Abstract
Leptin, a 16-kDa cytokine, has been implicated in several reproductive processes and disorders. Notably, elevated leptin levels in the peritoneal fluid of women with mild endometriosis has been demonstrated, suggesting a role for this cytokine in the early stages of disease establishment. To gain insight into the functional significance of leptin during the initial requisite proliferative and neovascularization events involved in endometriosis, we investigated the effect of disruption of in vivo leptin signaling on the establishment and/or maintenance of an endometriosis-like lesion in a syngeneic immunocompetent mouse model of endometriosis. Findings of this study show that the disruption of leptin signaling by ip injection of the pegylated leptin peptide receptor antagonist (LPrA) impairs the establishment of endometriosis-like lesions (derived from uteri of C57BL/6 female siblings) and results in a reduction of viable organized glandular epithelium, vascular endothelial growth factor-A expression, and mitotic activity. LPrA treatment resulted in a significant reduction of microvascular density in endometriosis-like lesions after continuous and acute courses. Endometriosis-like lesions (derived from tissue with functional leptin receptor) of Leprdb hosts (nonfunctional leptin receptor) were phenotypically similar to those of LPrA-treated mice. Our results confirm that leptin signaling is a necessary component in lesion proliferation, early vascular recruitment, and maintenance of neoangiogenesis in a murine model of endometriosis.
ENDOMETRIOSIS, a gynecological disorder primarily of reproductive-aged women, is characterized by the presence of endometrial tissue (glandular and stromal epithelium) in sites outside of the uterus (1). It is an estrogen-dependent disease associated with pelvic pain and infertility, and is estimated to affect 6–10% of the general female population and 15–50% of women with infertility and/or pelvic pain (2,3,4,5). Although the first written description of endometriosis by Von Rokitansky (6) appeared in 1860, the exact mechanism of disease establishment, persistence, and recurrence is not completely understood (7). The most commonly accepted etiology is retrograde menstruation of endometrial tissue through the fallopian tubes into the peritoneal cavity (8). Disease establishment and progression are thought to occur through a series of events, including attachment of endometrial tissue to surfaces in the peritoneal cavity, establishment of a vascular supply (angiogenesis), and proliferation in response to estradiol produced by the ovaries and endogenous aromatase p450 activity within ectopic endometrium (9,10,11,12). Although a majority of women have retrograde menstruation of some form, only a minority of these women have endometriosis (13). Thus, it is theorized that the endometrium of women with the disorder is abnormal, and that this population, therefore, has a predilection for the formation of ectopic disease (3).
Many studies have sought to elucidate other potential factors involved in disease pathogenesis. Aberrations in immune function are believed to play a primary role in this process. Studies have demonstrated increased activation of peritoneal macrophages and elevated peritoneal fluid levels of inflammatory cytokines, including monocyte chemotactic protein-1, IL-6, TNF-α, IL-1, IL-8, and RANTES, otherwise known as regulated upon activation, normal t cell expressed, and secreted protein (14,15,16) in women with endometriosis. Increased activation and chemoattraction of immune cells are thought to impair macrophage phagocytosis and natural-killer cell activity, potentially leading to decreased immune surveillance against the attachment of ectopic endometrium in the peritoneum (3,4,16).
The process of new blood vessel formation, known as angiogenesis, is integral to the growth of new tissue, and has been studied in relation to various pathological and normal processes, including tumor formation and corpus luteum formation (17). Many immune factors can influence vascular dynamics, and this effect can be amplified by the ever-changing hormone influence concomitant with the menstrual cycle, and through interaction with other cytokines. Elevated IL-8 (3), CD105 (18), and vascular endothelial growth factor (VEGF)-A (19,20,21) in ectopic endometrium and/or isolated eutopic endometrial cells indirectly implicates these angiogenic-related molecules in the recruitment and establishment of a vascular supply for ectopic endometrial lesions (22). The ability of several angiostatic agents such as antihuman VEGF antibody to inhibit increased vascular density and impair formation of endometriosis-like lesions in the chicken chorioallantoic membrane model supports the significance of angiogenesis during ectopic lesion formation (23).
Despite a plethora of reports detailing elevated concentrations of the aforementioned immune and angiogenic factors in animal models and in women with endometriosis, our current understanding of the specific in vivo function of immunological and neo-angiogenic molecules remains speculative. Another cytokine, leptin, possesses both immune and angiogenic properties (24,25,26). This 16-kDa adipocyte-derived product of the obese gene was originally described in metabolic regulation, and has now been recognized as a crucial factor in reproductive processes such as implantation, embryonic development, and placentation (27,28,29,30). Leptin has also been described as a significant regulator of IL-1 in human endometrial stromal cells (31,32), a stimulus for proinflammatory responses of CD 4+ T lymphocytes (33), and as an important in vitro and in vivo angiogenic factor (34,35). Interestingly, leptin concentrations are elevated in peritoneal fluid of women with endometriosis (26,36,37,38). The increase in peritoneal leptin levels is inversely proportional to disease extent, suggesting a potential role for leptin in the early stages of disease (34,36).
More recently, a leptin peptide receptor antagonist (LPrA) reduced expression of various endometrial cell-derived cytokines (28,39,40) and slowed cell proliferation in vitro. Furthermore, LPrA significantly reduced circulating VEGF levels and slowed the growth rate of mouse mammary tumors (41). Because the local obligatory immune and angiogenic events necessary for the establishment of endometriosis are unknown, leptin, with its capacity for involvement in two potentially critical facets of this disorder, is a desirable target to investigate specific in vivo cytokine function. Therefore, we hypothesized that disruption of leptin signaling, either by the administration of LPrA or by using mice deficient in the functional leptin receptor (Leprdb), would impair the establishment and development of ectopic endometrial tissue in a mouse endometriosis-like model. Our data provide evidence to support the role of leptin as a significant factor involved in the establishment of endometriosis-like lesions. More importantly, blockade of leptin receptor function and the disruption of leptin-associated downstream signaling pathways impair leptin-dependent angiogenic and immunological events necessary for the successful establishment of endometriosis-like lesions.
Materials and Methods
Reagents
Anti-VEGF goat polyclonal antibody (sc-152) against N terminus of VEGF-A (human origin), goat antirabbit and rabbit antigoat biotinylated secondary antibodies, and nonspecific goat and rabbit IgG were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA), anti-phospho-histone-3 rabbit polyclonal antibody against Thr11 residue (07-492; Upstate Cell Signaling Solutions, Charlottesville, VA), rat CD 31 ab (no. 557355; BD PharMingen, San Diego, CA), fluorescent secondary chicken antirat IgG2a ab (Alexa Fluor no. A21471; Molecular Probes-Invitrogen, Carlsbad, CA), fluorophore-labeled rabbit anti-green fluorescent protein (GFP) IgG antibody (A21311; Molecular Probes-Invitrogen), recombinant human VEGF (293-VE; R&D Systems, Minneapolis, MN), sFlt-1 Fc; and GFP adenovirus (Harvard Core Laboratory, Boston, MA).
OB-R inhibitors
Pegylated LPrA corresponding to helix III of leptin and a scrambled pegylated peptide, LPrASc, for negative control, were synthesized and purified as described (40). The peptides were dissolved in a sterile filtered vehicle solution composed of 0.05% dimethylsulfoxide (DMSO)-PBS. Normal goat IgGs (negative control) diluted in PBS were sterile filtered and also used.
Animal models.
Six- to 8-wk-old female C57BL6 wild-type (WT) (Charles River Laboratories, Wilmington, MA), EGFP (GFP or C57BL/6-Tg(ACTbEGFP)1Osb/J) (42), leptin receptor mutant (Leprdb or a/a + Leprdb/+ Leprdb), and leptin receptor mutant control (Leprdbm or m +/+ Leprdb) mice (43) (The Jackson Laboratory, Bar Harbor, ME) were housed in the animal facility at the Massachusetts General Hospital in accordance with the National Institutes of Health standards for the care and use of experimental animals. Rooms provided a controlled temperature range of (22–24 C) on a 14-h light, 10-h dark cycle. Mice were given food and water ad libitum. All animal procedures described here were approved by the Subcommittee on Research Animal Care at Massachusetts General Hospital.
Before uterine tissue transfer, donor mice were primed with pregnant mare serum gonadotropin (Sigma-Aldrich, St. Louis, MO) 10 IU by ip injection, and their uteri were removed and processed 41 hr later (WT sister syngeneic uterine tissue transfer). In Leprdb (db/db −/−) and Leprdbm (db control) uterine tissue transfer experiments, each animal donor was primed with a sc 17β-estradiol (E2) implant (44) for 5 d before uterine tissue transfer. This method of E2 priming was used in this circumstance to standardize uterine response to estradiol because Leprdb response to pregnant mare serum gonadotrophin may not be equivalent to that of Leprdbm mice. All animals were anesthetized with 2,2,2-tribromoethanol (99%) (Avertin 200 mg/kg body weight) (Sigma-Aldrich) ip before uterine tissue transfer (host animal) or euthanasia (donor animal).
The different animal model strategies used uterine tissue donors and hosts from: 1) wild type from C57BL6 WT female siblings of the same litter; 2) uterine tissue of GFP transgenic mice that were transferred into WT C57BL6 female siblings of the same litter for analysis of viability of endometriosis-like lesions via GFP lamp (Lightools Research, Encinitas, CA); 3) Leprdb uterine tissue transferred into Leprdbm host; and 4) Leprdbm tissue transferred into Leprdb host. All Leprdb and Leprdbm were from the same colony.
Preparation of hosts for receipt of uterine tissue transfer.
After Avertin (200 mg/kg body weight) ip anesthesia, female mice 6–8 wk of age underwent bilateral oophorectomy (sterile technique) through a 0.5-cm left dorsolateral skin incision and simultaneous placement of sc estradiol implant (44) 14 d before undergoing uterine tissue transfer. Peritoneal incision closure was performed with a 5–0 undyed Vicryl (Johnson and Johnson Gateway LLC, Irvine, CA) running suture, and the skin was reapproximated with surgical Autoclip staples (9 mm; Becton Dickinson, Franklin Lakes, NJ). Mice were placed in warmed cages until complete recovery from anesthesia.
Harvest of uterine tissue from donor and transfer to host.
After anesthesia with Avertin and euthanasia by cervical dislocation, the uterus was removed en bloc through a midline ventral laparotomy. Under a dissecting microscope, all extrauterine tissue was removed, and a longitudinal linear incision was made with dissecting microscissors (Roboz, Gaithersburg, MD) from one horn to the contralateral horn. The endometrium (with a moderate degree of underlying myometrium) was removed, washed once in 1 ml PBS, and minced.
The recipient mouse (host) was anesthetized with Avertin 200 mg/kg body weight ip. A right dorsolateral incision was made, and the peritoneal cavity was entered sharply with dissecting scissors (Roboz). The uterine tissue (from two mice uteri) suspension (in 500 μl PBS) was placed into the peritoneal cavity of the recipient host. An equivalent amount of minced tissue was transferred in all hosts. Peritoneal and skin closure, as well as recovery from anesthesia, was performed as previously described.
Animal treatments.
For syngeneic sister and GFP/WT models, animals were treated daily with 500 μl ip injection (27-gauge needle, Becton Dickinson) of either goat IgG (in PBS), vehicle (DMSO 0.0005%), LPrASc or LPrA (66 μm) beginning 1 d before uterine tissue transfer (n = 3 mice per treatment group). Concentrations of LPrA used in vivo were calculated from previous experiments (28). Animals were treated for either 7 or 14 d after uterine tissue transfer and were euthanized by cervical dislocation. To assess the effects of LPrA on ectopic uterine tissue, ectopic lesions (as well as native uteri) were photographed (Coolpix 5400; Nikon, Melville, NY) to capture in situ images of endometriosis-like lesions and relative qualitative fluorescence (GFP/WT syngeneic sister model). Portions of ectopic lesions and native uteri were fixed in 4% paraformaldehyde [phosphohistone 3 (PH3), VEGF-A, and GFP], and cryostat (for CD 31) for immunohistochemical analysis. Histological analysis (hematoxylin and eosin) of epithelial glandular and stromal components was also performed.
To assess the effects of peritoneal leptin signaling disruption on VEGF expression, VEGF add-back experiments were performed in both WT hosts receiving daily ip LPrA (as described previously) and in Leprdb hosts, by administering VEGF 10 μg ip for 7 d (beginning 1 d before tissue transfer). Ectopic and native uterus samples were collected and processed as previously described.
To assess the effect of VEGF on ectopic uterine tissue establishment, 1 × 108 plaque-forming unit adeno-sflt1 virus was injected by tail vein 4-h before uterine transfer in C57BL6 WT hosts (n = 3 mice). This virus and its control virus (adeno-GFP) have been described previously (obtained from the Harvard Vector Core Laboratory, Boston, MA) (45).
For the experiments conducted to analyze endometriosis-like CD 31 expression after leptin signaling disruption, the treatment groups (n = 3 mice per group) of recipient mice included daily ip LPrA (66 μm) and nonfunctional pegylated LPrASc (66 μm), respectively. Two time courses for each treatment group included either daily ip administration starting 1 d before uterine tissue transfer and continuing for 7 d (continuous) or beginning 5 d after uterine tissue transfer and continuing for 48 h (acute). For each experiment the weight of all animals was recorded before and after completion of uterine tissue transfer and/or treatment.
VEGF, PH3, and GFP immunohistochemical analysis
VEGF-A, PH3, and GFP were assessed by immunohistochemistry. Paraffin sections (7 μm) of ectopic lesions and native uteri were quenched of endogenous peroxides with H2O2 (3% in methanol) for 10 min, and samples were boiled in 10 mm sodium acetate + 10 mm sodium citrate (pH 6.0) for 10 min by microwave antigen retrieval to unmask antigen epitopes. Tissues were blocked [1% goat serum (PH3 and GFP), 1% rabbit serum (VEGF-A)], incubated in primary antibody (VEGF-A 1:200, PH3 5 μg/ml, GFP 1:200), diluted in 0.1 m Tris (pH 7.6), 0.55 m NaCl, 10 mm KCl, 0.1% Triton X-100, 0.02% BSA, and 1% specified species serum overnight at 4 C. For PH3 and VEGF-A, biotinylated secondary antibodies were used, tissue preparations were incubated with a streptavidin-biotin-peroxidase system according to the manufacturer’s directions (Vectastain, ABC-AP kit; Vector Laboratories, Burlingame, CA), developed in 3,3′diaminobenzidine (Sigma-Aldrich), counterstained with hematoxylin (Fischer Scientific, Pittsburgh, PA), and mounted with VectaMount (Vector Laboratories). Negative controls were also included in which primary antibodies were substituted by relevant species-matched IgGs.
For the quantitative and semiquantitative analysis of immunohistochemical markers, 10 high-powered fields (HPFs) per respective sample replicate were blindly analyzed microscopically without a priori knowledge of the treatment group identity. For PH3 quantification, the proportion (%) of the total epithelial glands staining for PH3 of all epithelial glands observed was calculated (one mouse per replicate) and compared in native endometrium and endometriosis-like lesions for all treatment groups. For VEGF-A, stromal and epithelial intensity was quantified and graded by two blinded observers as follows: marked intensity (2++) moderate intensity (2+), low intensity (+), trace intensity (tr), and no staining (0).
CD 31 analysis.
After euthanasia, endometriosis-like lesions were removed and fixed in optimal cutting temperature compound with liquid N2. Serial frozen sections (7 μm) were made on slides and stored at −80 C for 24 h. Before staining, slides were allowed to thaw at room temperature for 30 min. Sections were place in 100% acetone at room temperature for 15 min. Slides were immersed in 1% BSA in PBS for 8 min. Primary antirat CD 31 antibody (no. 557355; BD PharMingen) at 1:50 dilution (in 1% PBS/PBS) was applied to each section and incubated overnight at 4 C. Slides were rinsed with 0.1% BSA/PBS, and secondary fluorescent chicken antirat IgG2a (Alexa Fluor no. A21471) was applied at 1:500 dilution in 0.1% BSA/PBS/10% rabbit serum, and incubated at 37 C for 1 h. Slides were counterstained with Hoechst/TOPRO-3 (1 μg/ml) for 10 sec, washed with tap water for 3 min, mounted in aqueous mounting medium, and allowed to incubate at room temperature in the dark for 24 h. Three random HPFs (20×) per sample were selected; the mean microvascular density (MVD) (percentage of HPFs) was quantified (IP Labs Scanalytics Inc., Fairfax, VA) per treatment group from CD 31 secondary Ab fluorescent stain. Mean percentage ± sem for each group (n = 3 mice per group) was calculated.
Statistical analyses
Data were expressed mean ± sem of respective groups (experiments performed in triplicate). Data were analyzed using t test, χ2, and Fisher’s exact tests, or two-way ANOVA with post hoc Tukey test. P < 0.01 was designated as a statistically significant difference for ANOVA and P < 0.05 for comparison not using ANOVA. The experiments were repeated at least three times, and all samples were analyzed in duplicate.
Results
Multiple mouse models were used to test the functional significance of leptin in the establishment and/or maintenance of endometriosis-like lesions. WT mice that hosted ectopic endometrial tissue derived from their syngeneic sisters were killed after daily treatment (7 or 14 d) with a 500 μl ip injection of either goat IgG (in PBS), vehicle (DMSO 0.0005%), LPrASc, or LPrA (66 μm) beginning 1 d before uterine tissue transfer. Animals receiving LPrA displayed a unique ectopic lesion phenotype characterized by minimal visible gross vascularization and significant pallor, with a serpiginous chalky appearance. The endometriosis-like lesions of LPrA-treated animals displayed a predominance of fibrosis and sclerosis, with a paucity of endometrial glandular epithelium and stroma (Fig. 1). Conversely, lesions of the control and LPrASc-treated hosts were well vascularized grossly and circumscribed, had significant peritoneal attachment, and demonstrated endometrial glandular and stromal architecture typical of murine ectopic endometriosis-like lesions previously described (42). To complement the microscopic and macroscopic observations of the syngeneic WT experiments, the GFP/WT model was used to assess the relative degree of viable tissue (intensity of GFP) within ectopic lesions among treatment groups. Although lesions were similar in size among all respective groups, control and LPrASc-treated animals displayed greater intensity and distribution of fluorescence in lesions compared with the LPrA treatment group (the results can be found in supplemental Fig. 1, which is published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Histological analysis of lesions revealed findings similar to those observed in the syngeneic WT experiments.
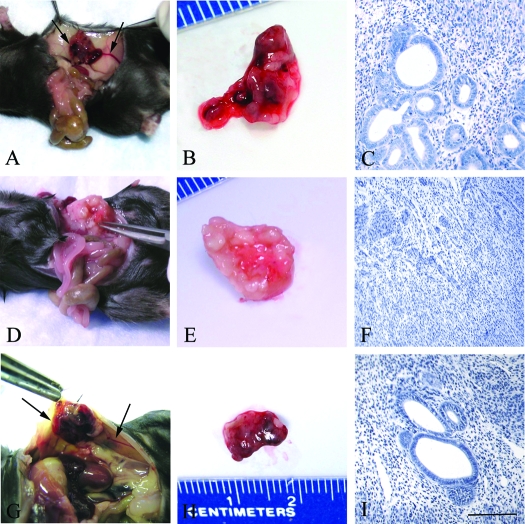
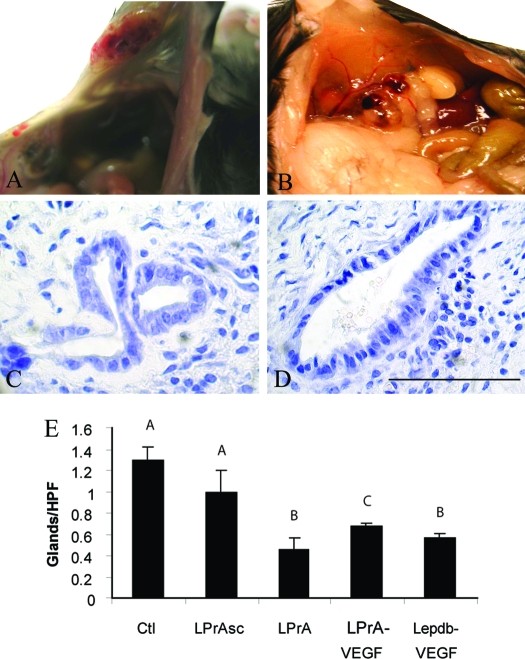
Figure 1.
Representative microscopic and macroscopic images of endometriosis-like lesions in C57BL6 WT female hosts after syngeneic uterine tissue transfer. Seven days after uterine tissue transfer, the control treatment group (daily ip PBS) (A and B) and LPrASc (G and H) (ip scrambled leptin peptide antagonist) displayed significant gross vascularity and hyperemia within the lesion and marked vascular recruitment along the periphery of the peritoneal attachment site (arrows). In contrast, lesions of animals treated daily with LPrA (ip leptin peptide antagonist) (D and E) displayed a phenotype characterized by marked pallor and minimal gross vascularity, with a mucoid appearance compared with controls. Microscopic examination (hematoxylin and eosin, ×40) showed that endometriosis-like lesions of LPrA-treated animals (F) displayed a predominance of fibrosis and reactive change, with a paucity of endometrial glandular epithelium and marked stromal obliteration compared with control (C) and LPrASc (I) animals. Scale bars, 100 μm.
To support further the role of leptin in the establishment and maintenance of endometriosis-like lesions, we used Leprdb and Leprdbm mice. Leprdb mice do not have a functional leptin receptor and have an infertile uterine phenotype. Comparison of relative uterine weights of estrous cycle matched genotypes before E2 exposure revealed a significantly smaller uterine weight in the Leprdb mice compared with Leprdbm (P < 0.05). In addition, Leprdb uteri were smaller in size relative to the WT controls and demonstrated a predominance of stromal epithelium microscopically (the results can be found in supplemental Fig. 2A). To overcome this, the mice were primed with a sc estradiol (E2) SILASTIC brand implant (Dow Corning, Corp., Midland, MI) for 5 d before undertaking endometrial transfer in the Leprdb/Leprdbm host model (supplemental Fig. 2B). After E2 exposure there was no statistical difference in uterine size or weight between genotypes. The proportion of glandular to stromal tissue in the Leprdb uteri was similar to Leprdbm after E2 exposure (supplemental Fig. 2A). Thus, estrogen supplementation allowed us to use these mice in a syngeneic transfer model as described previously. After uterine tissue transfer, Leprdb (recipient of Leprdbm uterine tissue) and Leprdbm (recipient of Leprdb uterine tissue) hosts were euthanized after 7 or 14 d. Seven days after uterine tissue transfer, Leprdbm hosts exhibited well-vascularized hyperemic lesions similar to control and LPrASc treatment WT hosts (Fig. 2). In contrast, Leprdb hosts displayed disorganized, fluctuant lesions with minimal visible evidence of neovascularization. At the longer time point (14 d), endometriosis-like lesions of Leprdb demonstrated marked purulence and chalky necrosis (Fig. 2). On microscopic examination, ectopic lesions derived from Leprdbm donors in Leprdb hosts demonstrated an amorphous free-floating phenotype with significant degrees of necrosis, glandular, and stromal breakdown, and no peritoneal attachment. Although lesions of Leprdbm were similar to WT control treatment hosts in overall histological appearance and glandular/stromal architecture, they did demonstrate a mild increase in glandular epithelial apoptotic bodies.
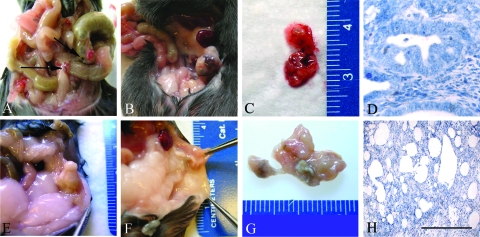
Figure 2.
Endometriosis-like lesions in Leprdbm (A–D) and Leprdb hosts (E–H). After uterine tissue transfer, each host type received uterine tissue from the female siblings of the opposite genotype of the same litter. Seven days after transfer, Leprdbm animals (received Leprdb tissue) displayed multiple vascular lesions (A), compared with the purulent nonvascular sites in Leprdb hosts (received Leprdbm tissue) (E). In long-term studies (14 d after transfer), lesions of the Leprdbm hosts appeared significantly more vascularized grossly and invasive at multiple sites (B and C) in contrast to Leprdb hosts, which did not display significant vascularization or mottling, and demonstrated marked purulence and necrosis (F and G). Microscopic comparison (hematoxylin and eosin) revealed glandular architecture and organization in Leprdbm hosts (D) similar to that seen in the WT syngeneic transfer model. Leprdb showed diffuse necrosis and glandular and stromal disruption (H). Microscopic images (hematoxylin and eosin), ×60. Scale bars, 100 μm.
The effect of LPrA treatment on the mitotic marker, PH3, and angiogenesis protein, VEGF-A, was assessed in endometriosis-like lesions by immunohistochemical analysis 7 d after transfer by analyzing 10 HPFs per respective sample replicate. In the WT syngeneic endometrial transfer model, expression of PH3 and VEGF was similar in native uteri of control, LPrASc-, and LPrA- treated hosts, respectively (Fig. 3A). Control and LPrASc treated groups clearly displayed PH3 expression within glandular epithelium, and displayed VEGF stromal and glandular staining. The proportion of PH3 staining glands of ectopic endometriosis-like lesion glands of control, LprASc, and LPrA treatment groups was 69.2 (27 of 39), 63.3 (19 of 30), and 21.4% (three of 14), respectively (Fig. 3B). LPrA-treated animals demonstrated a 3.5-fold reduction in the intensity of PH3 staining in endometriosis-like lesions compared with control-treated animals (21.4 vs. 69.2%, respectively; P < 0.01). VEGF-A expression in ectopic lesions of LPrA-treated hosts was minimal to trace and reduced 2-fold when compared with control and LPrASc groups, which displayed 2+ expression intensity (Fig. 4).
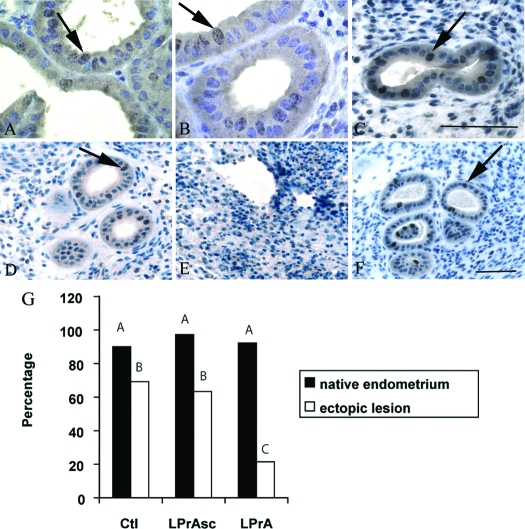
Figure 3.
Comparison of immunohistochemical PH3 expression in endometriosis-like lesions after 7-d LPrA treatment. Relative PH3 expression is demonstrated as seen in mitotic figures of native endometrium (60×) and endometriosis-like lesions (40×) within glandular epithelium (arrows). Similar PH3 expression was observed in the native endometrium of control (Ctl) (A), LPrA (B), and LPrASc- (C) treated animals. Minimal PH3 expression was observed in ectopic lesions after LPrA treatment (E) compared with lesions of control (D) and LPrASc (F) treatment animals. PH3 expression after LPrA treatment with quantification of the total percentage of epithelial glands with PH3 staining. G, Ten random HPFs per replicate were selected. The proportion of total glands staining for PH3 was calculated (one mouse per replicate). Relative proportion of PH3 expression in native endometrium was similar in all groups. PH3 expression was markedly reduced in LPrA-treated mice compared with control and LPrASc mice. Different letters represent differences at the level of P < 0.01 (χ2). Scale bars, 100 μm.
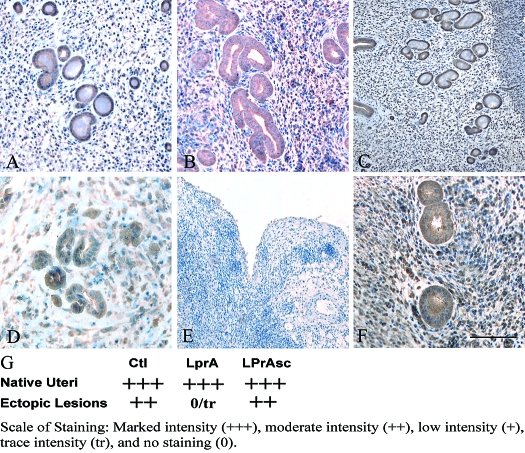
Figure 4.
Intensity of VEGF-A expression after LPrA treatment 7 d after uterine tissue transfer. Native endometrium VEGF-A expression was similar between control (Ctl) (A), LPrA- (B), and LPrASc- (C) treated groups. Marked reduction on ectopic lesions was demonstrated in the LPrA- (E) treated group compared with control (D) and LPrASc-treated animals (F). Quantification and comparison of VEGF-A expression intensity. G, Ten random HPFs per replicate were selected, and stromal and epithelial intensity was quantified with grading as follows: marked intensity (2++) moderate intensity (2+), low intensity (+), trace intensity (tr), and no staining (0). Endometriosis-like lesion VEGF-A expression intensity of LPrA-treated animals (trace to no staining) was reduced 2-fold when compared with control and LPrASc-treated animals (2+). Scale bars, 100 μm.
Because our previous work demonstrated that disruption of leptin signaling significantly affected VEGF levels, we sought to assess whether a VEGF receptor antagonist would have a similar effect on PH3 and VEGF expression. To accomplish this we administered adeno sFlt-1 (iv 1 × 108 plaque-forming unit) 4 h before uterine transfer. The tissue was harvested 7 d after uterine tissue transfer. On gross comparison it was evident that the endometriosis-like lesions of adeno sFlt-1-treated animals displayed marked pallor and minimal vascularity compared with controls (adeno-GFP) (the results can be found in supplemental Fig. 3). The ectopic lesions of adeno-GFP-treated animals demonstrated strikingly dense vascularity and consistent peritoneal attachment similar to control animals of the WT syngeneic model discussed previously. Similar to the ectopic lesion phenotype of LPrA-treated animals, histological examination of lesions derived from animals pretreated with adeno sFlt-1 showed minimal VEGF and PH3 staining within a background of fibrosis, necrosis, and obliterated glandular and stromal epithelial architecture (supplemental Fig. 3).
To investigate whether the blockade of leptin signaling by LPrA directly inhibits the ability of endogenous VEGF to promote the initial neovascularization of an ectopic lesion, we undertook several experiments in which recombinant VEGF was administered to hosts. These experiments (n = 3) included add-back by daily ip administration of VEGF (10 μg) to either WT hosts of the syngeneic transfer model, which were simultaneously receiving LPrA (as previously described), or Leprdb hosts for 7 d. After euthanasia, endometriosis-like lesions of the both animal host types displayed gross vascularity and peritoneal attachment comparable to control hosts of the WT syngeneic transfer model (Fig. 5A). Ten HPFs per endometriosis-like lesion per replicate were randomly selected and examined. LPrA-treated hosts receiving VEGF add-back had a smaller number of endometrial glands (0.67 ± 0.03 glands per HPF) compared with control (1.3 ± 0.12 glands per HPF) and LPrASc (1.0 ± 0.2 glands per HPF) groups. Significant evidence of necrosis, and glandular and stromal breakdown persisted in ectopic lesions of Leprdb hosts receiving VEGF. A very small proportion (0.56 ± 0.05 glands per HPF) of organized glandular epithelium was observed in these lesions compared with control, LPrASc, and wild type receiving add-back but was similar to LPrA-treated animals (0.46 ± 0.11 glands per HPF) (Fig. 5B)
Figure 5.
Demonstration of the effect of VEGF add-back on endometriosis-like lesion of Leprdb and LPrA-treated WT hosts. After uterine tissue transfer, recombinant VEGF (10 μg ip daily) add-back therapy was performed daily for 7 d. LPrA-treated WT (A and C) and Leprdb (B and D) hosts displayed appropriate grossly vascularized and well-circumscribed lesions with peritoneal attachment similar to control WT hosts. A smaller proportion of organized glandular epithelium was observed in these lesions during histological examination (hematoxylin and eosin, ×60), resulting in a partial recovery of the phenotype of endometriosis-like lesions in the Leprdb host. Comparison of the number of endometrial glands per HPF in control (Ctl), LPrASc, LPrA, LPrA treated with VEGF add-back (LPrA-VEGF), and leptin receptor mutation transgenic mice hosts receiving control mouse uterine tissue and VEGF-add-back (Lepdb-VEGF). E, Control and LPrASc groups were similar (1.3 ± 0.12 vs. 1 ± 0.2 glands per HPF). LPrA and Lepdb-VEGF were also similar (0.46 ± 0.11 vs. 0.56 ± 0.05 glands per HPF), but less than the three other remaining groups. LPrA VEGF (0.67 ± 0.03 glands per HPF) demonstrated less than control and LPrASc, but greater than LPrA and Lepdb-VEGF. Different letters represent differences at the level of P < 0.01. Scale bars, 100 μm.
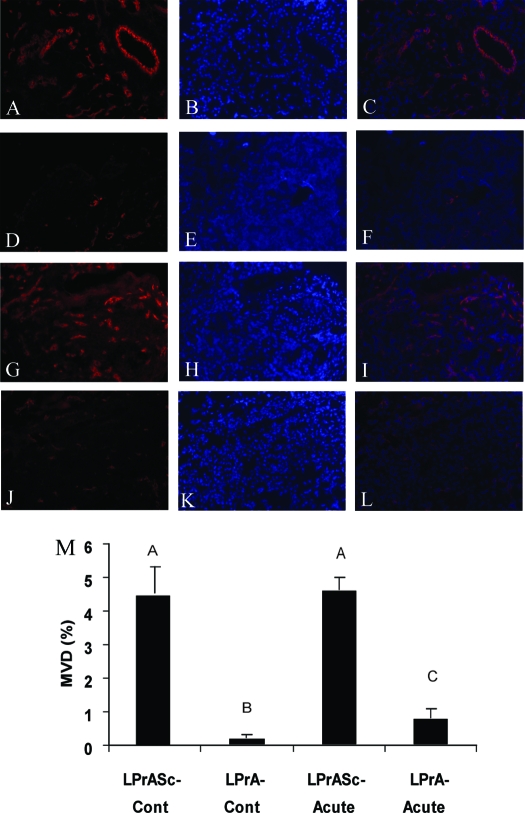
The effect of leptin signaling disruption on early vascular recruitment in endometriosis-like lesions was assessed by quantifying the MVD after CD 31 staining (Fig. 6A). When comparing animals of a continuous treatment group (LPrA or LPrASc beginning 1 d before uterine tissue transfer and continued daily until euthanasia 1 wk later), MVD (%) of the LPrA group (0.225 ± 0.09) was reduced significantly compared with LPrASc (4.55 ± 1.9) (P < 0.01) (Fig. 6B). In the acute treatment group (LPrA or LPrASc beginning 5 d after uterine tissue transfer and continuing daily for 48 h after until euthanasia and tissue harvest), MVD (%) was also decreased in the LPrA group (0.80 ± 0.29) compared with LPrASc (3.63 ± 1.2; P < 0.05). A greater proportion of endometrial glands were observed in LPrASc vs. LPrA in the continuous treatment arm (data not shown).
Figure 6.
Immunohistochemical analysis of CD 31 in murine endometriosis-like lesions after pegylated leptin peptide antagonist (LPrA) treatment. Inverted epifluorescent filtered microscopy images for the CD 31 secondary antibody (wavelength 594 nm) were used. Images include CD 31, Hoechst, and merged (CD 31 and Hoechst layered) for LPrASc (A–C) and LPrA (D–F) for continuous (Cont) treatment courses and acute time courses for LPrASc (G–I) and LPrA (J–L). Images demonstrated markedly reduced CD 31 fluorescent intensity in LPrA-treated endometriosis-like lesions compared with LPrASc in both the continuous and acute treatment time courses. Quantification of MVD in endometriosis-like lesions. M, Three random HPFs (20×) per sample were selected, and the mean MVD (percentage of HPFs) from CD 31 secondary Ab stain was assessed. Mean percentage ± sem for each group (n = 3 mice per group) was calculated. Respective values were compared using ANOVA and post hoc Tukey test. Different letters represent differences at the level of P < 0.01. Scale bars, 100 μm.
As a supplemental note, there were no differences in the mean change in animal weights in the control, LPrA, or LPrASc- treated groups before and after 7-d treatment (data not shown).
Discussion
Over the past century, the primary medical therapy for endometriosis has been either suppression of the hypothalamic pituitary axis and induction of systemic hypoestrogenism with GnRH agonists or the induction of a pseudopregnant state with oral contraceptive pills and progestins (46). The success of these therapies is based upon the assumption that endometriosis is exclusively influenced by ovarian sex steroids and attenuated by a marked reduction of systemic estrogen. Interestingly, a significant proportion of patients treated in this manner have persistent symptoms of endometriosis, implicating mechanisms independent of ovarian sex steroids in the pathogenesis and propagation of the disease. Due in part to the inconsistent efficacy of contemporary medical therapy, the well-recognized increase in cytokines, and the obvious requirement for adequate vascular recruitment to allow establishment of the ectopic lesions, there has been an increased effort to begin to identify cytokines that have immune, angiogenic, and/or cell adhesion properties that may be crucial to this disease process. Unfortunately, this effort is not mirrored by an increased effort to determine which cytokines are causal and which are consequential to the disease in in vivo models.
As mentioned previously, leptin is an interesting target because it possesses mitogenic and angiogenic properties (47,48). In several studies, elevated peritoneal fluid concentrations of leptin have been reported in women with endometriosis (49,50,51). Based on these findings, and the putative inflammatory and angiogenic roles of leptin, we hypothesized that this cytokine may play a significant role in the early events of angiogenesis and proliferation during the initial stages of disease development, establishment, and propagation.
Historically, human and mouse endometria have been successfully transplanted into the immunocompromised mouse (52). Because the functional contributions of specific cytokine factors in the establishment of and/or growth of ectopic endometrium have not been fully realized, the animal models used for this study were immunocompetent mouse models with congenic female siblings of the same litter. Given the proposed mitogenic mechanism of leptin, we first sought to examine the role of this cytokine in the establishment of endometriosis-like lesions. Initial experiments used leptin signaling blockade by use of the LPrA to assess its role in lesion establishment and viability. Given the short half-life of LPrA, the pegylated LPrA was used to increase bioavailability (41). Compared with controls the ectopic lesions of LPrA-treated animals displayed a unique phenotype. From a macroscopic standpoint, they were free floating with an amorphous appearance, and did not display peritoneal attachment. In addition, lesions displayed uniform pallor with no evidence of gross vascularization. Microscopically, there was a marked paucity of endometrial glands and stroma with a predominance of fibrosis and reactive change, and an absence of the typical endometriosis-like lesion architecture. In contrast, the control and vehicle control (LPrASc) lesions displayed consistent peritoneal attachment, significant gross hyperemia, and vascularization with a typical distribution of endometrial glands, stroma, and hemorrhage. These results suggest that disruption of leptin signaling can inhibit the establishment/development of endometrial tissue in the peritoneal cavity. Similar results have been shown in murine breast cancer models after disruption of leptin signaling. More specifically, LPrA treatment before cancer cell inoculation resulted in the attenuation of the growth of mammary tumors in mice (41). The Leprdb transgenic mouse, which lacks a functional leptin receptor, provided additional evidence on the effect of disrupting of systemic leptin-induced signaling on lesion establishment. Leprdb hosts, which received Leprdbm (normal leptin receptor function) tissue, displayed a microscopic phenotype similar to the LPrA-treated congenic C57BL6 host. One marked difference was the large degree of chalky necrosis and purulence seen in the Leprdb. The Leprdbm host, which received estrogen primed Leprdb uterine tissue, displayed a histological and gross phenotype similar to control-treated congenic C57BL6 hosts. The mice themselves did not appear to be ill and had no evidence of infection. Not only did these findings implicate a role for leptin in early ectopic endometrium establishment, they also suggested that the leptin receptor function of the host is a significant factor in determining the establishment of the endometriosis-like lesion.
Although LPrA treatment demonstrated disruption of lesion establishment, it was still necessary to elucidate possible mechanisms by which blockade of leptin signaling impaired the endometriosis-like lesion. It is well known that leptin is a mitogenic (32) and cytokine stimulatory factor in many in vitro systems (28,30,32). In addition, current evidence clearly supports the role of leptin in angiogenesis (35), including the up-regulation of VEGF and its receptor (41). To examine the effect of leptin blockade on proliferation and mitosis, the well-established mitotic marker, PH3, was used (53). LPrA-treated animals displayed similar PH3 and VEGF staining in native uteri compared with control and LPrASc-treated animals. However, LPrA-treated animals demonstrated a 3.5-fold reduction in the intensity of PH3 staining in endometriosis-like lesions compared with control-treated animals. In addition, VEGF expression of ectopic lesions was reduced by 50% in LPrA-treated animals compared with controls. After 14-d leptin signaling blockade, it is apparent that endometriosis-like lesion establishment may be partially dependent upon leptin regulation of mitogenic and angiogenic factors as evidenced by PH3 and VEGF-A expression.
Although leptin blockade was associated with reduced lesion VEGF-A expression, this endpoint may not sufficiently characterize leptin’s role in the events necessary for early small vessel recruitment. As demonstrated in previous studies, neutrophils and macrophages have the ability to infiltrate endometriosis-like lesions, and are able to secrete VEGF-A and promote angiogenesis within the first week of lesion establishment (54). Because many of these studies examining immune-dependent angiogenic events have been performed in immunocompromised mouse models, these results are difficult to extrapolate to an immunocompetent host model. Therefore, we also investigated the effect of leptin signaling on early small vessel recruitment by analyzing microvasculature density via CD 31 expression. CD 31 (also known as PECAM) is a cell adhesion molecule expressed at a high level in endothelial cells and has been used by several groups as a marker of early vessel recruitment (55,56,57). To define further the role of leptin in early neovascularization during and after lesion establishment, LPrA was used in a continuous treatment regimen beginning 1 d before uterine tissue transfer, and continuing until euthanasia and lesion harvest. MVD (%) was markedly decreased more than 20-fold in endometriosis-like samples in mice treated with LPrA compared with those receiving LPrASc. These results suggest that the early events necessary for ectopic uterine tissue to establish a vascular supply from the surrounding host tissue may be dependent, in part, to leptin signaling. To investigate the significance of leptin at a later stage in lesion development and maintenance of vascular supply, an acute LPrA treatment course was initiated 5 d after uterine tissue transfer and continued for 48 h until harvest. MVD was reduced 4.5-fold in the endometriosis-like lesion of the LPrA-treated group compared with LPrASc-treated animals.
This study demonstrates the function of a specific cytokine in the pathogenesis of ectopic endometrium growth in a mouse model. Contrary to many previous studies with immunocompromised mouse models using human endometrium (7,42,58), the use of congenic siblings (host-donor pairs) with the transfer of uterine tissue between sisters, may provide a more realistic perspective of leptin’s role in human endometriosis because the hosts are immunocompetent, and ectopic uterine tissue has been derived from the same species. The process of lesion establishment likely involves a complex interaction of immune, angiogenic, and proliferative factors (3). Leptin, which has been shown to have both angiogenic and immunogenic properties, may be an important modulator of the early vascular and immune events necessary to sustain the lesion. To this end, the pathogenesis and propagation of the disease are not solely dependent upon leptin or ovarian steroidogenesis but most certainly involve several factors.
As evidenced by the similar phenotype of endometriosis-like lesions in LPrA-treated and sflt-1-treated mice, VEGF-A is also an important regulator of neovascularization and lesion establishment (54,57). Experiments that used the add-back of VEGF after LPrA treatment or with systemic disruption of leptin signaling demonstrated endometriosis-like lesions somewhat similar to control or LPrASc-treated mice from a histological standpoint. Thus, in the VEGF add-back experiments, there was a partial phenotypical rescue observed in the endometriosis-like lesions of Leprdb and LPrA-treated hosts. Because VEGF add-back lesions did not identically resemble control-treated lesion from a macroscopic (vascularity) and microscopic (reduced proportion of epithelial glands) perspective, it may be implied that leptin most likely interacts with VEGF, as well as other factors to foster successful lesion establishment and maintenance.
In conclusion, disruption of leptin profoundly affected the successful establishment of endometriosis-like lesions, supporting the significance of leptin signaling in the establishment of ectopic uterine tissue in a syngeneic nonimmunocompromised model for endometriosis. The findings of this study further confirm that leptin has a role in the proliferative and angiogenic aspects of the establishment and maintenance of endometriosis-like lesions. As such, this may also be reflected in the human model of endometriosis, in which leptin is one of the many elevated peritoneal cytokines contributing to the inflammatory responses associated with the clinical aspects of endometriosis (e.g. dysmenorrhea, dyspareunia) or more cellular aspects (e.g. increased vascularity, invasive qualities, or adhesive qualities). Other cytokines that are regulated by leptin, such as TNF-α, IL-8, IL-1, and VEGF, have also been implicated as significant players. Thus, leptin’s influence may serve many roles, including promoting survival, proliferation, and vascularization of endometriotic lesions directly or through one or more of these other cytokines/growth factors. Given its immune, angiogenic, and mitogenic properties, leptin may serve as a valuable target to study the mechanisms by which endometriosis is established and propagated.
Supplementary Material
Acknowledgments
We thank Dr. Lawrence R. Zukerberg of the Department of Pathology at Massachusetts General Hospital for his histological/microscopic assessment. We also thank the Boston Biomedical Research Institute Core Facility for the preparation and purification of the peptide antagonists and Mrs. Michelle Forrestall Lee for her assistance in preparing the figures for this work.
Footnotes
This work was supported in part by Vincent Memorial Hospital Research Funds (to B.R.R. and A.K.S.), Harvard Medical School Center for Excellence in Research and Massachusetts General Hospital Executive Committee on Research (to A.K.S.), Advanced Medical Research Foundation (to B.R.R.), Contraceptive Research and Development Program Grant CIG-06-113 and CIG-07-114 (to R.R.G), National Institutes of Health Grant DK069621 (to M.P.), and Harvard Medical School Center of Excellence in Minority Health Disparities (to J.C.P).
Disclosure Statement: The authors have nothing to disclose.
First Published Online October 25, 2007
Abbreviations: DMSO, Dimethylsulfoxide; E2, 17β-estradiol; GFP, green fluorescent protein; HPF, high-powered field; Lepdb, deficient in the functional leptin receptor; LPrA, leptin peptide receptor antagonist; LPrASc, scrambled leptin peptide receptor; MVD, microvascular density; PH3, phosphohistone 3; VEGF, vascular endothelial growth factor; WT, wild type.
References
- Farquhar C 2007 Endometriosis. BMJ 334:249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensky TE, Liu DT 1980 Endometriosis: associations with menorrhagia, infertility and oral contraceptives. Int J Gynaecol Obstet 17:573–576 [DOI] [PubMed] [Google Scholar]
- Giudice LC, Kao LC 2004 Endometriosis. Lancet 364:1789–1799 [DOI] [PubMed] [Google Scholar]
- Houston DE 1984 Evidence for the risk of pelvic endometriosis by age, race and socioeconomic status. Epidemiol Rev 6:167–191 [DOI] [PubMed] [Google Scholar]
- Hadfield R, Mardon H, Barlow D, Kennedy S 1996 Delay in the diagnosis of endometriosis: a survey of women from the USA and the UK. Hum Reprod 11:878–880 [DOI] [PubMed] [Google Scholar]
- Von Rokitansky C 1860 Ueber uterusdrusen-neubildung in uterus and ovarilsarcomen. Z Ges Aertzte Wein 37:577–593 [Google Scholar]
- Bruner-Tran KL, Webster-Clair D, Osteen KG 2002 Experimental endometriosis: the nude mouse as a xerographic host. Ann NY Acad Sci 955:328–339 [DOI] [PubMed] [Google Scholar]
- Sampson JA 1927 Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 14:442–469 [Google Scholar]
- Bulun SE, Fang Z, Imir G, Gurates B, Tamura M, Yilmaz B, Langoi D, Amin S, Yang S, Deb S 2004 Aromatase and endometriosis. Semin Reprod Med 22:45–50 [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Chai D, Langoi D, Bulun SE 2003 Steroid receptor and aromatase expression in baboon endometriotic lesions. Fertil Steril 80(Suppl 2):820–827 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, Sebastian S 2002 Estrogen production and metabolism in endometriosis. Ann NY Acad Sci 955:75–85 [DOI] [PubMed] [Google Scholar]
- Bulun SE, Gurates B, Fang Z, Tamura M, Sebastian S, Zhou J, Amin S, Yang S 2002 Mechanisms of excessive estrogen formation in endometriosis. J Reprod Immunol 55:21–33 [DOI] [PubMed] [Google Scholar]
- Seli E, Berkkanoglu M, Arici A 2003 Pathogenesis of endometriosis. Obstet Gynecol Clin North Am 30:41–61 [DOI] [PubMed] [Google Scholar]
- Hornung D, Bentzien F, Wallwiener D, Kiesel L, Taylor RN 2001 Chemokine bioactivity of RANTES in endometriotic and normal endometrial stromal cells and peritoneal fluid. Mol Hum Reprod 7:163–168 [DOI] [PubMed] [Google Scholar]
- Khorram O, Taylor RN, Ryan IP, Schall TJ, Landers DV 1993 Peritoneal fluid concentrations of the cytokine RANTES correlate with the severity of endometriosis. Am J Obstet Gynecol 169:1545–1549 [DOI] [PubMed] [Google Scholar]
- Oosterlynck DJ, Cornillie FJ, Waer M, Vandeputte M, Koninckx PR 1991 Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertil Steril 56:45–51 [DOI] [PubMed] [Google Scholar]
- Folkman J 2000 Tumor angiogenesis. Ontario, Canada: B.C. Decker; 132–152 [Google Scholar]
- Hayrabedyan S, Kyurkchiev S, Kehayov I 2005 Endoglin (cd105) and S100A13 as markers of active angiogenesis in endometriosis. Reprod Biol 5:51–67 [PubMed] [Google Scholar]
- Takehara M, Ueda M, Yamashita Y, Terai Y, Hung YC, Ueki M 2004 Vascular endothelial growth factor A and C gene expression in endometriosis. Hum Pathol 35:1369–1375 [DOI] [PubMed] [Google Scholar]
- Liu Y, Lv L 2004 Mechanism of elevated vascular endothelial growth factor levels in peritoneal fluids from patients with endometriosis. J Huazhong Univ Sci Technolog Med Sci 24:470–472 [DOI] [PubMed] [Google Scholar]
- Del Carmen MG, Smith Sehdev AE, Fader AN, Zahurak ML, Richardson M, Fruehauf JP, Montz FJ, Bristow RE 2003 Endometriosis-associated ovarian carcinoma: differential expression of vascular endothelial growth factor and estrogen/progesterone receptors. Cancer 98:1658–1663 [DOI] [PubMed] [Google Scholar]
- Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, Jaffe RB, Taylor RN 1996 Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab 81:3112–3118 [DOI] [PubMed] [Google Scholar]
- Nap AW, Dunselman GA, Griffioen AW, Mayo KH, Evers JL, Groothuis PG 2005 Angiostatic agents prevent the development of endometriosis-like lesions in the chicken chorioallantoic membrane. Fertil Steril 83:793–795 [DOI] [PubMed] [Google Scholar]
- Suganami E, Takagi H, Ohashi H, Suzuma K, Suzuma I, Oh H, Watanabe D, Ojima T, Suganami T, Fujio Y, Nakao K, Ogawa Y, Yoshimura N 2004 Leptin stimulates ischemia-induced retinal neovascularization: possible role of vascular endothelial growth factor expressed in retinal endothelial cells. Diabetes 53:2443–2448 [DOI] [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM 1992 Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258:1798–1801 [DOI] [PubMed] [Google Scholar]
- Ryan IP, Tseng JF, Schriock ED, Khorram O, Landers DV, Taylor RN 1995 Interleukin-8 concentrations are elevated in peritoneal fluid of women with endometriosis. Fertil Steril 63:929–932 [PubMed] [Google Scholar]
- Gonzalez RR, Caballero-Campo P, Jasper M, Mercader A, Devoto L, Pellicer A, Simon C 2000 Leptin and leptin receptor are expressed in the human endometrium and endometrial leptin secretion is regulated by the human blastocyst. J Clin Endocrinol Metab 85:4883–4888 [DOI] [PubMed] [Google Scholar]
- Ramos MP, Rueda BR, Leavis PC, Gonzalez RR 2005 Leptin serves as an upstream activator of an obligatory signaling cascade in the embryo-implantation process. Endocrinology 146:694–701 [DOI] [PubMed] [Google Scholar]
- Gonzalez RR, Simon C, Caballero-Campo P, Norman R, Chardonnens D, Devoto L, Bischof P 2000 Leptin and reproduction. Hum Reprod Update 6:290–300 [DOI] [PubMed] [Google Scholar]
- Gonzalez RR, Leavis P 2001 Leptin upregulates β3-integrin expression and interleukin-1β, upregulates leptin and leptin receptor expression in human endometrial epithelial cell cultures. Endocrine 16:21–28 [DOI] [PubMed] [Google Scholar]
- Gonzalez RR, Devoto L, Campana A, Bischof P 2001 Effects of leptin, interleukin-1α, interleukin-6, and transforming growth factor-β on markers of trophoblast invasive phenotype: integrins and metalloproteinases. Endocrine 15:157–164 [DOI] [PubMed] [Google Scholar]
- Gonzalez RR, Leary K, Petrozza JC, Leavis PC 2003 Leptin regulation of the interleukin-1 system in human endometrial cells. Mol Hum Reprod 9:151–158 [DOI] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI 1998 Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394:897–901 [DOI] [PubMed] [Google Scholar]
- Matarese G, Alviggi C, Sanna V, Howard JK, Lord GM, Carravetta C, Fontana S, Lechler RI, Bloom SR, De Placido G 2000 Increased leptin levels in serum and peritoneal fluid of patients with pelvic endometriosis. J Clin Endocrinol Metab 85:2483–2487 [DOI] [PubMed] [Google Scholar]
- Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR 1998 Biological action of leptin as an angiogenic factor. Science 281:1683–1686 [DOI] [PubMed] [Google Scholar]
- Mahutte NG, Matalliotakis IM, Goumenou AG, Vassiliadis S, Koumantakis GE, Arici A 2003 Inverse correlation between peritoneal fluid leptin concentrations and the extent of endometriosis. Hum Reprod 18:1205–1209 [DOI] [PubMed] [Google Scholar]
- De Placido G, Alviggi C, Carravetta C, Pisaturo ML, Sanna V, Wilding M, Lord GM, Matarese G 2001 The peritoneal fluid concentration of leptin is increased in women with peritoneal but not ovarian endometriosis. Hum Reprod 16:1251–1254 [DOI] [PubMed] [Google Scholar]
- Matalliotakis IM, Koumantaki YG, Neonaki MA, Goumenou AG, Koumantakis GE, Kyriakou DS, Koumantakis EE 2000 Increase in serum leptin concentrations among women with endometriosis during danazol and leuprolide depot treatments. Am J Obstet Gynecol 183:58–62 [DOI] [PubMed] [Google Scholar]
- Gonzalez RR, Rueda BR, Ramos MP, Littell RD, Glasser S, Leavis PC 2004 Leptin-induced increase in leukemia inhibitory factor and its receptor by human endometrium is partially mediated by interleukin 1 receptor signaling. Endocrinology 145:3850–3857 [DOI] [PubMed] [Google Scholar]
- Gonzalez RR, Leavis PC 2003 A peptide derived from the human leptin molecule is a potent inhibitor of the leptin receptor function in rabbit endometrial cells. Endocrine 21:185–195 [DOI] [PubMed] [Google Scholar]
- Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, Sullivan BT, Sakamoto H, Olawaiye A, Serikawa T, Lynch MP, Rueda BR 2006 Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2). J Biol Chem 281:26320–26328 [DOI] [PubMed] [Google Scholar]
- Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y 1997 ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett 407:313–319 [DOI] [PubMed] [Google Scholar]
- Johnson LM, Sidman RL 1979 A reproductive endocrine profile in the diabetes (db) mutant mouse. Biol Reprod 20:552–559 [DOI] [PubMed] [Google Scholar]
- Zukerberg LR, DeBernardo RL, Kirley SD, D’Apuzzo M, Lynch MP, Littell RD, Duska LR, Boring L, Rueda BR 2004 Loss of cables, a cyclin-dependent kinase regulatory protein, is associated with the development of endometrial hyperplasia and endometrial cancer. Cancer Res 64:202–208 [DOI] [PubMed] [Google Scholar]
- Karumanchi SA, Stillman IE 2004 In vivo rat model of preeclampsia. Methods Mol Med 122:393–399 [DOI] [PubMed] [Google Scholar]
- Greenberg LH, Slayden OD 2004 Human endometriotic xenografts in immunodeficient RAG-2/γ(c)KO mice. Am J Obstet Gynecol 190:1788–1795 [DOI] [PubMed] [Google Scholar]
- Vignali M, Infantino M, Matrone R, Chiodo I, Somigliana E, Busacca M, Vigano P 2002 Endometriosis: novel etiopathogenetic concepts and clinical perspectives. Fertil Steril 78:665–678 [DOI] [PubMed] [Google Scholar]
- Kayisli UA, Mahutte NG, Arici A 2002 Uterine chemokines in reproductive physiology and pathology. Am J Reprod Immunol 47:213–221 [DOI] [PubMed] [Google Scholar]
- Halme J 1991 Role of peritoneal inflammation in endometriosis-associated infertility. Ann NY Acad Sci 622:266–274 [DOI] [PubMed] [Google Scholar]
- Pugsley MK 2001 Etanercept. Immunex. Curr Opin Investig Drugs 2:1725–1731 [PubMed] [Google Scholar]
- Fortin M, Lepine M, Merlen Y, Thibeault I, Rancourt C, Gosselin D, Hugo P, Steff AM 2004 Quantitative assessment of human endometriotic tissue maintenance and regression in a noninvasive mouse model of endometriosis. Mol Ther 9:540–547 [DOI] [PubMed] [Google Scholar]
- Zamah NM, Dodson MG, Stephens LC, Buttram Jr VC, Besch PK, Kaufman RH 1984 Transplantation of normal and ectopic human endometrial tissue into athymic nude mice. Am J Obstet Gynecol 149:591–597 [DOI] [PubMed] [Google Scholar]
- McKittrick E, Gafken PR, Ahmad K, Henikoff S 2004 Histone H3.3 is enriched in covalent modifications associated with active chromatin. Proc Natl Acad Sci USA 101:1525–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YJ, Lai MD, Lei HY, Wing LY 2006 Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology 147:1278–1286 [DOI] [PubMed] [Google Scholar]
- Cao G, O’Brien CD, Zhou Z, Sanders SM, Greenbaum JN, Makrigiannakis A, DeLisser HM 2002 Involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. Am J Physiol Cell Physiol 282:C1181–C1190 [DOI] [PubMed] [Google Scholar]
- Jackson DE 2003 The unfolding tale of PECAM-1. FEBS Lett 540:7–14 [DOI] [PubMed] [Google Scholar]
- Eggermont J, Donnez J, Casanas-Roux F, Scholtes H, Van Langendonckt A 2005 Time course of pelvic endometriotic lesion revascularization in a nude mouse model. Fertil Steril 84:492–499 [DOI] [PubMed] [Google Scholar]
- Witz CA, Monotoya-Rodriguez IA, Schenken RS 1999 Whole explants of peritoneum and endometrium: a novel model of the early endometriosis lesion. Fertil Steril 71:56–60 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.