whether the autoimmune response to IA-2 actually plays a role in the pathogenesis of type 1 diabetes. Moreover, the fact that 20% or more of patients with type 1 diabetes do not have autoantibodies to any of the known major autoantigens, and that it often takes years for the disease to develop after the first appearance of autoantibodies, raises the possibility that the autoimmune response might be a consequence of and secondary to other mechanisms that are responsible for, or at least contribute to, beta cell destruction. In the present report we show that overexpression of IA-2 puts insulin-secreting mouse insulinoma MIN-6 cells into a pre-apoptotic state and that exposure to a high concentration of glucose results in G2/M arrest and apoptosis. The mechanism(s) involved are described and the possible role of apoptosis, independent of or in conjunction with autoimmunity, as a cause of beta cell destruction is discussed.
Materials and methods
Reagents
Cell culture reagents were purchased from Biosource (Camarillo, CA, USA); pCMV-Tag3 mammalian expression vectors with G418 resistance gene from Stratagene (La Jolla, CA, USA); pGEX4T1 glutathione S-transferase (GST) fusion vector from Amersham Biosciences (Piscataway, NJ, USA); enhanced green fluorescence protein gene (pEGFP)-C3 vector and anti-EGFP antibody from BD Clontech (Palo Alto, CA, USA); Effectene transfection reagent and RNAiFect transfection reagent from Qiagen (Santa Clarita, CA, USA); mouse IA-2 antibody from Lad (Berlin, Germany); mouse anti-α-tubulin antibody, anti-flagellar antigen (FLAG) antibody and anti-GST antibody from Sigma (St Louis, MO, USA); anti-Akt/protein kinase B (PKB), anti-phospho-Akt/PKB, anti-phosphoinositide-dependent kinase (PDK)-1, anti-phospho-PDK-1, anti-mammalian target of rapamycin (mTOR), anti-p70S6 kinase, anti-phospho-p70S6 kinase, anti-glycogen synthetase kinase (GSK)-3β, anti-phospho-GSK-3β, anti-phosphatidylinositol phosphate 3'-phosphatase (PTEN), anti-phospho-PTEN, anti-phospho-tyrosine, anti-C-jun amino terminal kinase (JNK), anti-phospho-JNK, anti-caspase-3, 6, 7, 9, 12 antibody and anti-cMyc monoclonal antibody (MoAb), from Cell Signalling (Beverly, MA, USA); anti-PI3 kinase (p85) from Upstate (Charlottesville, VA, USA); anti-insulin receptor and anti-insulin receptor substrate 1 (IRS-1) from Calbiochem (San Diego, CA, USA); anti-caspase activated DNase (CAD) antibody from Santa Cruz Biotechnology (Santa Cruz, CA, USA); and tumour necrosis factor (TNF)-α and interferon (IFN)-γ from eBioscience (San Diego, CA, USA).
Plasmids
Primers for complementary IA-2 and sorting nexin (SNX)19 were synthesized by Integrated DNA Technology (Coralville, IA, USA). IA-2EC, IA-2IC and IA-2F vector constructs were made as reported previously [11], except the IA-2EC construct used in the present experiments contains a signal peptide. The forward and reverse primer sequences for IA-2EC were 5′-GGAATTCATGCGGCGCCCGCGGCGG-3′ and 5′-CCGCTCGAGCAAGATT TGGAGCCCTGC-3′. IA-2EC polymerase chain reaction (PCR) product was inserted into pCMV-Tag3 vector at EcoRI and XhoI sites. The forward and reverse primer sequences for SNX19 were 5′-CCGCTCGAGATGAAGACAGAAACAGTG3′ and 5′-CCGCTCGAGCTAAGAGGAGACACCCAT-3′. SNX19 PCR product was subcloned into pCMV-Tag2 and pEGFP-C3 vectors at XhoI sites. IA-2IC also was subcloned into pGEX4T1 vector at BamHI and XhoI sites. All plasmids were sequenced and no mutations were found.
Establishment of stable cell lines
MIN-6 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 25 mm d-glucose (high glucose), supplemented with 15% heat-inactivated fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in 95% air and 5% CO2. As reported previously [11], IA-2F (a.a. 1–979), IA-2EC (a.a 1–550) and IA-2IC (a.a. 600–979) was subcloned into pCMV-Tag3 vectors and introduced into mouse insulinoma MIN-6 cells using Effectene transfection reagent, and stably transfected cells were selected in 25 mM high glucose medium containing 500 µg/ml of G418. G418-resistant single clones were obtained by limiting dilution and IA-2 expression was confirmed by Western blot. Five different IA-2F transfected clonal cell lines and three different mock-transfected clonal cell lines were prepared successfully. Based on preliminary experiments, each of the IA-2F transfected cell lines showed a slower growth rate and greater G2/M arrest and apoptosis than the mock-transfected cell lines. A representative IA-2F transfected (no. 3) and mock-transfected (no. 1) cell line was chosen and used through the current experiments. pCMV-Tag3 vector itself (mock) also was transfected into MIN-6 cells and single clones were selected by the same method. Unless stated otherwise, cells were kept at high glucose concentration (25 mM) and cells from the first several passages (i.e. 3–6) were used in all the current experiments. PC12 and NIH3T3 cells were maintained as described above with slight modification.
Cell proliferation assay
A total of 1 × 104 cells/ml of IA-2F, IA-2IC, IA-2EC and mock transfected MIN-6 cells were seeded into 96-well and 1 × 104 cells/ml into 12-well culture plates and incubated for 8 days in 25 mM (high) glucose media. Cell proliferation in 96-well plates was measured by a bromodeoxyuridine (BrdU) cell proliferation assay kit (Calbiochem) or counted in 12-well plates by trypan blue staining for 8 days.
Flow cytometry analysis
Mock, IA-2F and SNX19 transfected MIN-6 cells or IA-2 siRNA, SNX19 siRNA and non-silencing siRNA transfected MIN-6 cells were cultured in six-well culture plates at 3 mM (low) or 25 mM (high) glucose. For cell cycle analysis, cells were cultured for 2 days and fixed with 70% ethanol. After washing with phosphate-buffered saline (PBS), the cells were treated with RNase for 30 min and 50 µg/ml propidium iodide (Calbiochem) was added and cells were analysed by flow cytometry (Becton-Dickinson, Franklin Lakes, NJ, USA). For detection of apoptosis, the cells were cultured for 3 days under low and high glucose conditions and apoptotic cells were detected by the annexin V-fluorescein isothiocyanate (FITC) apoptosis kit (Calbiochem) using flow cytometry. Fas expression on cells was detected by anti-mouse Fas antibody (BD Pharmigen).
DNA fragmentation
After culture for 3 days in 3 mM or 25 mM glucose, attached and floating cells were collected. Both fragmented and high molecular weight DNA were extracted from the cells with a suicide-track DNA ladder isolation kit (Calbiochem) and the extracts were applied to a 1·5% agarose gel.
Transient transfection of plasmids into mammalian cells
A total of 6 × 105 NIH3T3 cells, MIN-6 cells and PC12 cells were seeded onto a six-well cell culture plate 24 h before transfection with 2 µg of IA-2 expression vectors and/or SNX19 expression vector by Effectine transfection reagent; 48 h after transfection, the cells were used for immunoprecipitaion or Western blot analysis.
Construction of IA-2 siRNA
IA-2 siRNA and non-silencing siRNA were prepared by Qiagen, as reported previously [11]. Target sequences were derived from the cDNA sequences of human and mouse IA-2: 5′-AAGTCTGTATTCAGGATGGCTT-3′, corresponding to nucleotide (nt) 152–173 of human IA-2 mRNA and nt 206–227 of mouse IA-2 mRNA. Non-silencing siRNA target (random sequence) was 5′-AATTCTCCGAACGTGTCACG TT-3′. SNX19 siRNA and non-silencing siRNA were purchased from Ambion (Austin, TX, USA). Target sequences for mouse SNX19 was 5′-GGTCAATTGCACCGCCAACTT-3′. All sequences were subjected to Basic Local Alignment Search Tool (blast) searches to ensure that there were no matches with known sequence of other genes.
siRNA transfection
A total of 6 × 105 non-transfected MIN-6 cells, MIN-6 mock-transfected cells and MIN-6 IA2F-transfected cells were seeded in a six-well culture plate 24 h before transfection with 2 µg IA-2 siRNA or 2 µg non-silencing siRNA by RNAiFect transfection reagent; 48 h after siRNA transfection, IA-2 expression was confirmed by Western blot.
Western blot
Cells were washed twice with PBS, detached from plates with trypsin-ethylenediamine tetraacetic acid (EDTA), collected, washed two more times with PBS and then sonicated in lysis buffer. Equivalent amounts of protein were resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) on 4–12% acrylamide gels (Invitrogen, Carlsbad, CA, USA) and transferred to polyvinylidene difluoride (PVDF) membranes (Invitrogen), followed by immunoblotting with antibodies to detect respective proteins.
Phosphorylation
To detect phosphorylation of Akt/PKB, PDK-1, mTOR, GSK-3β, p70S6 kinase and PTEN, IA-2F transfected and mock-transfected MIN-6 cells were seeded in six-well culture plates and cultured for 2 days under high or low glucose conditions. IA-2F transfected and non-transfected MIN-6 cells also were cultured for 2 days under high or low glucose conditions after transfection with IA-2 siRNA or non-silencing siRNA. The cells then were collected, lysed and phosphorylation was analysed by Western blot with specific anti-phospho antibodies to respective proteins.
Immunoprecipitation
To detect tyrosine phosphorylated insulin receptor (IR), IRS-1 and PI3 kinase (p85), mock and IA-2F transfected MIN-6 cells cultured for 2 days in 25 mM glucose media were collected, washed and lysed. Cell lysates were immunoprecipitated for 3 h at 4°C with anti-IR antibody (Calbiochem), anti-IRS-1 antibody (Calbiochem) and PI3 kinase (p85) antibody (Upstates) in the presence of protein A-Sepharose CL-4B (Amersham Biosciences), and then tyrosine phosphorylated proteins were detected with specific anti-phospho-tyrosine antibody (Cell Signalling). NIH3T3 cells transfected with FLAG-tagged SNX19 and/or c-Myc-tagged IA-2IC were used to detect interacting proteins. MIN-6 cells were used to detect endogenous interacting proteins. Cells lysates were immunoprecipitated by a protein G imunoprecipitation kit (Sigma) with antibodies to IA-2, FLAG, c-Myc or SNX19, followed by SDS-PAGE and Western blot.
Fractionation of insulin-containing vesicles
MIN-6 cells were fractionated to separate insulin-containing vesicles on a Percoll gradient [12]. In brief, MIN-6 cells were cultured in six 75 cm2 flasks in 25 mM high glucose DMEM media for 1 week. The cells were collected in 10 ml of insulin-containing vesicle separation buffer with 275 mM sucrose, 10 mM 2-(N-morpholino)-ethane-sulphonate (MES), 1 mM ethyleneglycol-bis-(β-aminoethyl ether)-N,N,N′,N′-tetra-acetate (EGTA), 0·2 mM benzamidine, 0·2 M phenylmethylsulphonyl fluoride (PMSF), 10 µg/ml leupeptin, 10 µg/ml antipain and 10 µg/ml pepstatin, then homogenated with 23- and 27-gauge needles. The homogenate was centrifuged at 1700 g for 10 min and the supernatant was layered on 27% (wt/vol) Percoll, centrifuged at 35 000 g for 50 min at 4°C in Beckman type 50·2TI (Beckman Instruments, Palo Alto, CA, USA). The top 10 ml were discarded, 1·0 ml fractions were collected, washed twice in buffer, centrifuged at 13 000 g for 15 min, the pellets were resuspended in buffer and analysed by Western blot.
Anti-SNX19 antibody
Rabbit anti-serum to SNX19 was prepared by Biosource. The target peptide was ESKPQTEGKKASKSRLRFC, corresponding to a.a. 714–731 of mouse SNX19. A blast search showed no matches to other proteins, including other members of the sorting nexin family.
Protein/lipid overlay assay
PIP-Strip membranes (Echelon, Salt Lake City, UT, USA) were blocked in 0·1% Tween-20-Tris-buffered saline (TBS) plus 3% fatty acid free bovine serum albumin (BSA) (Sigma) for 1 h at room temperature, then incubated overnight at 4°C with 0·5 µg/ml EGFP-SNX19 and/or GST-IA-2IC in 0·1% Tween-20-TBS plus 3% fatty acid free BSA. The membranes were washed gently three times with the same buffer, incubated with anti-EGFP MoAb or anti-GST antibody for 1 h at room temperature, and then incubated with anti-mouse IgG-horseradish peroxidase (HRP) to detect the bound proteins using the ECL system (Amersham Biosciences). The SNX19 protein used in these experiments was obtained from three sources; SNX19-transfected NIH3T3 cells; SNX19-transformed Escherichia coli BL21; and SNX19 expressed in the in vitro transcription/translation system.
PI3 kinase assay
PI3 kinase was prepared by immunoprecipitation with anti-p85 PI3 kinase antibody (Upstates) from 1 × 107 MIN-6 cells and then incubated with PtdIns(4,5)P2 substrate for 3 h at room temperature in the presence GST-IA-2IC and/or EGFP-SNX19 proteins. The amount of PtdIns(3,4,5)P3 produced from PtdIns(4,5)P2 was measured by PI3 kinase enzyme-linked immunosorbent assay (ELISA) kit (Echelon).
TNF-α- and IFN-γ- induced apoptosis
Mock- and IA-2F transfected MIN-6 cells were seeded into six-well plates and cultured for 18 h at low and high glucose concentrations in the presence and absence of 10 ng/ml of TNF-α or IFN-γ. Apoptotic cells were detected by flow cytometry staining with annexin V-FITC and propiodium iodide.
Statistical analysis
All data are expressed as mean ± standard error. Student's t-test was used to determine statistical significance.
Results
Overexpression of IA-2 results in G2/M arrest
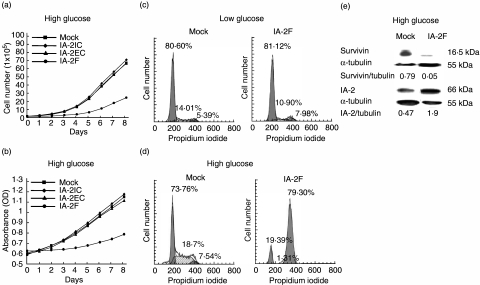
Previously we established stable MIN-6 beta cell lines transfected with the extracellular (a.a. 1–550), intracellular (a.a. 600–979) and full length (a.a. 1–979) of IA-2 [11]. Western blots showed that these transfected cells expressed two to four times more of the extracellular (IA-2EC), intracellular (IA-2IC) and full length of IA-2 (IA-2F) than the mock transfected cells. In the current experiments we examined the growth rate of these cells. As seen in Fig. 1a,b, the growth rate of IA-2F transfected cells was markedly inhibited compared to mock, IA-2IC and IA-2EC transfected cells as determined by both trypan-blue staining (Fig. 1a) and BrdU cell proliferations assays (Fig. 1b). In all subsequent experiments IA-2F was used. To investigate where growth was blocked in the cycle cell, mock and IA-2F transfected cells were incubated for 2 days under low and high glucose conditions and then analysed by flow cytometry (Fig. 1c,d). Under low glucose conditions mock and IA-2F transfected cells showed no difference, but under high glucose there was a dramatic block at the G2/M stage in the IA-2F transfected cells. Western blot analysis showed that survivin, which is required for progression past G2/M [13], was decreased substantially in the IA-2F compared to the mock transfected cells (Fig. 1e).
Fig. 1.
IA-2 transfected mouse insulinoma MIN-6 cells undergo growth arrest in high glucose media. (a, b) Growth rate of IA-2 and mock transfected MIN-6 cells as determined by (a) trypan blue staining and (b) bromodeoxyuridine (BrdU) cell proliferation. (c, d) Cell cycle analysis by flow cytometry of mock and IA-2F transfected MIN-6 cells under (c) low and (d) high glucose conditions. (e) Survivin expression in mock and IA-2F transfected MIN-6 cells as determined by Western blot.
Overexpression of IA-2 results in apoptosis
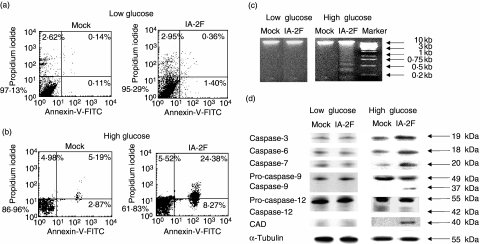
To see whether the cells at G2/M arrest went on to apoptosis, cells were cultured for 3 days under low and high glucose conditions. Figure 2a shows that there was essentially no difference in the number of apoptotic cells as evaluated by annexin-V at low glucose concentrations when the mock and IA-2F transfected cells were compared. However, at high glucose concentrations, 32% of the IA-2F transfected cells expressed annexin-V compared to only 8% of the mock transfected cells (Fig. 2b). Moreover, at high glucose concentrations, the IA-2F transfected, but not mock transfected, cells showed apoptotic DNA fragmentation (Fig. 2c). Further evidence for apoptosis came from examining caspase activity by Western blots. As seen in Fig. 2d, caspase 3, 6, 7, 9, but not 12, were activated and caspase activated DNase (CAD) expression was increased in IA-2F transfected cells when cultured at high glucose concentrations. No increase was observed in mock transfected cells or cells cultured at low glucose concentrations. Thus, IA-2 overexpression establishes a pre-apoptotic state which, in the presence of high glucose, induces G2/M arrest and apoptosis.
Fig. 2.
IA-2 transfected mouse insulinoma MIN-6 cells undergo apoptosis in high glucose media. (a, b) Flow cytometry detection of apoptotic cells as determined by uptake of propidium iodide and by staining with anti-annexin V-fluorescein isothiocyanate (FITC) in (a) low and (b) high glucose media in mock and IA-2F transfected cells. (c) DNA fragmentations of cells in low and high glucose media. (d) Caspase-3, 6, 7, 9, 12 and caspase activated DNase (CAD) activity detected by Western blot in low and high glucose media.
IA-2 siRNA treatment prevents G2/M arrest and apoptosis
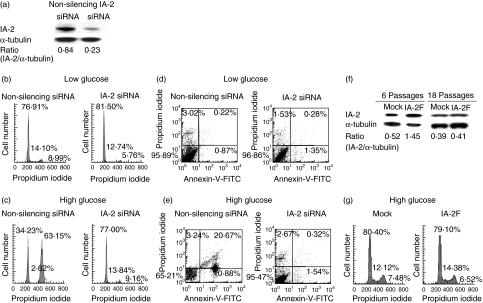
To determine if IA-2-induced G2/M arrest and apoptosis could be prevented, cells were treated with IA-2 siRNA or non-silencing siRNA. As seen in Fig. 3a, siRNA knocked down IA-2 expression by approximately 75% and completely prevented G2/M arrest (Fig. 3b,c) and apoptosis (Fig. 3d,e) upon exposure to high glucose. Non-silencing siRNA had no effect. After many months of culturing IA-2 transfected cells IA-2 could no longer be detected (Fig. 3f), and when this happened the cells no longer showed G2/M arrest upon exposure to a high concentration of glucose (Fig. 3g).
Fig. 3.
IA-2 siRNA treatment prevents G2/M arrest and apoptosis in IA-2 transfected mouse insulinoma MIN-6 cells. (a) IA-2 expression as determined by Western blot 48 h after transfection with IA-2 siRNA and non-silencing siRNA. (b, c) Cell cycle analysis of IA-2F transfected MIN-6 cells as determined by flow cytometry 48 h after transfection with IA-2 siRNA or non-silencing siRNA in low and high glucose media. (d, e) Detection of apoptotic cells by flow cytometry 72 h after exposure of IA-2F transfected MIN-6 cells to IA-2 siRNA and non-silencing siRNA in low and high glucose media. (f, g) Long-term passage of IA-2F transfected MIN-6 cells kicks out IA-2 as shown by (f) Western blot and (g) when this occurs (e.g. by the 18th passage) glucose-induced G2/M arrest is prevented.
Overexpression of IA-2 inhibits Akt phosphorylation
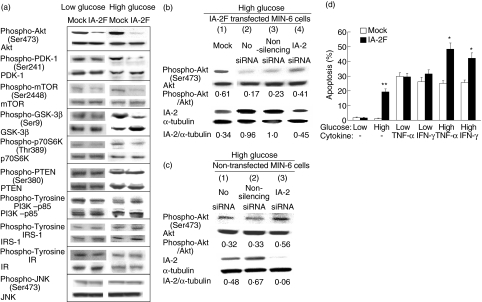
In an attempt to determine the key molecules involved in IA-2F-induced cell cycle arrest and apoptosis, several different signalling pathways were examined. Because decreased Akt/PKB phosphorylation has been linked to beta cell apoptosis, we focused on this pathway [14,15]. Western blot analysis of mock and IA-2F transfected cells cultured at high glucose revealed decreased phosphorylation of Akt/PKB and PDK-1 and the downstream kinases mTOR, GSK-3β and p70S6K, with no apparent change in protein expression, in the IA-2F transfected compared to the mock transfected cells (Fig. 4a). In contrast, the phosphorylation of PTEN, PI3 kinase-p85, IRS-1 and IR were not affected (Fig. 4a). At low glucose, the phosphorylation of Akt/PKB was decreased slightly in the IA-2F transfected cells with no changes in the downstream kinases. Phosphorylation of proteins in the JNK pathway also were not affected (Fig. 4a), nor were there any substantial differences in the expression of Fas on IA-2 transfected compared to mock transfected cells (data not shown).
Fig. 4.
Phosphorylation of proteins involved in the insulin signalling pathway. (a) Phosphorylation of Akt/protein kinase B (PKB), phosphoinositide-dependent kinase (PDK)-1, mammalian target of rapamycin (mTOR), glycogen synthetase kinase (GSK)-3β, p70S6 kinase (p70S6K), phosphatidylinositol phosphate 3'-phosphatase (PTEN), PI3 kinase (PI3K), insulin receptor substrate (IRS)-1 and insulin receptor (IR) in mock and IA-2F transfected mouse insulinoma MIN-6 cells in high and low glucose media. (b) Phosphorylation of Akt/PKB in IA-2F transfected MIN-6 cells, or (c) non-transfected MIN-6 cells in the presence of high glucose 48 h after exposure to IA-2 siRNA and non-silencing siRNA. (d) Apoptosis as measured by flow cytometry with anti-annexin V and propidium iodine in the presence of tumour necrosis factor (TNF)-α and interferon (IFN)-γ at low or high glucose concentrations in mock and IA-2F transfected MIN-6 cells. Mean ± s.e. of three separate experiments. *P < 0·05, **P < 0·01.
Further evidence that the decrease in Akt/PKB phosphorylation was related to IA-2F overexpression was obtained by treating IA-2F transfected cells with IA-2 siRNA. Figure 4b shows that treatment with IA-2 siRNA suppressed IA-2 expression by about 50% and increased Akt/PKB phosphorylation by twofold (lane 4 versus lane 2). Non-silencing siRNA (lane 3) had no effect on IA-2 expression or Akt phosphorylation compared to the untreated controls (lane 2). These findings with IA-2F overexpression raised the possibility that endogenous IA-2 also might influence Akt/PKB phosphorylation. Figure 4c shows that treatment of non-transfected MIN-6 cells with IA-2 siRNA suppressed endogenous IA-2 expression by nearly 85% (lane 3 versus lane 1) and increased Akt/PKB phosphorylation by close to 1·8-fold (lane 3 versus lane 1). Non-silencing siRNA (lane 2) had no significant effect on IA-2 expression or Akt/PKB phosphorylation compared to the untreated control (lane 1). The findings from both transfected and non-transfected MIN-6 cells show that IA-2 can influence Akt phosphorylation.
TNF-α and IFN-γ are known inducers of apoptosis [16]. To determine if overexpression of IA-2 could enhance cytokine-induced apoptosis, IA-2F transfected cells were cultured with low or high glucose in the presence of TNF-α or IFN-γ. Figure 4d shows that under low glucose conditions there was no difference in the percentage of mock and IA-2F transfected cells showing apoptosis when exposed to TNF-α or IFN-γ. However, under high glucose conditions, the percentage of IA-2F transfected cells showing apoptosis was significantly higher than the percentage of mock transfected cells when exposed to TNF-α or IFN-γ. These findings show that overexpression of IA-2 promotes both cytokine and glucose-induced apoptosis in beta cells.
SNX19 interacts with IA-2
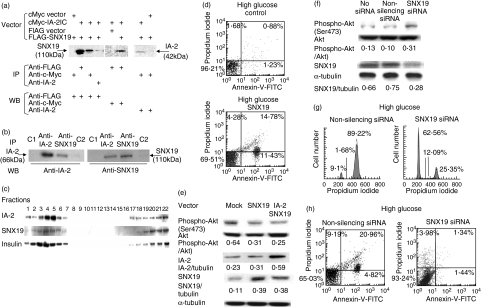
To elucidate the mechanism by which IA-2 might regulate Akt/PKB phosphorylation, we looked for IA-2 interacting proteins by screening several human libraries with the yeast two-hybrid system [17]. About a dozen interacting proteins were identified, but the one that we found of most interest was SNX19, because SNX19 has a Phox (PX) domain [18–20] that could bind phosphatidylinositol (PtdIns), whereas none of the other IA-2 interacting proteins are known to directly phosphorylate or dephosphorylate Akt/PKB or PDK-1. Figure 5a shows that the intracellular domain of IA-2 binds to full length SNX19 in mammalian cells. FLAG-tagged full length SNX19 and c-Myc-tagged IA-2IC were co-transfected into NIH3T3 cells, and the cell lysates were pulled down with anti-FLAG, anti-c-Myc or anti-IA-2 antibody followed by SDS-PAGE and Western blot analysis. Western blots showed that IA-2IC was pulled down with SNX19 and that SNX19 was pulled down with IA-2IC. Figure 5b shows that in MIN-6 cells, endogenous IA-2 binds to endogenous SNX19. Cell lysates of MIN-6 cells were immunoprecipated with anti-IA-2 or anti-SNX19 antibody and Western blots revealed that antibody to SNX19 pulled down IA-2 and antibody to IA-2 pulled down SNX19. Fractionation of cell lysates showed that IA-2 and SNX19 co-localized in the same fractions in which insulin was found (Fig. 5c), suggesting that SNX19 bound to DCV through IA-2.
Fig. 5.
Sorting nexin (SNX)19 interacts and co-localizes with IA-2 and SNX19 siRNA increases Akt/phosphoinositide-dependent kinase (PDK)-1 phosphorylation and inhibits G2/M arrest and apoptosis. (a) Lysates of NIH3T3 cells co-transfected with anti-flagellar antigen (FLAG)-tagged SNX19 and c-Myc-tagged IA-2IC (a.a. 600–979) were immunoprecipitated with anti-FLAG antibody, anti-c-Myc antibody or anti-IA-2 antibody followed by Western blot analysis. (b) Endogenous interaction of IA-2 and SNX19 in mouse insulinoma MIN-6 cells (non-transfected) detected by immunoprecipitation with anti-IA-2 or anti-SNX19 antibody followed by Western blot. (c) Fractionation of lysates from non-transfected MIN-6 cells followed by Western blot analysis with anti-IA-2, -SNX19 and -insulin antibody. (d) MIN-6 cells were transiently transfected with SNX19 and analysed 48 h later by flow cytometry for apoptosis in the presence of high glucose. (e) PC12 cells were transiently transfected in the presence of high glucose with SNX19 or co-transfected with SNX19 and IA-2 and 48 h later Akt/PKB phosphorylation was determined by Western blot. (f) Non-IA-2 transfected MIN-6 cells were transfected with SNX19 siRNA or non-silencing siRNA and Akt/protein kinase B (PKB) phosphorylation determined by Western blot. (g h) IA-2F transfected MIN-6 cells were transfected with SNX19 siRNA or non-silencing siRNA and the effect on (g) cell cycle and (h) apoptosis was determined by flow cytometry in the presence of high glucose.
SNX19 inhibits Akt/PKB phosphorylation resulting in apoptosis
To determine if SNX19 itself could induce apoptosis, MIN-6 cells were transfected transiently with SNX19. Two days later approximately 26% of SNX19 transfected cells were found to be annexin-V positive, whereas only 2% of the mock transfected cells expressed annexin-V (Fig. 5d). Transfection with SNX19 also led to a threefold or greater increase in the expression of SNX19 (Fig. 5e) and a 50% or greater decrease in Akt/PKB phosphorylation in both SNX19 and SNX19/IA-2 transfected cells compared to the mock transfected cell (Fig. 5e). Conversely, treatment of MIN-6 cells with SNX19 siRNA resulted in about a 60% decrease in SNX19 expression and a 2·5–3·0-fold increase in Akt/PKB phosphorylation (Fig. 5f). Non-silencing siRNA had no effect on SNX19 expression or Akt/PKB phosphorylation. SNX19 siRNA also prevented the IA-2 induced pre-apoptotic cells from undergoing G2/M arrest and apoptosis (Fig. 5g,h) upon exposure to high glucose. Again, non-silencing siRNA has no effect on G2/M arrest or apoptosis.
SNX19/IA-2 binds to PtdIns
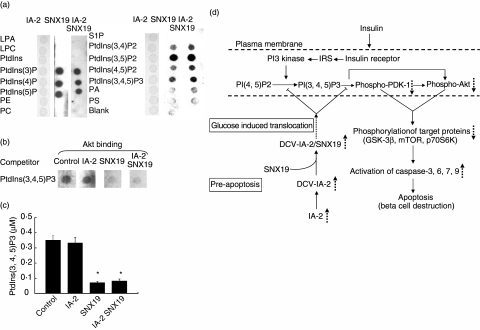
To study the molecular basis by which SNX19 decreases Akt/PKB phosphorylation we explored the ability of SNX19 to bind to PtdIns by use of a protein-lipid overlay assay. As seen in Fig. 6a, SNX19 bound to all the phosphorylated PtdIns tested. Similar results were obtained in three separate experiments using different SNX19 preparations (data not shown). In contrast, IA-2 did not bind to any of the PtdIns nor did the combination of IA-2/SNX19 inhibit the binding of SNX19 to PtdIns, suggesting that IA-2 might serve as a scaffolding molecule on DCV to bring SNX19 to PtdIns in the plasma membrane. In addition, the well-known binding of Akt to PtdIns (Fig. 6b, lane 1) was inhibited by SNX19 and SNX19/IA-2 (lanes 3, 4), but not by IA-2 (lane 2). Evidence that the binding of SNX19 to PtdIns inhibits the conversion of PtdIns(4,5)P2 to PtdIns(3,4,5)P3 by PI3 kinase is illustrated in Fig. 6c. In the presence of IA-2, the conversion of PtdIns(4,5)P2 to PtdIns(3,4,5)P3 was the same as in the control, whereas in the presence of SNX19 or IA-2/SNX19 there was nearly a 75% reduction in the conversion.
Fig. 6.
Binding of sorting nexin (SNX)19 to PtdIns. (a) Purified IA-2, SNX19 or a combination of the two was overlaid on a membrane spotted with different PtdIns and binding was determined. (b) Competitive Akt/protein kinase B (PKB) binding to PtdIns(3,4,5)P3 in the presence or absence of IA-2 and/or SNX19. (c) SNX19, but not IA-2, inhibits the conversion of PtdIns(4, 5)P2 to PtdIns(3, 4, 5)P3 by PI3 kinase as determined by enzyme-linked immunosorbent assay (ELISA). Mean ± s.e. of three separate experiments. *P < 0·05. (d) Schematic diagram depicting the proposed mechanism by which overexpression of IA-2 leads to apoptosis. Overexpression of IA-2 increases the number of DCV in mouse insulinoma MIN-6 cells [11]. SNX19 binds to the cytoplasmic domain of IA-2 and in the presence of high glucose dense core vesicles (DCV) are translocated to the plasma membrane. As there are more DCV in IA-2 transfected cells this would result in the delivery of more SNX19 to the plasma membrane, where SNX19 could block the conversion of PI(4,5)P2 to PI (3,4,5)P3 and inhibit the phosphorylation of phosphoinositide-dependent kinase (PDK)-1 and Akt/protein kinase B (PKB). This in turn would down regulate the phosphorylations of proteins in the PDK-1 and Akt/PKB signalling pathway resulting in the activation of caspases and beta cell apoptosis. LPA, lysophosphatidic acid; LPC, lysophosphocholine; PE, phosphatidylethanolamine; PC, phosphatidylcholine; S1P, sphingosine-1-phosphate; PA, phosphatidic acid; PS, phosphatidylserine.  , increase,
, increase,  , decrease,
, decrease,  , inhibition,
, inhibition,  , translocation.
, translocation.
Discussion
Transfection with IA-2 puts MIN-6 cells into a pre-apoptotic state which, upon exposure to high glucose, results in G2/M arrest and apoptosis. Glucose-induced apoptosis/toxicity has been known for years and a variety of mechanisms have been proposed [15,21–23]. In our studies exposure of IA-2 transfected MIN-6 cells, but not mock transfected cells, to a high concentration of glucose resulted in a decrease in the phosphorylation of proteins in the Akt pathway. Our studies also showed that decreased Akt/PKB phosphorylation led to the activation of caspases 3, 6, 7 and 9 which in turn resulted in cell death. Treatment of the transfected cells with IA-2 siRNA prior to exposure to high glucose prevented the decrease in phosphorylation and, in fact, increased Akt/PKB phosphorylation in non-transfected cells. From these studies we conclude that the Akt/PKB signalling pathway plays a major role in the transition from the IA-2 pre-apoptotic state to apoptosis and that glucose is necessary but, in the absence of IA-2, not sufficient for the transition.
Insight into one possible mechanism by which IA-2 sets the pre-apoptotic stage for glucose-induced apoptosis comes from identifying SNX19 as an IA-2 interacting protein. Based on sequence, SNX19 is a new member of the sorting nexin family which consists of a diverse group of cellular trafficking proteins [24]. SNX19 is 992 a.a. in length and has a PX-associated (PXA) domain and a PX domain. The PX domains of SNX are known to bind to PtdIns [25,26]. The function of SNX19 previously was not known. Our experiments showed that under high glucose conditions transfection of cells with SNX19 resulted in a decrease in the phosphorylation of Akt/PKB and caused apoptosis. Conversely, treatment with SNX19 siRNA increased Akt phosphorylation and prevented apoptosis. Thus, the biological effects of SNX19 are similar to and parallel IA-2. Because IA-2 and SNX19 interact with each other it was not clear whether the decrease in phosphorylation and glucose-induced apoptosis in the IA-2 pre-apoptotic cell was due to IA-2 or SNX19. The demonstration that SNX19, but not IA-2, binds to several PtdIns, inhibits the conversion of PtdIns(4, 5)P2 to PtdIns(3, 4, 5)P3 and blocks the binding of PtdIns(3, 4, 5)P2 to Akt/PKB argues that SNX19 is a key molecule in the IA-2 pre-apoptotic cell (Fig. 6d).
Based on the SNX19 findings it would appear that IA-2, which is a transmembrane component of DCV, serves in the capacity of a scaffolding protein to which SNX19 attaches and then is transported with the DCV to other parts of the cells. In this context, we recently showed that IA-2 is a regulator of DCV number and insulin secretion [11]. Overexpression of IA-2 resulted in a sixfold increase in glucose-induced insulin secretion and approximately a threefold increase in the number of DCV in the cell. The increase in vesicle number appears to be due to IA-2 induced stabilization of DCV, which were found to have a half-life nearly twice as great in the IA-2 transfected cells as in the mock transfected cells. The current study shows that SNX19 co-localizes with IA-2 and insulin and therefore appears to be linked to DCV, where it is attached to the cytoplasmic domain of IA-2. Exposure to a high concentration of glucose results in the trafficking of DCV from the cytoplasm to the plasma membrane [27,28]. Therefore, cells overexpressing IA-2 contain more DCV which, in the presence of high glucose, can translocate more SNX19 to the plasma membrane where SNX19 can bind to PtdIns(4,5)P2 or PtdIns(3,4,5)P3 and thereby decrease phosphorylation and down-regulate the Akt pathway (Fig. 6d). It is of interest that glucose-induced toxicity generally occurs in cells exposed for a long period of time to high glucose [29]. In the case of MIN-6 cells, which require high glucose for growth and survival, high glucose does not result in apoptosis in the absence of IA-2 overexpression. Thus, the mechanism(s) for classic glucose-induced toxicity and the apoptosis that occurs in IA-2 transfected cells appear to have a different basis.
Aberrant expression of IA-2 also might be an explanation for the difficulties encountered by a number of laboratories [30,31], including our own, in establishing and maintaining permanent IA-2 cell lines. In our hands, tranfection of fibroblasts (i.e. NIH3T3, Chinese hamster ovary (CHO) and HEK293), which do not contain DCVs, with IA-2F linked to a signal peptide resulted in apoptosis. Under certain conditions, transfection of MIN-6 cells with the extracellular domain of IA-2, but lacking a signal peptide, could also result in apoptosis (unpublished data). In both these cases there was aberrant expression of IA-2: in one case, because of the absence of DCV, and in the other because of the absence of a signal peptide. Thus the extracellular domain of IA-2 might have apoptotic signalling properties independent of SNX19, but not seen when the gene is properly expressed and processed.
Although it is accepted widely that the autoimmune response in humans is responsible for beta cell destruction, much of the evidence comes from animal models [32]. The present study points to an alternative non-immunological mechanism to explain how at least some beta cells may be destroyed. Genetic factors that influence the expression of IA-2 (e.g. transcription factors) or hormones (e.g. insulin) that enhance the expression of IA-2 [33,34] might put beta cells into a pre-apoptotic state. Environmental factors (e.g. high glucose) then could force the pre-apoptotic cells into apoptosis. This process may take place over many years, with only a few beta cells being destroyed at any one time, but resulting in the gradual loss of the beta cell reserve. The autoimmune response thus may be a consequence of this apoptotic process [35,36], especially in individuals with high-risk haplotypes for type 1 diabetes [37,38], and may contribute to the pathogenesis of the disease, but may not be the primary trigger or cause of the disease.
The findings presented here with IA-2 link a major autoantigen with apoptosis. Based on the IA-2 model, it is possible that overexpression or aberrant expression of other host proteins might also put beta cells into a pre-apoptotic state, which upon exposure to environmental factors and/or host factors could activate apoptotic pathways and result in non-immunological destruction of beta cells and type 1 diabetes. Autoantigen-induced, but non-immunologically mediated processes also might play a role in other autoimmune diseases.
Acknowledgments
We thank Drs H. Nishizaka and H. Kusaba for providing several antibodies and Dr Z. Galdzicki for his support.
References
- 1.Notkins AL, Lernmark A. Autoimmune type 1 diabetes: resolved and unresolved issues. J Clin Invest. 2001;108:1247–52. doi: 10.1172/JCI14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Notkins AL. Immunologic and genetic factors in type 1 diabetes. J Biol Chem. 2002;277:43545–8. doi: 10.1074/jbc.R200012200. [DOI] [PubMed] [Google Scholar]
- 3.Lan MS, Lu J, Goto Y, et al. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol. 1994;13:505–14. doi: 10.1089/dna.1994.13.505. [DOI] [PubMed] [Google Scholar]
- 4.Lu J, Notkins AL, Lan MS. Isolation, sequence and expression of a novel mouse brain cDNA, mIA-2, and its relatedness to members of the protein tyrosine phosphatase family. Biochem Biophys Res Commun. 1994;204:930–6. doi: 10.1006/bbrc.1994.2549. [DOI] [PubMed] [Google Scholar]
- 5.Magistrelli G, Toma S, Isacchi A. Substitution of two variant residues in the protein tyrosine phosphatase-like PTP35/IA-2 sequence reconstitutes catalytic activity. Biochem Biophys Res Commun. 1996;227:581–8. doi: 10.1006/bbrc.1996.1549. [DOI] [PubMed] [Google Scholar]
- 6.Xie H, Zhang B, Matsumoto Y, et al. Autoantibodies to IA-2 and IA-2beta in insulin-dependent diabetes mellitus recognize conformational epitopes: location of the 37- and 40-kDa fragments determined. J Immunol. 1997;159:3662–7. [PubMed] [Google Scholar]
- 7.Cai T, Krause MW, Odenwald WF, et al. The IA-2 gene family: homologs in Caenorhabditis elegans, Drosophila and zebrafish. Diabetologia. 2001;44:81–8. doi: 10.1007/s001250051583. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Li Q, Xie H, et al. Identification of a second transmembrane protein tyrosine phosphatase, IA-2beta, as an autoantigen in insulin-dependent diabetes mellitus: precursor of the 37-kDa tryptic fragment. Proc Natl Acad Sci USA. 1996;93:2307–11. doi: 10.1073/pnas.93.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Q, Borovitskaya AE, DeSilva MG, et al. Autoantigens in insulin-dependent diabetes mellitus: molecular cloning and characterization of human IA-2 beta. Proc Assoc Am Physicians. 1997;109:429–39. [PubMed] [Google Scholar]
- 10.Saeki K, Zhu M, Kubosaki A, et al. Targeted disruption of the protein tyrosine phosphatase-like molecule IA-2 results in alterations in glucose tolerance tests and insulin secretion. Diabetes. 2002;51:1842–50. doi: 10.2337/diabetes.51.6.1842. [DOI] [PubMed] [Google Scholar]
- 11.Harashima S, Clark A, Christie MR, et al. The dense core transmembrane vesicle protein IA-2 is a regulator of vesicle number and insulin secretion. Proc Natl Acad Sci USA. 2005;102:8704–9. doi: 10.1073/pnas.0408887102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arden C, Harbottle A, Baltrusch S, et al. Glucokinase is an integral component of the insulin granules in glucose-responsive insulin secretory cells and does not translocate during glucose stimulation. Diabetes. 2004;53:2346–52. doi: 10.2337/diabetes.53.9.2346. [DOI] [PubMed] [Google Scholar]
- 13.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–10. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 14.Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–45. [PubMed] [Google Scholar]
- 15.Bernal-Mizrachi E, Fatrai S, Johnson JD, et al. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Invest. 2004;114:928–36. doi: 10.1172/JCI20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens LA, Thomas HE, Ming L, et al. Tumor necrosis factor-alpha-activated cell death pathways in NIT-1 insulinoma cells and primary pancreatic beta cells. Endocrinology. 1999;140:3219–27. doi: 10.1210/endo.140.7.6873. [DOI] [PubMed] [Google Scholar]
- 17.Hu YF, Zhang HL, Cai T, et al. The IA-2 interactome. Diabetologia. 2005;48:2576–81. doi: 10.1007/s00125-005-0037-y. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Seet LF, Hanson B, et al. The Phox homology (PX) domain, a new player in phosphoinositide signaling. Biochem J. 2001;360:513–30. doi: 10.1042/0264-6021:3600513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickson LM, Rhodes CJ. Pancreatic beta-cell growth and survival in the onset of type 2 diabetes: a role for protein kinase B in the Akt? Am J Physiol Endocrinol Metab. 2004;287:E192–8. doi: 10.1152/ajpendo.00031.2004. [DOI] [PubMed] [Google Scholar]
- 20.Carlton J, Bujny M, Rutherford A, et al. Sorting nexins − unifying trends and new perspectives. Traffic. 2005;6:75–82. doi: 10.1111/j.1600-0854.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 21.Maedler K, Sergeev P, Ris F, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–60. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donath MY, Storling J, Maedler K, et al. Inflammatory mediators and islet beta-cell failure: a link between type 1 and type 2 diabetes. J Mol Med. 2003;81:455–70. doi: 10.1007/s00109-003-0450-y. [DOI] [PubMed] [Google Scholar]
- 23.Robertson RP, Harmon J, Tran PO, et al. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53:S119–24. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- 24.Worby CA, Dixon JE. Sorting out the cellular functions of sorting nexins. Nat Rev Mol Cell Biol. 2002;3:919–31. doi: 10.1038/nrm974. [DOI] [PubMed] [Google Scholar]
- 25.Sato TK, Overduin M, Emr SD. Location, location, location: membrane targeting directed by PX domains. Science. 2001;294:1881–5. doi: 10.1126/science.1065763. [DOI] [PubMed] [Google Scholar]
- 26.Ellson CD, Andrews S, Stephens LR, et al. The PX domain: a new phosphoinositide-binding module. J Cell Sci. 2002;115:1099–105. doi: 10.1242/jcs.115.6.1099. [DOI] [PubMed] [Google Scholar]
- 27.Leung YM, Sheu L, Kwan E, et al. Visualization of sequential exocytosis in rat pancreatic islet beta cells. Biochem Biophys Res Commun. 2002;292:980–6. doi: 10.1006/bbrc.2002.6712. [DOI] [PubMed] [Google Scholar]
- 28.Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46:1029–45. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- 29.Poitout V, Robertson RP. Secondary beta-cell failure in type 2 diabetes − a convergence of glucotoxicity and lipotoxicity. Endocrinology. 2002;143:339–42. doi: 10.1210/endo.143.2.8623. [DOI] [PubMed] [Google Scholar]
- 30.Hermel JM, Dirkx R, Jr, Solimena M. Post-translational modifications of ICA512, a receptor tyrosine phosphatase-like protein of secretory granules. Eur J Neurosci. 1999;11:2609–20. doi: 10.1046/j.1460-9568.1999.00677.x. [DOI] [PubMed] [Google Scholar]
- 31.Papakonstantinou T, Myers MA, Jois J, et al. Expression of protein tyrosine phosphatase-like molecule ICA512/IA-2 induces growth arrest in yeast cells and transfected mammalian cell lines. J Autoimmun. 2001;17:51–61. doi: 10.1006/jaut.2001.0516. [DOI] [PubMed] [Google Scholar]
- 32.Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med. 1999;5:601–4. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 33.Lee MS, Dirkx R, Jr, Solimena M, et al. Stabilization of the receptor protein tyrosine phosphatase-like protein ICA512 in GH4C1 cells upon treatment with estradiol, insulin, and epidermal growth factor. Endocrinology. 1998;139:2727–33. doi: 10.1210/endo.139.6.6039. [DOI] [PubMed] [Google Scholar]
- 34.Lobner K, Steinbrenner H, Roberts GA, et al. Different regulated expression of the tyrosine phosphatase-like proteins IA-2 and phogrin by glucose and insulin in pancreatic islets: relationship to development of insulin secretory responses in early life. Diabetes. 2002;51:2982–8. doi: 10.2337/diabetes.51.10.2982. [DOI] [PubMed] [Google Scholar]
- 35.Casciola-Rosen L, Rosen A, Petri M, et al. Surface blebs on apoptotic cells are sites of enhanced procoagulant activity: implications for coagulation events and antigenic spread in systemic lupus erythematosus. Proc Natl Acad Sci USA. 1996;93:1624–9. doi: 10.1073/pnas.93.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosen A, Casciola-Rosen L. Autoantigens as substrates for apoptotic proteases. implications for the pathogenesis of systemic autoimmune disease. Cell Death Differ. 1999;6:6–12. doi: 10.1038/sj.cdd.4400460. [DOI] [PubMed] [Google Scholar]
- 37.She JX. Susceptibility to type I diabetes: HLA-DQ DR revisited. Immunol Today. 1996;17:323–9. doi: 10.1016/0167-5699(96)10014-1. [DOI] [PubMed] [Google Scholar]
- 38.Klein J, Sato A. The HLA system. Second of two parts. N Engl J Med. 2000;343:782–6. doi: 10.1056/NEJM200009143431106. [DOI] [PubMed] [Google Scholar]