Abstract
Data indicate that appendicectomy for intra-abdominal inflammation protects against inflammatory bowel disease (IBD). This suggests an important role for the appendix in mucosal immunity. There is no established model of appendicitis. We therefore developed a murine model of appendicitis and examined the effect of inflammation on appendiceal lymphocyte constituents. The caecal patch of specific pathogen-free (SPF)-Balb/c mice was transformed into an obstructed ‘appendiceal pouch’ by standardized suction and band ligation. Mice were killed and ‘pouches’ removed for histology and phenotypic analysis of leucocytes by flow cytometry. Serum C-reactive protein (CRP) was determined by enzyme-linked immunosorbent assay. All ‘pouches’ developed features resembling human appendicitis – mucosal ulceration, transmural inflammation with neutrophils, lymphocytes and occasional eosinophils, and serositis. These changes were most evident between days 7 and 10. There was significant elevation of serum CRP (8·0 ± 0·3 ng/ml to 40·0 ± 3·1 ng/ml; P < 0·01), indicating systemic inflammation. Following the initial neutrophil-predominant response, there was an increase in CD4− (15·3% ± 1·2% to 31·0 ± 2·0%; P < 0·01) and CD8− T lymphocytes (3·7% ± 0·6% to 9·2 ± 0·8%; P < 0·01). CD25− forkhead box P3 (FoxP3)− regulatory T lymphocytes were increased by 66% (P < 0·01). Furthermore, significant increases in CD8− FoxP3− regulatory T lymphocytes were restricted to younger mice (age < 10 weeks, P < 0·003). This is the first description of a murine model of appendicitis. Inflammation resulted in T lymphocyte accumulation associated with an increase in regulatory T lymphocytes, which might explain the age-dependent protective phenomenon. Further exploration will provide insights into the mechanisms of intestinal immune homeostasis and the immunopathogenesis of IBD.
Keywords: appendicitis, appendix, regulatory T lymphocytes
Introduction
The human appendix has long been regarded as a useless, vestigial organ, despite the fact that it contains abundant lymphoid tissue and is constantly exposed to intraluminal bacterial flora. It is derived embryologically from the caecum, and is characterized by a large number of well-organized lymphoid follicles and germinal centres which contain more B lymphocytes than T lymphocytes [1]. However, it constitutes only a small part of the gut-associated lymphoid tissue (GALT) in humans. Despite embryological, histological and phenotypic similarities (which may suggest a similarly insignificant function), the rabbit appendix is known to play an important role in both systemic and mucosal immune function [2–6].
Appendicitis is still the most common abdominal surgical emergency in humans, with a lifetime risk of approximately 7% in females and 9% in males. Acute appendicitis can occur at any time from infancy to old age, but the peak age of incidence is in the second and third decades [7,8]. Despite many years of clinical observation, the exact aetiology of appendicitis remains obscure. The most widely proposed mechanism is primary obstruction of the lumen by faecolith, fibrous band or lymphoid hyperplasia, followed by secondary bacterial infection triggering acute inflammation. Alternatively, primary viral or bacterial infection might be the underlying pathogenesis. Other factors considered important include hygiene, diet, primary ischaemia of the appendix, trauma, genetic factors (a familial tendency to experience appendicitis is rarely seen [9]) and type I hypersensitivity (in some cases of appendicitis, an eosinophilic infiltrate is the only histological feature) – all are still controversial. Whatever the inciting cause, all these possibilities are thought to lead to bacterial overgrowth and acute inflammation which is responsible for the clinical manifestations.
Although the histological changes of acute appendicitis are well documented, immunological examination of appendicitis specimens has been scanty. Immunohistochemical staining showed that CD4− T lymphocytes are present in the appendix of all patients undergoing appendicectomy, whereas B lymphocytes, natural killer cells and CD8− T lymphocytes are found in the vast majority of perforated appendicitis specimens, in comparison to approximately 50% of non-perforated appendicitis samples [10,11].
Recently, the role of the appendix in human mucosal immune function was emphasized by the demonstration that appendicectomy for intra-abdominal inflammatory conditions, including appendicitis and mesenteric adenitis, was protective against development of ulcerative colitis (odds ratio: 0·312) [12–18] and potentially also against Crohn's disease (odds ratio: 0·8) [16,18]. This protective phenomenon is age-dependent, as it is restricted to patients having surgery before the age of 20 years. In addition, studies in three different murine models of colitis have demonstrated that removal of the normal appendix prevented the development of colitis, including in T cell receptor-α mutant mice [19], dextran sulphate sodium (DSS)-induced colitis [20] and colitis induction in severe combined immunodeficiency (SCID) mice by adoptive transfer of effector CD62L−CD4− T lymphocytes (which migrate preferentially to the appendix) [21]. Therefore, the appendix also appears to play an important role in the pathogenesis of inflammatory bowel disease in these models.
As there is no established animal model of appendicitis, we developed a murine model by constructing an appendiceal equivalent involving formation of a pouch from the caecal patch and then obstructing this murine ‘appendix’. The appendiceal pathology in the optimized model closely resembles human appendicitis, and has allowed us to examine the impact of inflammation on the appendiceal lymphocyte constituents. This model will allow studies to provide insights into the immunological consequences of appendicitis, including clues to the pathogenesis of inflammatory bowel disease.
Methods and materials
Mice
Male, specific pathogen-free (SPF), Balb/c mice were purchased from the Animal Resource Centre in Perth, Western Australia. These animals were kept in the holding and care facility at the University of New South Wales under SPF conditions in physical containment (PC) level 2 rooms. They were housed in filtered plastic cages in groups of five to six. The mice were allowed to acclimatize to the living conditions for at least 1 week before commencement of experiments.
All experiments were approved and monitored by the University of New South Wales Animal Care and Ethics Committee.
Induced appendicitis
In separate experiments, groups of three mice at different ages (weeks 4, 6, 8, 10, 11 and 12) were utilized. The day before induction of appendicitis, the abdominal wall of the mice was shaved. The mice were anaesthetized by intraperitoneal administration of xylazine (5 mg/kg) and ketamine (100 mg/kg) reconstituted in sterile phosphate-buffered saline (PBS). The mice were secured subsequently onto a surgical platform.
The skin of the abdomen was cleaned with antiseptic solution (Povidine-iodine, 10%w/v; Orion Laboratories Pty Ltd, Welshpool, Australia) and covered with sterile fenestrated surgical drapes. Subsequently a left-sided laparotomy incision of less than 1 cm was performed. The caecum was identified, mobilized out of the abdomen and kept moist by applying Dulbecco's PBS (Invitrogen, New York, USA) periodically. The caecal patch (the murine equivalent of human appendix) (Fig. 1a) was identified, and transformed into an ‘appendiceal pouch’ by standardized continuous aspiration using the tip of a 1-ml pipette (Gilson Classic Pipetman; Gilson Inc., Middleton, WI, USA) applied directly onto the patch. This suction allowed formation of the appendiceal pouch inside the lumen of the pipette tip. The diameter of the pipette tip (internal diameter, 2 mm) and the amount of suction (pipette at 1 ml volume setting) applied were optimized in preliminary experiments to minimize local trauma to the serosa, and to ensure that the lumen of the caecum was in continuity with the pouch. Obstruction of this ‘appendiceal pouch’ was achieved by loading a sterile ligation rubber band (Kilroid ligation ring; Astra Tech AB, Molndal, Sweden) to the neck of the pouch (Fig. 1b). This obstructed ‘appendiceal pouch’ was returned to the peritoneal cavity and phosphate-buffered saline, 1 ml, was sprayed into the abdominal cavity. The wound was closed in layers with 4–0 Dexon II sutures (Sherwood Davis & Geck, St Louis, MO, USA).
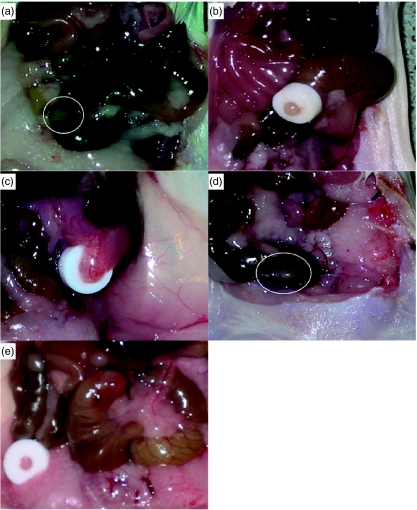
Fig. 1.
Macroscopic appearance of the murine caecal patches (the murine equivalent of the human appendix). (a) Normal murine caecal patch (circled); (b) pale, oedematous appendiceal pouch formation with ligation band in-situ; (c) appendicitis with surrounding adhesions; (d) sham-operated mouse with a ‘normal’-appearing caecal patch; (e) antibiotic-treated mouse with minimal surrounding adhesions.
A subset of mice underwent appendicitis induction with broad-spectrum antibiotic cover (antibiotic-treated mice). Enrofloxacin, 5 mg/kg, was administered subcutaneously daily from day 0 after mice arrived from the animal resource centre until killed, in an attempt to alter the intestinal flora.
The mice were then hydrated with 1 ml subcutaneous normal saline and were allowed to recover in the left lateral position. The mice were monitored daily for generalwell-being, grooming, weight gain or loss, wound infection, sepsis, bowel obstruction or development of an ileus. Normal saline (0·9%) was administrated subcutaneously daily to maintain hydration until mice returned to their preoperative clinical status.
Mice were then killed at different time-points (days 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 14 and 18 after formation of the appendiceal pouch) to allow the pouch to be removed and processed for histological examination by standard haematoxylin and eosin (H&E) staining for features resembling human appendicitis.
Additional experiments were performed to examine the impact of age-related inflammation on the appendiceal lymphocyte constituents by determining their phenotype in flow cytometry. The appendiceal lymphocytes were isolated as described previously [22], with some minor modifications. Briefly, the appendiceal pouch was dissected away from adhesions and divided from the caecum at the neck of the pouch. Faecal matter was removed from the pouch which was then bathed in Hank's balanced salt solution (HBSS)/0·75 mM ethylenediamine tetraacetic acid (EDTA)/10% heat-inactivated fetal bovine serum (HI-FBS)/1% penicillin and streptomycin for 10 min to remove the epithelium. The supernatant was then discarded. The pouch was mechanically disaggregated over a 40-µm cell strainer with RPMI-1640/10% HI-FBS/l-glutamine/25 mM herpes/2% penicillin and streptomycin (Invitrogen, New York, USA), to isolate appendiceal lymphocytes. The lymphocytes were stained with monoclonal antibodies for cell surface markers including those for T and B lymphocytes, or permeabilized for staining of intracellular markers according to the manufacturer's protocol. Regulatory T cells were identified by staining for forkhead box P3 (FoxP3), a member of the forkhead/winged helix family of transcription factors which has been demonstrated to be fundamental to the function of regulatory T lymphocytes [23,24]. Apart from the FoxP3 staining kit (eBioscience, San Diego, CA, USA), all antibodies were obtained from Becton Dickinson Bioscience (NJ, USA). Thereafter, the phenotype of the appendiceal lymphocytes was examined by flow cytometry (FACsort, Becton Dickinson Bioscience) and analysed with the Cell QuestPro program (BD Bioscience).
Control/sham mice
Balb/c mice of the same age as the test animals underwent surgery using the same general procedures, including mobilization of the caecum out of the abdominal cavity and formation of the appendiceal pouch by standardized aspiration (i.e. sustained same degree of manipulation, trauma and exposure to extra-peritoneal environment) and peritoneal application of PBS, but without band ligation (that is, without continuous obstruction). In addition, a single rubber band was placed inside the abdominal cavity next to the caecal patch as a control for foreign body reaction.
C reactive protein (CRP) measurement
At the time of sacrifice, blood was collected via direct cardiac puncture and serum was separated for measurement of CRP by enzyme-linked immunosorbent assay (ELISA) using a mouse CRP ELISA kit (Immunology Consultants Laboratory, Newburg, OR, USA).
Statistical analysis
Data are expressed as mean ± s.e.m. Comparison of the two experimental groups of mice (e.g. lymphocyte phenotyping from normal appendices versus lymphocytes phenotyping from inflamed appendices) within the same age group was made using the Mann–Whitney test. Differences were considered to be significant if P < 0·05. Age-related change in lymphocyte phenotype in inflamed appendices was assessed by Kruskal–Wallis test. Change was considered significant if P < 0·05.
Results
Clinical monitoring
Apart from some initial weight loss and a period of poor grooming which resolved rapidly, all mice remained well post-operatively without any clinical evidence of systemic sepsis, bowel obstruction or ileus formation.
Macroscopic appearance
On day 1 after formation of pouches, the obstructed appendiceal pouches became pale and oedematous with ‘ballooning’ of the pouch as disease progressed (data not shown) over subsequent days. Considerable surrounding adhesions developed after day 5 which persisted throughout all time-points (Fig. 1c), whereas the appendices from sham-operated mice remained uninflamed and free of any adhesions (Fig. 1d). In addition, approximately 10% of the obstructed appendiceal pouches (resected at day 7) were associated with abscess formation. No foreign body reaction in the peritoneal cavity was elicited by the rubber band alone.
The appendiceal pouches from the antibiotic-treated mice appeared pale and had minimal surrounding adhesions (Fig. 1e).
Histology
Histological examination revealed relatively normal follicular structure with minimal inflammatory infiltrate in obstructed appendiceal pouches for the first 3 days (Figs 2 and 3a–d). By day 4, there was mucosal ulceration and a mild, predominantly neutrophilic infiltrate in the mucosa (data not shown). Florid inflammation with a mixture of neutrophilic and lymphocytic infiltrate was seen from day 7 onwards and this persisted throughout all time-points thereafter. H&E staining of appendiceal pouches from day 7 (n = 10) confirmed histological features resembling human appendicitis, including mucosal ulceration (Fig. 3e), transmural infiltration of neutrophils, lymphocytes and occasional eosinophils (Fig. 3f,g) and serositis (Fig. 3h). As this day 7 time-point was most representative of human appendicitis at the histological level, it was used for subsequent analysis of infiltrating cell phenotype. There were no histological features of ischaemic necrosis at any of the time-points.
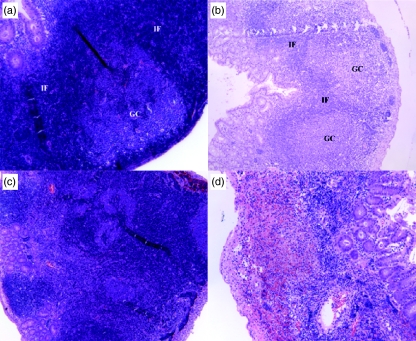
Fig. 2.
Histological images showing the progressive changes of lymphoid structure in murine appendix during inflammation. GC: germinal centre; IF: interfollicular T cell area (a) at 48 h of appendicitis showing normal lymphoid follicle, (b) at 72 h of appendicitis, normal follicular architecture is still apparent, (c) at 96 h of appendicitis, follicular structure is very distorted, (d) at day 7 of appendicitis, lymphoid follicular structure is no longer recognizable.
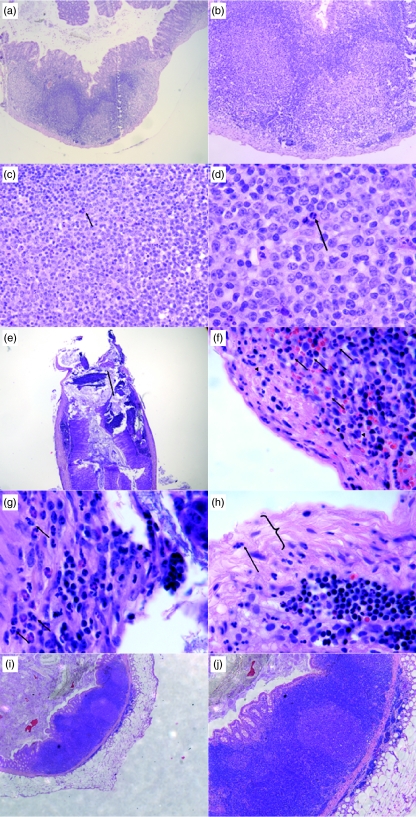
Fig. 3.
Histological features of inflamed murine appendices (appendicitis) at day 3 (a–d) and day 7 (e–h) by haematoxylin and eosin stain, (a) at day 3, the appendix retained its normal architecture with lymphoid follicles with minimal inflammatory infiltrates, (b) at high power, the mucosa remains intact and no evidence of serositis, (c) only few neutrophils ( ) are evidence in the lymphoid area, (d) high power of neutrophils (
) are evidence in the lymphoid area, (d) high power of neutrophils ( ), (e) mucosal ulceration (
), (e) mucosal ulceration ( ), (f) inflammatory infiltrate at day 7 with neutrophils (→) and lymphocytes (▸) in submucosa, (g) in muscularia propria and (h) serositis with fibrinous deposits (
), (f) inflammatory infiltrate at day 7 with neutrophils (→) and lymphocytes (▸) in submucosa, (g) in muscularia propria and (h) serositis with fibrinous deposits ( ) and neutrophilic infiltration (→), (i) low power of appendiceal pouch from antibiotic-treated mice showing relative preservation of normal lymphoid architecture with minimal inflammatory infiltration, (j) high power of (i).
) and neutrophilic infiltration (→), (i) low power of appendiceal pouch from antibiotic-treated mice showing relative preservation of normal lymphoid architecture with minimal inflammatory infiltration, (j) high power of (i).
Histological examination of appendiceal pouches from antibiotic-treated mice showed less inflammatory infiltrate and retained relatively normal lymphoid follicular architecture (Fig. 3i,j).
The appendices from sham-operated mice showed normal histology without any microscopic features of inflammation.
Phenotype of appendiceal lymphocytes in inflamed appendix
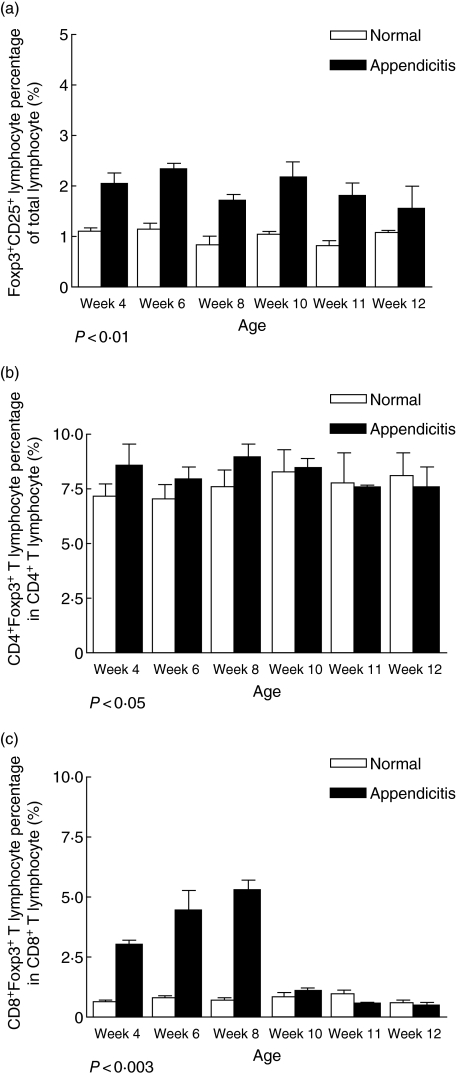
As a result of the obstruction and subsequent inflammatory response, significant changes in the phenotype of appendiceal lymphocyte subpopulations were observed in pouches resected on day 7. Analysis showed a significant increase in total lymphocyte numbers isolated from inflamed appendices when compared with normal appendices (from 0·3 to 0·4 ± 0·02–0·05 × 106 per mg of tissue to 0·5–0·7 ± 0·02–0·08 × 106 per mg of tissue; P < 0·05). There was a marked increase in CD4− and CD8− T lymphocyte populations (from 15·3 ± 1·2% to 31·1 ± 2·1%; P < 0·01 and from 3·8 ± 0·6% to 9·2 ± 0·8%; P < 0·01, respectively) across all age groups (Fig. 4a,b) with reciprocal reduction of CD45R/B220− B lymphocytes from 71·7 ± 3·1% to 27·9 ± 4·5%; P < 0·01 (Fig. 4c). Intracellular expression of FoxP3 was examined as an indicator of change in putative regulatory T lymphocyte numbers. In the appendicitis-affected mice, FoxP3− T lymphocytes were increased significantly (from 1·5 ± 0·1% to 2·5 ± 0·4%, P < 0·01) across all time-points (Fig. 5a,b). When subpopulations of FoxP3− T lymphocytes were assessed, there was no significant change in the proportion of CD4− FoxP3− T lymphocytes with age in inflamed appendices. However, CD8−FoxP3− T lymphocyte in inflamed appendices increased significantly, but only in younger mice (age < 10 weeks), P < 0·003 (Fig. 5c).
Fig. 4.
Phenotype of murine appendiceal lymphocytes in normal (□) and inflamed appendices (▪) across different age groups. (a) CD4− T lymphocyte, (b) CD8− T lymphocyte and (c) CD45R/B220 B lymphocyte. Bars represent the mean value calculated from results of three to four experiments and each experiment contains at least three mice. Error bars represent s.e.m. Difference is considered significant if P < 0·05.
Fig. 5.
Comparison of proportions of regulatory T lymphocytes in murine appendices between normal (□) and test mice [with appendicitis (▪)]. (a) Forkhead box P3 (FoxP3)− regulatory T lymphocyte, (b) CD4− FoxP3− regulatory T lymphocyte and (c) CD8− FoxP3− regulatory T lymphocyte. Bars represent the mean value calculated from results of three to four experiments and each experiment contains at least three mice. Error bars represent s.e.m. Difference is considered significant if P < 0·05.
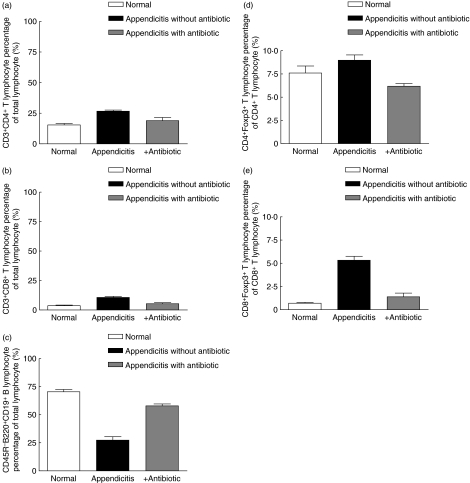
Phenotyping of appendiceal lymphocytes from antibiotic-treated mice showed qualitatively similar but quantitatively less marked increases in CD4− and CD8− T lymphocytes (Fig. 6a,b) and reciprocal reduction of B lymphocytes (Fig. 6c). However, there was no increase in CD4−FoxP3− and CD8−FoxP3− T lymphocytes (Fig. 6d,e).
Fig. 6.
Comparison of lymphocyte constituents of normal appendices (□), inflamed murine appendices (▪) and murine appendices with antibiotic treatment ( ). (a) CD4− T lymphocytes, (b) CD8− T lymphocyte, (c) CD45R-B220−CD19− B lymphocytes, (d) CD4−forkhead box P3 (FoxP3)− T lymphocyte, (e) CD8−FoxP3− T lymphocyte.
). (a) CD4− T lymphocytes, (b) CD8− T lymphocyte, (c) CD45R-B220−CD19− B lymphocytes, (d) CD4−forkhead box P3 (FoxP3)− T lymphocyte, (e) CD8−FoxP3− T lymphocyte.
CRP levels
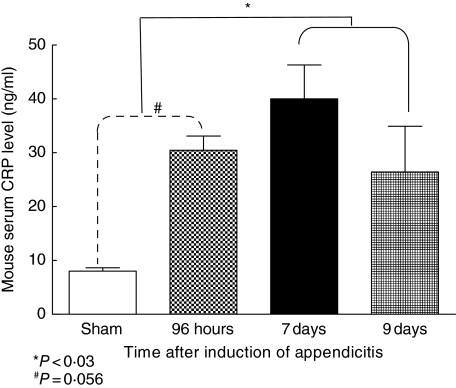
A significant (P < 0·01) increase in serum CRP levels was observed in mice with appendicitis (40·0 ± 3·1 ng/ml) compared to sham-operated mice, which had low and unchanging levels (8·0 ± 0·3 ng/ml; Fig. 7).
Fig. 7.
Comparison of serum C reactive protein (CRP) levels between sham (□) and test mice [with appendicitis ( , ▪,
, ▪,  )] measured by enzyme-linked immunosorbent assay from different time-points after formation of appendiceal pouches. Mean value of measured serum level of CRP from three mice and error bars represent s.d. Difference is considered significant if P < 0·05.
)] measured by enzyme-linked immunosorbent assay from different time-points after formation of appendiceal pouches. Mean value of measured serum level of CRP from three mice and error bars represent s.d. Difference is considered significant if P < 0·05.
Discussion
Human epidemiological data showing appendicectomy is protective against the development of inflammatory bowel disease indicate that the human appendix is likely to play an important role in gastrointestinal mucosal immune function, similar to the rabbit appendix [1–4,6]. Given the paucity of data in the literature on the function of the human appendix [1,5] and the difficulty in studying human appendicitis, we created a novel murine model of appendicitis.
The appendicitis model described here has many of the features of human appendicitis, namely mucosal ulceration, transmural infiltration with neutrophils and lymphocytes and serositis. This appendiceal inflammation is associated with elevation of the serum inflammatory marker, CRP, confirming a systemic response. Furthermore, in relation to studies of the pathogenesis of inflammatory bowel disease, the appendiceal inflammation induced substantial changes in the lymphocyte population in the murine appendix.
In humans, acute appendicitis presents typically with abdominal pain, but the classical symptom complex of anorexia and peri-umbilical pain followed by nausea, right lower quadrant pain and vomiting occurs in only 50% of cases [25]. This inconsistency in clinical presentation is related to the degree of inflammation, the age of the patient and variations in the anatomical position of the appendix. In histologically confirmed cases of acute appendicitis resulting in appendicectomy, 60–70% of patients had uncomplicated, suppurative appendicitis on histopathological assessment. Complicated appendicitis including perforation, gangrene and abscess formation made up the remaining 30–40% of cases [25]. Suppurative appendicitis is characterized by a transmural leucocyte infiltration featuring polymorphonuclear neutrophils and, to a lesser extent, lymphocytes, accompanied by mucosal ulceration [9]. Commonly, the appendix also features oedema, fibrino-purulent serositis, mural microabscess formation and vascular microthrombi. In addition, eosinophils have been shown to be commonly present [26]. Apart from the lower rate of abscess formation, our model mimics closely each of these features of human condition at the histological level.
Detailed examination of the leucocyte subpopulations infiltrating these inflamed murine appendices during the late phase (≥ 7 days) revealed a significant change in the lymphocyte constituents, with increases in both CD4− and CD8− T lymphocytes, including FoxP3−CD25− regulatory T lymphocytes which could be related to clonal expansion of subpopulations of lymphocytes in response to bacterial invasion. This is supported further by the lack of regulatory T lymphocyte expansion in antibiotic-treated mice. Intestinal regulatory T lymphocytes are believed to play an important role in modulating gastrointestinal mucosal immune responses [27–29], and have been implicated in both murine and human colitis. Murine studies have shown that depletion of regulatory T lymphocytes results in the development of colitis, which can be prevented by injection of regulatory T lymphocytes [30–33]. These regulatory T lymphocytes have been shown to populate the lamina propria of the colon [28]. In human inflammatory bowel disease (IBD), an initial study showed an increase in the proportion of CD4− regulatory T lymphocytes in the lamina propria of colon from patients with IBD [29]. However, a more recent study showed that CD8− regulatory T lymphocytes are deficient in number and defective in function in the lamina propria of the colon from patients with IBD [27]. In fact, our data have shown that there is a significant age-related difference in CD8−FoxP3− T lymphocytes in inflamed appendices, with younger mice only affected; this may explain the age-dependent protective phenomenon of appendicectomy for intra-abdominal inflammatory conditions against development of colitis. While these data await confirmatory studies, there is a strong belief that regulatory T lymphocytes play an important protective role in the pathogenesis of IBD. Accordingly, the accumulation of regulatory T cells documented in our murine model of appendicitis provides in principle support for the relevance of these cells to the mechanism of protection against later development of inflammatory bowel disease seen in human epidemiological studies.
The late phase of appendicitis in this model which features an increase in the lymphocytic infiltration into the appendix is consistent with the leucocyte response during the transition from acute into chronic inflammation [34]. Persistence of the irritant in the form of overgrowth of luminal bacteria may drive this evolution to chronic inflammation. This shift may also result from an adaptive cellular immune response to antigens derived from bacterial invasion secondary to breakdown of the mucosal barrier during appendicitis. The simultaneous increase in FoxP3− regulatory T lymphocytes may therefore represent a means of auto-regulation to prevent excessive effector immune responses which might lead to undesirable tissue damage induced by bacterial or cross-reactive self-antigens [35]. This notion is again supported by the effect of antibiotics in this appendicitis model. Alternatively, the accumulation of mononuclear cells, including CD4− and CD8− T cells with FoxP3− regulatory cells, may represent a non-specific manifestation of the late (resolving) phase of acute inflammation [34].
Further exploration of this model of appendicitis in association with murine models of colitis has the potential to provide significant insights into the pathogenesis of inflammatory bowel disease. In particular, our current studies are focused upon an analysis of migration pathways of appendiceal regulatory T cells, in particular trafficking to intestine where they might proliferate to mediate protection against inflammation.
References
- 1.Dasso JF, Objahon H, Bach H, Anderson AO, Moje RG. A morphological and immunohistological study of the human and rabbit appendix for comparison with the avian bursa. Dev Comp Immunol. 2000;24:797–814. doi: 10.1016/s0145-305x(00)00033-1. [DOI] [PubMed] [Google Scholar]
- 2.Becker RS, Knight KL. Somatic diversification of immunoglobulin heavy chain VDJ genes: evidence for somatic gene conversion in rabbits. Cell. 1990;63:987–97. doi: 10.1016/0092-8674(90)90502-6. [DOI] [PubMed] [Google Scholar]
- 3.Dasso JF, Howell MD. Neonatal appendectomy impairs mucosal immunity in rabbits. Cellular Immunol. 1997;182:29–37. doi: 10.1006/cimm.1997.1216. [DOI] [PubMed] [Google Scholar]
- 4.Reynaud CA, Garcia C, Hein WR, Weill IC. Hypermutation generating the sheep immunoglobulin repertoire is an antigen-independent process. Cell. 1995;80:115–25. doi: 10.1016/0092-8674(95)90456-5. [DOI] [PubMed] [Google Scholar]
- 5.Spencer J, Finn T, Isaacson PG. Gut associated lymphoid tissue: a morphological and immunocytochemical study of the human appendix. Gut. 1985;26:672–9. doi: 10.1136/gut.26.7.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinstein PD, Anderson AO, Mage RG. Rabbit IgH sequences in appendix germinal centers: VH diversification by gene conversion-like and hypermutation mechanisms. Immunity. 1994;1:647–59. doi: 10.1016/1074-7613(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 7.Larner AJ. The aetiology of appendicitis. Br J Hosp Med. 1988;39:540–2. [PubMed] [Google Scholar]
- 8.Walker AR, Segal I. What causes appendicitis? J Clin Gastroenterol. 1990;12:127–9. [PubMed] [Google Scholar]
- 9.Carr NJ. The pathology of acute appendicitis. Ann Diagn Pathol. 2000;4:46–58. doi: 10.1016/s1092-9134(00)90011-x. [DOI] [PubMed] [Google Scholar]
- 10.Kuga T, Taniguchi S, Inone T, Zampo N, Esato K. Immunocytochemical analysis of cellular infiltrates in human appendicitis. Surg Today. 2000;30:1083–8. doi: 10.1007/s005950070005. [DOI] [PubMed] [Google Scholar]
- 11.Rivera-Chavez FA, Wheeler H, Lindberg G, Munford RS, O'Keefe GE. Regional and systemic cytokine responses to acute inflammation of the vermiform appendix. Ann Surg. 2003;237:408–16. doi: 10.1097/01.SLA.0000055274.56407.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frisch M, Biggar RJ. Appendectomy and protection against ulcerative colitis. N Engl J Med. 2001;345:222–3. doi: 10.1056/NEJM200107193450316. author reply 223–4. [DOI] [PubMed] [Google Scholar]
- 13.Frossard JL, de Peyer R, Hadengue A. Appendectomy and protection against ulcerative colitis. N Engl J Med. 2001;345:223. author reply 223–4. [PubMed] [Google Scholar]
- 14.Koutroubakis IE, Vlachonikolis IG. Appendectomy and the development of ulcerative colitis: results of a metaanalysis of published case–control studies. Am J Gastroenterol. 2000;95:171–6. doi: 10.1111/j.1572-0241.2000.01680.x. [see comment] [DOI] [PubMed] [Google Scholar]
- 15.Koutroubakis IE, Vlachonikolis IG, Kouroumalis EA. Role of appendicitis and appendectomy in the pathogenesis of ulcerative colitis: a critical review. Inflamm Bowel Dis. 2002;8:277–86. doi: 10.1097/00054725-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Lopez Ramos D, Gabriel R, Cantero Perona J, Moreno Otero R, Fernandez Bermejo M, Mata Jimenez J. Association of MALTectomy (appendectomy and tonsillectomy) and inflammatory bowel disease: a familial case–control study. Rev Esp Enferm Dig. 2001;93:303–14. [PubMed] [Google Scholar]
- 17.Lowenfels AB, Maisonneuve P. Appendectomy and protection against ulcerative colitis. N Engl J Med. 2001;345:223. author reply 223–4. [PubMed] [Google Scholar]
- 18.Radford-Smith GL, Edwards JE, Pardie DM, et al. Protective role of appendicectomy on onset and severity of ulcerative colitis and Crohn's disease. Gut. 2002;51:808–13. doi: 10.1136/gut.51.6.808. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizoguchi A, Mizoguchi E, Chiba C, et al. Cytokine imbalance and autoantibody production in T cell receptor-alpha mutant mice with inflammatory bowel disease. J Exp Med. 1996;183:847–56. doi: 10.1084/jem.183.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieglstein CF, Cerwinka WH, Laroux FS, et al. Role of appendix and spleen in experimental colitis. J Surg Res. 2001;101:166–75. doi: 10.1006/jsre.2001.6223. [DOI] [PubMed] [Google Scholar]
- 21.Farkas SA, Homung M, Sattler C, et al. Preferential migration of CD62L cells into the appendix in mice with experimental chronic colitis. Eur Surg Res. 2005;37:115–22. doi: 10.1159/000084543. [DOI] [PubMed] [Google Scholar]
- 22.Davies MD, Parrott DM. Preparation and purification of lymphocytes from the epithelium and lamina propria of murine small intestine. Gut. 1981;22:481–8. doi: 10.1136/gut.22.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [see comment] [DOI] [PubMed] [Google Scholar]
- 24.Walker MR, Kasprowioz DS, Gersuk VH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112:1437–43. doi: 10.1172/JCI19441. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohmann C, Yang Q, Franke C. Diagnostic scores for acute appendicitis. Eur J Surg. 1995;161:273–81. Abdominal Pain Study Group. [PubMed] [Google Scholar]
- 26.Aravindan KP. Eosinophils in acute appendicitis: possible significance. Indian J Pathol Microbiol. 1997;40:491–8. [PubMed] [Google Scholar]
- 27.Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol. 2005;174:5814–22. doi: 10.4049/jimmunol.174.9.5814. [DOI] [PubMed] [Google Scholar]
- 28.Uhlig HH, Coombes J, Mottet C, et al. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852–60. doi: 10.4049/jimmunol.177.9.5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makita S, Kanai T, Oshima S, et al. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173:3119–30. doi: 10.4049/jimmunol.173.5.3119. [DOI] [PubMed] [Google Scholar]
- 30.Leach MW, Bean AG, Manze S, Coffman RL. Inflammatory bowel disease in C.B-17 scid mice reconstituted with the CD45RBhigh subset of CD4+ T cells. Am J Pathol. 1996;148:1503–15. [PMC free article] [PubMed] [Google Scholar]
- 31.Morrissey PJ, Chawier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993;178:237–44. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powrie F, Correa-Oliveria R, Mansez S, Coffman RL. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powrie F, Leach MW, Manze S, Coddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461–71. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 34.Stephenson T. Inflammation. In: Underwood J, editor. General and systemic pathology. 4. Edinburgh: Churchill-Livingstone; 2004. pp. 201–21. [Google Scholar]
- 35.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–60. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]