Abstract
Complications induced by the chemotherapeutic agent cisplatin, such as neuropathy and cachexia, occur frequently, are often dose limiting, and have an impact on quality of life and survival in cancer patients. The recently discovered hormone ghrelin is a potent GH secretagogue with orexigenic and neuroprotective properties that may prevent or ameliorate these complications. The objective of this study was to determine the effects of ghrelin administration on mechanical hyperalgesia, anorexia, and cachexia induced by cisplatin. Adult male Sprague-Dawley rats were given cisplatin, ghrelin, ghrelin-cisplatin, or vehicle ip. Food intake and body weight were measured daily. Behavioral tests to assess the development of hyperalgesia were conducted by measuring mechanical and thermal sensitivity. Plasma ghrelin and IGF-I levels were also measured. Our results indicate that ghrelin coadministration inhibited the development of cisplatin-induced mechanical hyperalgesia, anorexia, and cachexia induced by cisplatin. Although ghrelin treatment had no effect on plasma IGF-I levels in control rats, it prevented the decrease in IGF-I levels induced by cisplatin. The attenuation of cisplatin-induced mechanical hyperalgesia induced by ghrelin was correlated with the prevention of cisplatin-induced lowering of IGF-I. In conclusion, ghrelin administration may be useful in the treatment or prevention of chemotherapy induced neuropathy and cachexia. Attenuation of mechanical hyperalgesia in the rat by the hormone ghrelin provides a unique model for elucidating the mechanisms involved, which are essential toward our understanding of these complications.
THE TREATMENT OF cancer usually encompasses the use of cytotoxic agents, including platinum-based drugs such as cisplatin. However, the administration of cisplatin is associated with significant neurotoxicity manifested as peripheral neuropathy, cachexia (involuntary weight loss), and anorexia that often exacerbate the pain, cachexia, and anorexia induced by the tumor itself.
Cisplatin-induced neuropathy causes significant distress to patients and is often a dose-limiting side effect interfering with normal daily activities (1). Patients usually present increased touch detection, paresthesias, and dysesthesias in the affected areas, including the extremities (2). Anorexia and loss of body weight and lean body mass are present in up to 80% of cancer patients. These symptoms are major contributors to the high mortality seen in this population and may indeed be the direct cause of death in some patients (3). The pathophysiological mechanisms underlying the development of neuropathy and cachexia in this setting have not been fully elucidated. However, a decrease in IGF-I is associated with both cancer-induced cachexia and cisplatin-induced neuropathy (4,5).
Ghrelin, an octanoylated 28-amino acid peptide secreted mainly from the stomach, is a potent GH secretagogue, and its administration increases not only GH secretion but also food intake and body weight in animals and humans (6,7). Subjects with cancer-induced cachexia have increased levels of ghrelin (4), and ghrelin infusion increases appetite in subjects with cancer (8). Ghrelin also increases food intake and body weight in experimental models of cancer-related cachexia (9). More recently, ghrelin also has been shown to have neuroprotective properties and to prevent neuronal apoptosis (10). Based on ghrelin’s GH secretagogue, neuroprotective and orexigenic properties, we postulated that it would be effective against cisplatin-induced neuropathy and cachexia.
Accordingly, we tested the effects of exogenous ghrelin administration in this setting.
Materials and Methods
Twelve-week-old male Sprague Dawley rats weighing between 230 and 270 g (Harlan, Houston, TX) were randomized to receive vehicle (saline), cisplatin, ghrelin + cisplatin and ghrelin. Cisplatin was purchased from Sigma-Aldrich (St. Louis, MO), and rodent ghrelin was purchased from Phoenix Pharmaceuticals (Belmont, CA).
The dose of cisplatin was 0.5 mg/kg given daily at 0830 h from d 0–2 ip, and the dose for ghrelin was 0.8 mg/kg twice daily ip at 0800 and 1700 h for 14 d. This particular regimen of cisplatin was chosen because the total dose is similar to the dose of cisplatin administered to humans during a “cycle” of cisplatin (11), and we had determined in preliminary experiments that this regimen induces a mild but significant degree of weight loss and hyperalgesia similar to that induced in humans.
Animals were individually housed, acclimated to their cages and human handling for 5 d before the experiments were started and maintained on a 12-h light, 12-h dark cycle (lights on at 0600 h). Food and water were given ad libitum. All experiments were conducted with the approval of the Institutional Animal Care and Use Committee, and were in compliance with the National Institutes of Health Guidelines for Use and Care of Laboratory Animals.
Body weight and food consumption were assessed daily by weighing the food and animals before the morning injection. Body weight changes were expressed as percent change from baseline, and food consumption was expressed cumulatively as grams of food consumed during cisplatin administration (d 0–3) and after the cisplatin cycle was completed (d 3–13).
In a subgroup of animals (n = 4 per group), plasma total ghrelin levels were measured at baseline before injections, and again 6 and 24 h after cisplatin and ghrelin administration, as we have previously described (12). IGF-I levels were measured at baseline, and again on d 1 and 13 upon completion of the experiment (n = 4 per group). The peptide was extracted using acid-methanol and measured by a RIA kit for rat IGF-I from Diagnostic System Laboratories, Inc. (Webster, TX).
Behavioral testing
Behavioral tests to assess the development of neuropathy were conducted in a quiet room at 22.8 C as previously described (13). All the tests were conducted before injecting the animals by an investigator blinded to treatment allocation.
To test mechanical sensitivity, rats were placed on a wire mesh, loosely restrained under a Plexiglas cage, and allowed to accommodate for at least 10 min. A series of von Frey monofilaments (range of bending force: 0.61, 1.18, 2.87, 8.88, and 14.96 g) were tested in ascending order to generate response frequency functions for each animal. Each von Frey filament was applied five times to the midplantar side of each hind paw from beneath for about 1 sec. The values for each animal were averaged across all animals in each group to yield a group stimulus-response function.
Response threshold was defined as the lowest force filament that evoked a 50% or greater response frequency. This value was later averaged across all animals in each group to yield the group response threshold.
Thermal sensitivity was tested using a radiant heat source applied to the plantar surface of the hind paw as previously described (14).
SPSS 12.00 software for Windows (SPSS, Inc., Chicago, IL) was used for all statistical analyses. Parameters are expressed as mean ± sem. Statistical comparisons for longitudinal data (mechanical threshold and body weight) were performed using repeated measures ANOVA with time, treatment, and the interaction between them as effects. Post hoc analyses were done using the Tukey test. Area under the curve (AUC) was calculated using the trapezoidal rule. Pearson’s correlations, or nonparametric Spearman’s correlation when the data were not normally distributed, were obtained between variables of interest. P values of 0.05 or smaller were considered significant.
Results
Behavioral tests
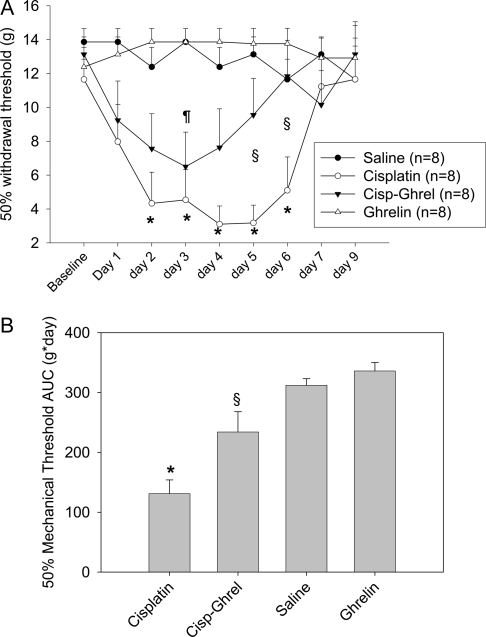
To assess the development of peripheral neuropathy, behavioral tests measuring mechanical hyperalgesia were performed daily from d 1–7 and on d 9. The 50% withdrawal threshold at baseline was similar for all groups (Fig. 1A). Animals in the cisplatin-treated group showed a decrease in the withdrawal threshold, indicating a hyperresponse to monofilament stimuli, from d 2–6 when compared with the other groups and from d 1–6 when compared with the same group at baseline. Animals receiving cisplatin-ghrelin showed a smaller decrease in the withdrawal threshold compared with saline-treated animals that was only significantly decreased on d 3.
Figure 1.
A, Fifty percent mechanical threshold per group. *, P < 0.05 for cisplatin vs. saline group. §, P < 0.05 for cisplatin vs. cisplatin-ghrelin (Cisp-Ghrel) group.¶, P < 0.05 for cisplatin-ghrelin vs. saline group. B, Fifty percent mechanical threshold AUC per group. *, P < 0.05 for cisplatin vs. all other groups. §, P < 0.05 for cisplatin-ghrelin vs. all other groups.
When the 50% withdrawal threshold was compared between ghrelin-cisplatin and cisplatin-treated animals, there was a remarkable difference in the 50% withdrawal threshold for d 5 and 6 (P < 0.05), indicating that mechanical hyperalgesia was markedly attenuated by ghrelin. Vehicle- and ghrelin-treated animals did not experience significant changes in 50% mechanical threshold throughout the duration of the experiment. The 50% withdrawal threshold AUC was calculated to quantify the severity of the mechanical hyperalgesia. Animals receiving cisplatin experienced a significant reduction in the 50% withdrawal threshold AUC compared with all other groups, and ghrelin coadministration significantly improved the hyperalgesia induced by cisplatin (Fig. 1B).
There was no significant difference in latency of hind paw withdrawal responses to radiant heat stimuli in cisplatin-treated animals. Similarly, other groups treated showed no change in response to thermal stimuli (data not shown). This study confirmed that cisplatin does not affect thermal sensitivity, at least during the period tested, as it has been shown elsewhere (15).
Body weight
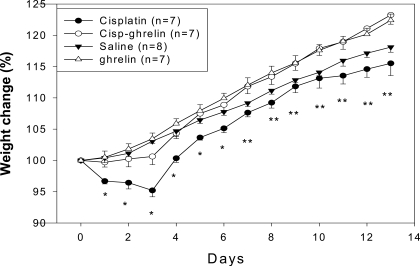
During the first 3 d of the experiment when cisplatin was being administered, animals in the cisplatin group lost weight significantly (−4.8 ± 1%), whereas animals receiving saline, ghrelin, or cisplatin-ghrelin gained weight (3.1 ± 0.4, 3.5 ± 0.9, and 0.6 ± 1.3%, respectively; ANOVA, P < 0.001; Fig. 2). After cisplatin administration was stopped (d 3–13), cisplatin-treated animals gained weight, but their body weight remained significantly lower than the body weight in animals receiving saline until d 7. There were no significant differences between animals receiving ghrelin-cisplatin and saline-treated controls at any of the time points measured during the study.
Figure 2.
Body weight gain per group. *, P < 0.05 for cisplatin vs. all other groups. **, P < 0.05 for cisplatin vs. ghrelin and cisplatin-ghrelin (Cisp-ghrelin)-treated animals, not vs. saline-treated animals.
Food intake
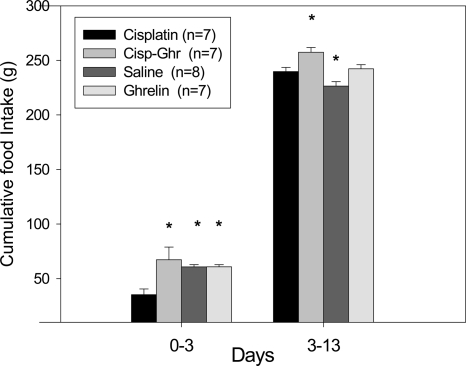
Animals in the cisplatin-treated group had a significant decrease in food intake during the first 3 d of the experiment. Ghrelin coadministration completely normalized food intake, making it indistinguishable from that of ghrelin- or saline-treated animals (35.3 ± 5.2, 60.8 ± 2.1, 60.9 ± 1.8, and 67.3 ± 11.6 g for cisplatin, saline, ghrelin, and ghrelin-cisplatin treated groups, respectively (ANOVA, P < 0.009; Fig. 3).
Figure 3.
Cumulative food intake per group divided in d 0–3 and 3–13. *, P < 0.05 compared with cisplatin-treated animals. Cisp-Ghr, Cisplatin-ghrelin.
During the recovery phase (d 3–13), cisplatin-treated animals increased their food intake, although it remained significantly lower than the food intake for cisplatin-ghrelin treated animals (240 ± 3.8 g for cisplatin group, 257 ± 4 g for cisplatin-ghrelin group, 227 ± 4 g for saline group, and 242 ± 2.8 g for ghrelin group; ANOVA, P < 0.001).
Ghrelin and IGF-I levels
There was no difference in ghrelin levels at baseline between groups (Table 1). At 6 h, plasma ghrelin increased in all groups, but it only reached statistical significance for the cisplatin-ghrelin and ghrelin groups when compared with baseline. At 24 h, ghrelin levels were significantly higher than baseline only in the cisplatin-treated animals.
Table 1.
Plasma ghrelin and IGF-I levels
| Group | Baseline ghrelin (ng/ml) | 6-h ghrelin (ng/ml) | 24-h ghrelin (ng/ml) | Baseline IGF-I (mg/ml) | 24-h IGF-I (mg/ml) | 13-d IGF-I (mg/ml) |
|---|---|---|---|---|---|---|
| Saline (n = 4) | 1.68 ± 0.13 | 2.22 ± 0.21 | 1.51 ± 0.13 | 1.24 ± 0.01 | 1.58 ± 0.25 | 0.96 ± 0.19 |
| Cisplatin (n = 4) | 1.50 ± 0.13 | 2.41 ± 0.25 | 2.1 ± 0.12a,b | 1.69 ± 0.15 | 1.09 ± 0.09a,b | 1.17 ± 0.1 |
| Cisplatin-ghrelin (n = 4) | 1.56 ± 0.2 | 2.59 ± 0.26a | 1.88 ± 0.24 | 1.59 ± 0.07 | 1.25 ± 0.21 | 1.31 ± 0.17 |
| Ghrelin (n = 4) | 1.49 ± 0.15 | 2.62 ± 0.33a | 2.09 ± 0.21b | 1.45 ± 0.22 | 1.54 ± 0.07 | 0.85 ± 0.08 |
P < 0.05 compared with same treatment group at baseline.
P < 0.05 compared with saline-treated animals.
IGF-I levels at baseline were the same between all groups. However, on d 1, IGF-I levels were significantly lower in cisplatin-treated animals but remained stable in the other groups. Upon conclusion of the experiment on d 13, there were no differences in IGF-I levels for each group compared with baseline or between groups.
Correlation analyses
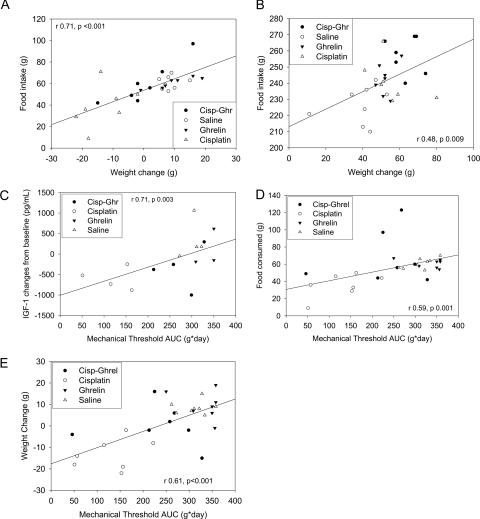
Body weight changes directly correlated with food intake during cisplatin administration (d 0–3, Fig. 4A) and during the recovery phase (d 3–13, Fig. 4B). Fifty percent mechanical threshold AUC directly correlated with IGF-I level changes at 24 h, and with food intake and body weight changes from d 0–3 and 3–13 (Fig. 4, C–E).
Figure 4.
A, Correlation between food intake and body weight changes (d 0–3). B, Correlation between food intake and body weight changes (d 3–13). C, Correlation between mechanical threshold and IGF-I changes at 24 h. D, Correlation between mechanical threshold and food intake (d 0–3). E, Correlation between mechanical threshold and weight change (d 0–3). Cisp-Ghr, Cisplatin-ghrelin; Ghrel, ghrelin.
Discussion
The rat model we used illustrates two of the most serious complications induced by cisplatin: neuropathy and cachexia. These side effects occur frequently, are often dose limiting, and severely affect the quality of life in patients with cancer. Because there are currently no Food and Drug Administration-approved treatments for these complications, the development of new therapies is desperately needed.
Cisplatin has direct neurotoxic effects that often cause incapacitating neuropathy. In this study we show that a single course of cisplatin administered in doses similar to those used in humans for the treatment of cancer, induces mechanical hyperalgesia. Hyperalgesia as detected by behavioral testing has been one of the most sensitive tools to assess the development of sensory neuropathy in both human and animal models of chemotherapy induced peripheral neuropathy (2,16,17) because severe damage is necessary before changes are evident in nerve conduction studies or histological studies. Remarkably, the hormone ghrelin ameliorated this mechanical hyperalgesia, as indicated by a marked improvement in the 50% mechanical threshold. Although cisplatin significantly decreased this threshold as early as 24 h after starting treatment and maintained these effects for 6 d, ghrelin coadministration reduced the intensity and the duration of the neuropathic symptoms by approximately 50%. To our knowledge, this is the first report showing that the hormone ghrelin is protective against mechanical hyperalgesia.
Several mechanisms could be responsible for ghrelin’s neuroprotective property. A decrease in IGF-I expression in nociceptive neurons in the dorsal root ganglia has been linked to the development of painful peripheral neuropathy (18). Indeed, IGF-I treatment has prevented the development of chemotherapy induced neuropathy (5). Ghrelin induces GH secretion through direct effects on GHRH neurons and pituitary somatotrophs by activating GH secretagogue receptor 1a (GHS-R1a) (19). Furthermore, chronic activation of GHS-R1a by a ghrelin mimetic increases IGF-I levels (20,21) that are often reduced in subjects with cancer (4). In our study low IGF-I levels were correlated with the development of cisplatin-induced hyperalgesia, and ghrelin administration inhibited both cisplatin-induced reduction in IGF-I levels and mechanical hyperalgesia. This suggests that exogenous ghrelin elicits beneficial effects by maintaining IGF-I levels.
IGF-I levels were significantly decreased by cisplatin administration. Moreover, the degree of IGF-I reduction seen in this setting is similar to the reduction in IGF-I we and others have reported in humans with cancer-related cachexia (4). As weight loss causes a decrease in IGF-I levels, the inhibitory effect of cisplatin on IGF-I levels could be mediated through its negative impact on appetite and body weight, although a direct effect of cisplatin on IGF-I production cannot be excluded. The decrease in IGF-I induced by cisplatin was prevented by coadministration of ghrelin, which could be a result of ghrelin’s GH secretagogue activity and/or prevention of weight loss. Although 13-d ghrelin administration did not increase IGF-I levels in control animals, this was not surprising because healthy rats of this age have high basal IGF-I levels that are not increased further by ghrelin administration. One possible explanation for this is that the feedback mechanism between GH and IGF-I would prevent the increase of IGF-I above normal levels. Nevertheless, it is clear that coadministration of ghrelin prevented the inhibitory effect of cisplatin on IGF-I production.
Alternative mechanisms involve the putative antiinflammatory properties of IGF-I and ghrelin. Cisplatin is also known to induce a proinflammatory response (22), which is thought to play a role in inducing neuropathy (2). Cisplatin preferentially binds to DNA in dorsal root ganglia neurons and induces apoptosis (23). Because of its antiinflammatory and antiapoptotic properties, IGF-I would be expected to attenuate cisplatin toxicity (24). Besides mediating its effect through maintaining IGF-I production, an additional contributing factor could be that ghrelin has inhibited nuclear factor-κB activation and apoptosis in several tissues (10,25). An intriguing possibility is that the antiinflammatory and antiapoptotic effects of ghrelin, in combination with its effect on IGF-I production, contribute to the prevention of cisplatin-induced peripheral neuropathy.
Cisplatin has been shown to be a highly emetogenic chemotherapeutic agent in humans and in experimental models (26), leading to a decrease in appetite and subsequently to weight loss. In addition to ameliorating mechanical hyperalgesia, we also demonstrate that administration of exogenous ghrelin prevents the development of cisplatin-induced anorexia and cachexia. The administration of ghrelin stabilized weight loss during cisplatin treatment and induced rapid weight gain after the cycle of cisplatin was completed, leading to a complete recovery 48 h after the last dose of cisplatin was given, whereas cisplatin-treated animal only partially recovered their weight that remained significantly lower than in saline-treated animals until d 7. This difference could be at least partially explained by the differences in food intake during the recovery phase. Although cisplatin-treated animals consumed more food than their saline-treated counterparts, animals that had received ghrelin-cisplatin maintained their advantage in food intake, leading to a faster and complete recovery.
Ghrelin has been postulated to have antiemetic properties by virtue of its prokinetic properties in the gastrointestinal tract. Two recent reports demonstrated ghrelin’s antiemetic properties in similar models using cisplatin (27,28), although they failed to show an effect on body weight. The discrepancies between our findings and those previously reported can be explained by the different doses and dose regimens of cisplatin and ghrelin used. Whether the antagonistic effect of ghrelin on cisplatin-induced anorexia and weight loss is a consequence of the direct stimulatory effect of ghrelin on hypothalamic neurons regulating appetite and/or an indirect effect mediated through ghrelin’s prokinetic properties remains to be determined.
The strong correlation between food intake and body weight changes suggests that an increase in food intake is likely to be the main mechanism underlying the prevention of weight loss in this setting, although other mechanisms such as a decrease in energy expenditure cannot be excluded. Ghrelin stimulates appetite and induces weight gain by activating the GHS-R1a in specific hypothalamic neurons. Ghrelin also ameliorates tumor-induced cachexia in animals and improves appetite in human subjects with cancer (8,9). Furthermore, activation of the GHS-R1a by a ghrelin mimetic induced positive nitrogen balance in a group of noncancerous calorie-restricted subjects, suggesting that ghrelin-induced weight gain involves other mechanisms besides an increase in food intake (i.e. decreased energy expenditure) (29).
To establish the effect of cisplatin on endogenous ghrelin production, circulating plasma ghrelin levels were measured 6 and 24 h after the initial injection of cisplatin in a subset of the animals. Although ghrelin levels were higher at 6 h than at baseline in each group, there were no major differences between groups, arguing against a direct effect of cisplatin on ghrelin synthesis. However, this possibility cannot be excluded because the study was probably not sufficiently powered to detect changes in ghrelin levels between groups. This uniform increase in ghrelin levels at 6 h also could be explained by a normal ghrelin circadian rhythm (30). Given the very short half-life of ghrelin in the circulation (30 min) (31), ghrelin levels at 24 h could only represent endogenous ghrelin. At this time point, endogenous levels of ghrelin were modestly, but significantly elevated (P < 0.05) compared with baseline in cisplatin-treated animals. The increase in plasma ghrelin levels induced by cisplatin after 24 h is consistent with our observations in humans suffering from cancer-induced cachexia (4), and suggests that ghrelin elevation is a compensatory response to the anorexia and weight loss.
In summary, coadministration of the hormone ghrelin was shown to prevent the development of mechanical hyperalgesia, cachexia, and anorexia induced by cisplatin. Activation of GHS-R1a by ghrelin or its mimetics shows promise in the treatment of peripheral neuropathy and cachexia in cancer patients. At the same time, elucidating the mechanisms underlying these exciting observations will prove useful in improving our understanding of these conditions.
Acknowledgments
We thank Dr. Mark Asnicar for his valuable input and assistance.
Footnotes
This work was funded by a Merit Review Entry Program Grant from the Department of Veterans Affairs (to J.M.G.), a South Central Network Career Development Award from the Department of Veterans Affairs (to J.M.G.), a grant from the National Institutes of Health R-01 NS39933 (to P.M.D.), and a grant from the Ted Nash Life Long Foundation (R.G.S.). Ghrelin peptide used in these experiments was provided by Sapphire Therapeutics.
All authors contributed equally to this publication.
Disclosure Statement: J.M.G., J.P.C., and P.M.D. have nothing to declare. R.G.S. has received lecture fees and consults for Elixir Pharmaceuticals, Inc.
First Published Online October 25, 2007
Abbreviations: AUC, Area under the curve; GHS-R1a, GH secretagogue receptor 1a.
References
- Hovestadt A, van der Burg ME, Verbiest HB, van Putten WL, Vecht CJ 1992 The course of neuropathy after cessation of cisplatin treatment, combined with Org 2766 or placebo. J Neurol 239:143–146 [DOI] [PubMed] [Google Scholar]
- Cata JP, Weng HR, Lee BN, Reuben JM, Dougherty PM 2006 Clinical and experimental findings in humans and animals with chemotherapy-induced peripheral neuropathy. Minerva Anestesiol 72:151–169 [PubMed] [Google Scholar]
- Maltoni M, Nanni O, Pirovano M, Scarpi E, Indelli M, Martini C, Monti M, Arnoldi E, Piva L, Ravaioli A, Cruciani G, Labianca R, Amadori D 1999 Successful validation of the palliative prognostic score in terminally ill cancer patients. Italian Multicenter Study Group on Palliative Care. J Pain Symptom Manage 17:240–247 [DOI] [PubMed] [Google Scholar]
- Garcia JM, Garcia-Touza M, Hijazi RA, Taffet G, Epner D, Mann D, Smith RG, Cunningham GR, Marcelli M 2005 Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab 90:2920–2926 [DOI] [PubMed] [Google Scholar]
- Apfel SC, Kessler JA 1996 Neurotrophic factors in the treatment of peripheral neuropathy. Ciba Found Symp 196:98–108 [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR 2001 Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 86:5992 [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR 2001 Ghrelin causes hyperphagia and obesity in rats. Diabetes 50:2540–2547 [DOI] [PubMed] [Google Scholar]
- Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, Frost GS, Ghatei MA, Coombes RC, Bloom SR 2004 Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab 89:2832–2836 [DOI] [PubMed] [Google Scholar]
- Hanada T, Toshinai K, Kajimura N, Nara-Ashizawa N, Tsukada T, Hayashi Y, Osuye K, Kangawa K, Matsukura S, Nakazato M 2003 Anti-cachectic effect of ghrelin in nude mice bearing human melanoma cells. Biochem Biophys Res Commun 301:275–279 [DOI] [PubMed] [Google Scholar]
- Chung H, Kim E, Lee DH, Seo S, Ju S, Lee D, Kim H, Park S 2007 Ghrelin inhibits apoptosis in hypothalamic neuronal cells during oxygen-glucose deprivation. Endocrinology 148:148–159 [DOI] [PubMed] [Google Scholar]
- Johnson BE, Crawford J, Downey RJ, Ettinger DS, Fossella F, Grecula JC, Jahan T, Kalemkerian GP, Kessinger A, Koczywas M, Langer CJ, Martins R, Marymont MH, Niell HB, Ramnath N, Robert F, Williams Jr CC, National Comprehensive Cancer Network (NCCN)2006 Small cell lung cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw 2006 4:602–622 [DOI] [PubMed] [Google Scholar]
- Sun Y, Garcia JM, Smith RG 2007 Ghrelin and growth hormone secretagogue receptor expression in mice during aging. Endocrinology 148:1323–1329 [DOI] [PubMed] [Google Scholar]
- Cata JP, Weng HR, Dougherty PM 2004 Cyclooxygenase inhibitors and thalidomide ameliorate vincristine-induced hyperalgesia in rats. Cancer Chemother Pharmacol 54:391–397 [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J 1988 A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88 [DOI] [PubMed] [Google Scholar]
- Krarup-Hansen A, Helweg-Larsen S, Schmalbruch H, Rorth M, Krarup C 2007 Neuronal involvement in cisplatin neuropathy: prospective clinical and neurophysiological studies. Brain 130(Pt 4):1076–1088 [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Cata JP, Burton AW, Vu K, Weng HR 2007 Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. J Pain Symptom Manage 33:166–179 [DOI] [PubMed] [Google Scholar]
- Ling B, Authier N, Balayssac D, Eschalier A, Coudore F 2007 Behavioral and pharmacological description of oxaliplatin-induced painful neuropathy in rat. Pain 128:225–234 [DOI] [PubMed] [Google Scholar]
- Craner MJ, Klein JP, Black JA, Waxman SG 2002 Preferential expression of IGF-I in small DRG neurons and down-regulation following injury. Neuroreport 13:1649–1652 [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang P, Zheng H, Smith RG 2004 Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA 101:4679–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JM, Polvino WJ 2007 Effect on body weight and safety of RC-1291, a novel, orally available ghrelin mimetic and growth hormone secretagogue: results of a phase I, randomized, placebo-controlled, multiple-dose study in healthy volunteers. Oncologist 12:594–600 [DOI] [PubMed] [Google Scholar]
- Smith RG, Van der Ploeg LH, Howard AD, Feighner SD, Cheng K, Hickey GJ, Wyvratt Jr MJ, Fisher MH, Nargund RP, Patchett AA 1997 Peptidomimetic regulation of growth hormone secretion. Endocr Rev 18:621–645 [DOI] [PubMed] [Google Scholar]
- Basu S, Sodhi A 1992 Increased release of interleukin-1 and tumour necrosis factor by interleukin-2-induced lymphokine-activated killer cells in the presence of cisplatin and FK-565. Immunol Cell Biol 70(Pt 1):15–24 [DOI] [PubMed] [Google Scholar]
- Ozturk G, Erdogan E, Anlar O, Kosem M, Taspinar M 2005 Effect of leukemia inhibitory factor in experimental cisplatin neuropathy in mice. Cytokine 29:31–41 [DOI] [PubMed] [Google Scholar]
- Ramesh G, Reeves WB 2004 Salicylate reduces cisplatin nephrotoxicity by inhibition of tumor necrosis factor-alpha. Kidney Int 65:490–499 [DOI] [PubMed] [Google Scholar]
- Li WG, Gavrila D, Liu X, Wang L, Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C, Weintraub NL 2004 Ghrelin inhibits proinflammatory responses and nuclear factor-κB activation in human endothelial cells. Circulation 109:2221–2226 [DOI] [PubMed] [Google Scholar]
- Hesketh PJ, Van Belle S, Aapro M, Tattersall FD, Naylor RJ, Hargreaves R, Carides AD, Evans JK, Horgan KJ 2003 Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. Eur J Cancer 39:1074–1080 [DOI] [PubMed] [Google Scholar]
- Liu YL, Malik NM, Sanger GJ, Andrews PL 2006 Ghrelin alleviates cancer chemotherapy-associated dyspepsia in rodents. Cancer Chemother Pharmacol 58:326–333 [DOI] [PubMed] [Google Scholar]
- Rudd JA, Ngan MP, Wai MK, King AG, Witherington J, Andrews PL, Sanger GJ 2006 Anti-emetic activity of ghrelin in ferrets exposed to the cytotoxic anti-cancer agent cisplatin. Neurosci Lett 392:79–83 [DOI] [PubMed] [Google Scholar]
- Murphy MG, Plunkett LM, Gertz BJ, He W, Wittreich J, Polvino WM, Clemmons DR 1998 MK-0677, an orally active growth hormone secretagogue reverses diet-induced catabolism. J Clin Endocrinol Metab 83:320–325 [DOI] [PubMed] [Google Scholar]
- Murakami N, Hayashida T, Kuroiwa T, Nakahara K, Ida T, Mondal MS, Nakazato M, Kojima M, Kangawa K 2002 Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol 174:283–288 [DOI] [PubMed] [Google Scholar]
- ThidarMyint H, Yoshida H, Ito T, Kuwayama H 2006 Dose-dependent response of plasma ghrelin and growth hormone concentrations to bovine ghrelin in Holstein heifers. J Endocrinol 189:655–664 [DOI] [PubMed] [Google Scholar]