Abstract
The gastrin-enterochromaffin-like (ECL) cell-parietal cell axis is known to play an important role in the regulation of gastric acid secretion. Somatostatin, acting on somatostatin receptor type 2 (SSTR2), interferes with this axis by suppressing the activity of the gastrin cells, ECL cells, and parietal cells. Surprisingly, however, freely fed SSTR2 knockout mice seem to display normal circulating gastrin concentration and unchanged acid output. In the present study, we compared the control of acid secretion in these mutant mice with that in wild-type mice. In SSTR2 knockout mice, the number of gastrin cells was unchanged; whereas the numbers of somatostatin cells were reduced in the antrum (−55%) and increased in the oxyntic mucosa (35%). The ECL cells displayed a reduced expression of histidine decarboxylase and vesicle monoamine transport type 2 (determined by immunohistochemistry), and an impaired transformation of the granules to secretory vesicles (determined by electron microscopic analysis), suggesting low activity of the ECL cells. These changes were accompanied by an increased expression of galanin receptor type 1 in the oxyntic mucosa. The parietal cells were found to respond to pentagastrin or to vagal stimulation (evoked by pylorus ligation) with increased acid production. In conclusion, the inhibitory galanin-galanin receptor type 1 pathway is up-regulated in the ECL cells, and the direct stimulatory action of gastrin and vagal excitation is enhanced on the parietal cells in SSTR2 knockout mice. We suggest that there is a remodeling of the neuroendocrine mechanisms that regulate acid secretion in these mutant mice.
GASTRIC ACID SECRETION is regulated by endocrine and paracrine pathways via gastrin, histamine, and somatostatin, and by neural pathways via acetylcholine and neuropeptides, e.g. galanin, gastrin-releasing peptide, or pituitary adenylate cyclase-activating polypeptide (1,2,3,4,5). Circulating gastrin, produced and released by antral G cells, stimulates enterochromaffin-like (ECL) cells in the oxyntic mucosa of the stomach through gastrin receptors, also referred to as cholecystokinin (CCK) 2 receptors. As a result, the ECL cells release histamine, which in turn stimulates the parietal cells via histamine H2 receptors to cause acid secretion (the so-called gastrin-ECL cell-parietal cell axis) (6). As expected, gene disruption of either of these elements results in an impaired acid secretion, e.g. gastrin knockout mice, gastrin receptor knockout mice, histidine decarboxylase (HDC) knockout mice, and H2 receptor knockout mice (5).
Somatostatin in the stomach is produced in D cells in the antrum (open-type cells) as well as in the oxyntic mucosa (close-type cells). Somatostatin is known to act via five distinct receptor subtypes. Several studies have shown that the somatostatin receptor found in the ECL and parietal cells is of the subtype 2 [somatostatin receptor type 2 (SSTR2)] (7,8,9,10,11,12,13). In addition, neurons of the enteric nervous system were found to express STTR2 (7,9). Recently, we have shown that SSTR2 is the receptor subtype that mediates the effects of somatostatin on gastric acid secretion (14,15).
Previously, we reported that SSTR2 knockout mice were normogastrinemic in the freely fed state but that they displayed an increased basal acid secretion (14,15). These observations do not seem to support the view that somatostatin plays a key role in the regulation of gastric acid secretion. On the other hand, it illustrates the complexity and plasticity of the regulatory mechanisms of gastric acid secretion, allowing different pathways to be either suppressed or dominate, depending on the circumstances (16). Thus, in the present study, we attempt to identify the control mechanism that regulates acid secretion in SSTR2 knockout mice. To this end, the endocrine, paracrine, and neural pathways were studied and compared in SSTR2 knockout vs. wild-type mice.
Materials and Methods
Animals
Adult mice (3–6 months of age) were used. SSTR2-deficient mice were generated by gene targeting in mouse embryonic stem cells using a neomycin cassette with the entire SSTR2 gene on a 129 Sv/C57BL6 hybrid background (17). Wild-type and knockout mice were genotyped by Southern blot analysis and knockout mice maintained as inbred colonies. Mice used in the present study were born from different litters; they all descended from genotyped littermates obtained through inbreeding. Their general appearance, behavior, and gross morphology appeared indistinguishable from those of wild-type mice (14,15,17). Mice were maintained under controlled conditions of temperature (22 C) and humidity (60%), with food (Harlan Ibérica S.A., Barcelona, Spain) and tap water ad libitum. Mice were either freely fed or fasted for 16–18 h with free access to water (as specified). Pilot experiments showed no differences in gastric acid secretion and gastric histology between male and female mice, therefore, both genders were used. Animals were handled in accordance with the regulations of the American Physiological Society.
Chemicals
Pentagastrin (Peptavlon; Ayerst Laboratories, New York, NY), histamine (Sigma-Aldrich, St. Louis, MO), somatostatin-14 (Peptides Intl., Louisville, KY), and rat galanin (Peninsula Labs. Inc., Belmont, CA) were dissolved in 0.9% saline. All solutions were prepared immediately before each experiment. Doses of compounds were selected according to our previous studies in mice (3,4,14,15).
Histology
Tissue specimens were collected from the oxyntic (corpus) and antral regions of the glandular stomach from freely fed SSTR2 knockout and wild-type mice. Specimens were rinsed in saline solution and fixed in 4% formaldehyde. Tissues were embedded in paraffin, and multiple 4-μm thick sections were obtained from each specimen and stained for hematoxylin-eosin following standard procedures. Microscopic examination was performed using an optical microscope, and digitalized images were obtained with a digital camera (Nikon DXM1200; Nikon Corp., Tokyo, Japan). The morphometric analysis was performed on digitalized images using the image analysis software ImageJ (Research Services Branch, National Institute of Mental Health, Bethesda, MD). The thickness of the mucosa, submucosa, and muscular layer was measured in 10 randomly selected areas per each section of corpus and antrum, and repeated in five to 10 sections for each animal.
Immunohistochemistry
Tissue specimens were collected from the corpus of freely fed SSTR2 knockout and wild-type mice, and fixed for 8–12 h at 4 C in 4% 1-ethyl-3(3-dimethylaminopropyl)-carbodiimide hydrochloride (Sigma-Aldrich) for the visualization of histamine or in 4% formaldehyde for the visualization of all other antigens. The immunohistochemical analysis was performed using various primary antibodies to visualize the endocrine cells (Table 1). The specimens for histamine immunohistochemistry were rinsed with 0.1 m PBS (pH 7.4) containing 20% sucrose. Frozen tissue sections were cut perpendicularly to the mucosal surface in a cryostat (Bright, Huntington, UK) set at 10-μm thickness and thawed onto gelatin-coated glass slides. The sections were incubated with antihistamine antibody overnight at 4 C, visualized by goat antirabbit fluorescein isothiocyanate conjugated serum for 1 h at room temperature, and examined by fluorescence microscopy. The specimens for the other antigens were formaldehyde fixed, paraffin embedded, and cut at 4-μm thickness. The sections were mounted on super-frost glass slides (Dako, Glostrup, Denmark). Paraffin was removed with xylene, and then sections were pressure cooked for 5 min in EDTA-Tris-buffered saline (pH 9). The automatic immunostaining method was used. Briefly, the sections were transferred to a Dako AutoStainer (Universal Staining System, E172566, model LV-1, serial no. DC 3400-9114-03; Dako), and staining was performed automatically using the detection system (code no. K4011; Dako EnVision System). Immunoreactivity was visualized by 3′3-diaminobenzidine HCl chromogen and examined using light microscopy or per oxyntic gland.
Table 1.
Primary antibodies used in immunohistochemical analysis
| Antibody | Cell type | Working dilution | Product code | Source |
|---|---|---|---|---|
| Chromogranin A | Endocrine cells, including ECL cells | 1:500 | 20086 | ImmunoStar, Inc., Hudson, WI |
| Pancreastatin | ECL cells | 1:1000 | K9006 | Euro-Diagnostica, Malmö, Sweden |
| HDC | ECL cells | 1:1500 | K9506 | Euro-Diagnostica |
| Histamine | ECL cells | 1:1000 | 8431 | Euro-Diagnostica |
| VMAT2 | ECL cells | 1:5000 | B-GP-280-1 | Phoenix Pharmaceuticals, Burlingame, CA |
| GalR1 | ECL cells | 1:100 | GALR11-A | Alpha Diagnostics Inc. Intl., San Antonio, TX |
| Galanin | Enteric nerves | 1:100 | GAL51-S | Alpha Diagnostics Inc. |
| Ghrelin | A-like cells | 1:7000 | H-031–31 | Phoenix Pharmaceuticals |
| Somatostatin | D cells | 1:1000 | M09204 | Dako |
| Serotonin | EC cells | 1:50 | M0758 | Dako |
| H+,K+-ATPase | Parietal cells | 1:100 | Dr. L. Friis-Hansen, Rigshospitalet, Copenhagen, Denmark | |
| PCNA | Proliferating cells | 1:100 | M0879 | Dako |
| PGP9.5 | Endocrine cells | 1:100 | Z5116 | Dako |
| Gastrin | G cells | 1:500 | CURE Ab E5 | Center for Ulcer Research and Education: Digestive Diseases Research Center, Antibody Core, Los Angeles, CA |
For the detection of gastrin immunoreactive cells, tissue specimens were collected from the antral region of the stomach of freely fed SSTR2 knockout and wild-type mice, fixed in 4% formaldehyde (6–7 h, at 4 C), and cryoprotected in a 10% sucrose solution in PBS [0.1 m (pH 7.4), 12–15 h at 4 C). Frozen sections (12-μm thickness) were immunostained using a monoclonal antigastrin as primary antiserum and a rhodamine-conjugated goat antimouse IgG as secondary antiserum (1:500; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Immunoreactive cells were counted under fluorescence or light microscope and expressed as number per millimeter length of mucosa (horizontal measurement), per field, or per oxyntic gland.
Electron microscopy
Small tissue specimens were taken from the oxyntic mucosa of freely fed SSTR2 knockout and wild-type mice, and immediately immersed in 2% glutaraldehyde in 0.1 m PBS (pH 7.2). After 6 h, the specimens were transferred to 1% OsO4 and post fixed for 1 h, dehydrated in graded acetone, and embedded in Epoxy (TAAB Laboratories Equipment Ltd., Berkshire, UK). Ultrathin sections of 60- to 80-nm thickness were cut on a LKB MK III Ultrotome (LKB, Bromma, Sweden), contrasted with uranyl acetate and lead citrate, and examined in a Philips CM10 transmission electron microscope (Philips, Eindhoven, The Netherlands). ECL cells were identified by their characteristic cytoplasmic granules/vesicles, and electron micrographs (×13,900) were taken for morphometric analysis (18). Granules are membrane-enclosed organelles (diameter 50–250 nm) with an electron-dense core and a thin electron-lucent halo separating the membrane from the dense core. The diameter of the dense core exceeded 50% of the diameter of the entire organelle. Vesicles are membrane-enclosed, electron-lucent organelles without dense core or with a small, often eccentrically located, dense core. The diameter of the dense core was less than 50% of the organelle. Vesicles were further classified as previously described (19) into two populations based on their size and the presence or absence of dense core: 1) secretory vesicles with a diameter of 125–500 nm with a dense core; and 2) clear, electron-lucent microvesicles with a diameter of 25–125 nm, lacking a dense core. Photographs of cells were used for morphometric analysis as described previously (20,21). Twenty-six to 28 ECL cells from each group of mice were analyzed planimetrically. The cell profile area, nuclear profile area, cytoplasm profile area, and volume densities of nucleus and of various types of secretory organelles in each ECL cell were determined. The parietal cells were identified by their characteristic cytoplasmic tubulovesicles and/or secretory canaliculi (22,23).
Gastric acid secretion
Gastric acid secretion was monitored as previously described (3,4,14,15) in fasted wild-type and SSTR2 knockout mice anesthetized with urethane (1.25 g/kg, about 0.2 ml, ip). The trachea was cannulated to ensure a clear airway and the esophagus ligated. Thereafter, the pylorus was ligated through an abdominal midline incision. The gastric lumen was rinsed until clean with warm 0.9% saline, and a double-lumen gastric cannula was inserted through an incision in the nonglandular portion of the stomach. A catheter (30-gauge needle inserted into polyethylene E-10 tubing; Baxter, Irvine, CA) was placed into the ileal vein for constant iv infusion of saline (0.1 ml/h). A 30- to 45-min period was allowed for stabilization. Thereafter, the following substances were infused iv: somatostatin-14 (20 μg/kg·h for 1 h), pentagastrin (16 μg/kg·h for 2 h), histamine (5 mg/kg·h for 2 h), galanin (10 μg/kg·h for 1 h), or saline (0.1 ml/h). Doses and route of substance administration were based on our previous dose-response studies in mice (3,4,14,15). Gastric acid secretion was determined by continuous intragastric perfusion with warm saline (pH 7.0) (0.3 ml/min) and collection of the effluents that were back titrated to pH 7.0 (0.001 n NaOH) with an automatic titrator (Radiometer, Copenhagen, Denmark). Gastric acid secretion was determined during a 2- (pentagastrin and histamine) or 1-h period (somatostatin and galanin). To compensate for the differences in basal secretion, in some cases, the acid response was calculated as net acid output (i.e. difference between total gastric acid output during the time considered and the rate of basal secretion during the same period).
In refeeding experiments, mice were fasted for 16–18 h and fed ad libitum with the standard mouse diet for 1 h before they were euthanized by cervical dislocation. The stomach from each mouse was dissected out, the lumen was washed with saline (pH 7.0) (3 ml), and the gastric contents plus the washout solution were collected and centrifuged (3500 rpm, 10 min, 4 C). Thereafter, the supernatant was used for pH measurement and determination of total acid content (μmol) by titration with 0.01 n NaOH.
In pylorus ligation experiments, mice were fasted for 16–18 h and subjected to pylorus ligation under a 5- to 6-min halothane anesthesia (Fluothane; AstraZeneca, Södertälje, Sweden). The animals regained consciousness 4–5 min after the surgery and were maintained undisturbed in their home cages without food or water for a 2-h period. Thereafter, the animals were euthanized by cervical dislocation. The gastric juice was collected, and the acid output (μmol/2 h) was determined by titration with 0.01 n NaOH.
Statistical analysis
Values are expressed as mean ± sem. Differences between two groups were determined by the paired or unpaired Student’s t test, as appropriate. Differences between multiple groups were determined by ANOVA, followed, whenever necessary, by a Student-Newman-Keuls multiple comparisons test. Data were considered statistically significant when P was less than 0.05.
Results
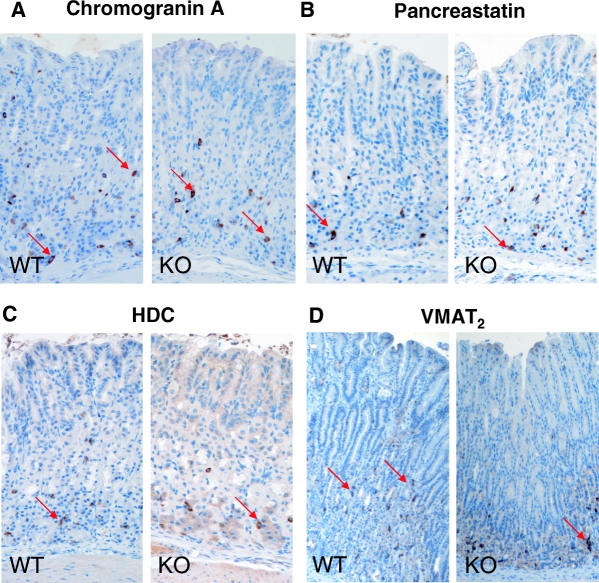
No gross differences in the stomach were noted between wild-type and SSTR2 knockout mice, as shown by similar organ weight (absolute or relative to the total body weight) and macroscopic or microscopic mucosal morphology (Table 2). The density of proliferating cells in the oxyntic mucosa, revealed by proliferating cell nuclear antigen (PCNA) immunostaining, was similar in wild-type (43.8 ± 3.9 number/mm) and SSTR2 knockout mice (47.3 ± 2.8 number/mm). There were no differences in terms of density in chromogranin A-immunoreactive cells and pancreastatin-immunoreactive cells in the oxyntic mucosa between wild-type and SSTR2 knockout mice (Fig. 1, A and B, and Table 3). However, in the oxyntic mucosa of SSTR2 knockout mice, the density of somatostatin immunoreactive cells was increased by 35% (P < 0.05), whereas the density of somatostatin immunoreactive cells in the antrum was reduced by 55% (P < 0.05) compared with wild-type animals. The density of gastrin cells was similar in the two strains (Table 3). In addition, other endocrine cells, including the ghrelin cells (Table 3), serotonin immunoreactive enterochromaffin (EC) cells, and PGP9.5 immunoreactive endocrine cells were unchanged in number (data not shown).
Table 2.
Gastric morphometry in wild-type (WT) and SSTR2 knockout (KO) mice
| Mice | Body weight (g) | Stomach weight (mg) | Antrum thickness (μm)a
|
Corpus thickness (μm)a
|
||||
|---|---|---|---|---|---|---|---|---|
| Mucosa | Submucosa | Muscular | Mucosa | Submucosa | Muscular | |||
| WT (n = 6) | 22.9 ± 0.3 | 186 ± 8 | 283 ± 10 | 73 ± 10 | 121 ± 14 | 301 ± 16 | 68 ± 10 | 119 ± 10 |
| KO (n = 6)b | 23.5 ± 0.4 | 201 ± 5 | 276 ± 5 | 63 ± 8 | 137 ± 12 | 306 ± 12 | 67 ± 5 | 122 ± 14 |
Data are mean ± sem of multiple measurements for each region and tissue layer, performed in multiple samples collected from the number of animals indicated.
There is no significant difference between WT and KO mice in any of the measurements.
Figure 1.
Representative transverse sections of the oxyntic mucosa showing immunostaining for chromogranin A (A), pancreastatin (B), HDC (C), and VMAT2 (D) in epithelial cells in wild-type (WT) and SSTR2 knockout (KO) mice. Examples of immunoreactive cells are indicated by arrows. Magnification, ×200.
Table 3.
Endocrine and parietal cells in the oxyntic mucosa, and gastrin and somatostatin cells in the antral mucosa of wild-type (WT) and SSTR2 knockout (KO) mice
| Mice | Oxyntic mucosa
|
Antral mucosa
|
|||||
|---|---|---|---|---|---|---|---|
| Chromogranin A-ir cells (no./mm) | Pancreastatin-ir cells (no./mm) | Somatostatin-ir cells (no./mm) | Ghrelin-ir cells (no./mm) | H+,K+-ATPase-ir cells (no./gland) | Gastrin-ir cells (no./field) | Somatostatin-ir cells (no./field) | |
| WT (n = 6) | 66.4 ± 10.1 | 32.2 ± 3.7 | 9.3 ± 0.7 | 23.1 ± 1.8 | 9.1 ± 0.8 | 9.8 ± 1.9 | 12.0 ± 2.2 |
| KO (n = 9) | 57.7 ± 4.2a | 29.1 ± 1.6a | 12.6 ± 1.1b | 21.4 ± 1.3a | 8.9 ± 1.1a | 9.0 ± 0.4a | 4.8 ± 0.5b |
Values are expressed as means ± sem of the number of animals indicated. no./field, Number of immunoreactive cells per field (× 40); no./gland, number of immunoreactive cells per the oxyntic gland. no./mm, number of immunoreactive (ir) cells per millimeter of mucosa (horizontal measurement).
Not significant.
P < 0.05 vs. respective value in WT mice.
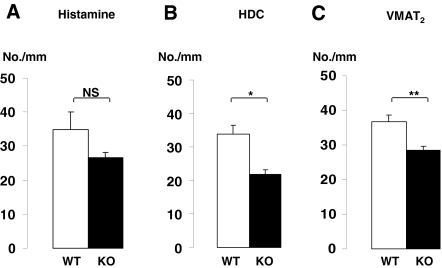
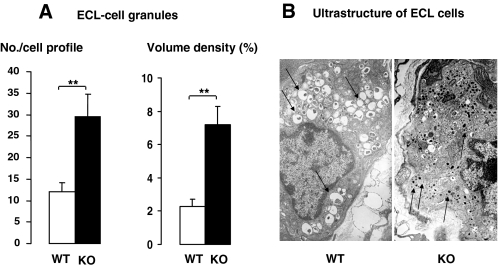
In SSTR2 knockout mice, histamine immunoreactive ECL cells tended to be reduced in number, whereas the density of HDC immunoreactive ECL cells was significantly reduced (Figs. 1C and 2). Moreover, ECL cell granules are known to harbor vesicle monoamine transport type 2 (VMAT2), and there was a reduced number of VMAT2-immunoreactive cells in SSTR2 knockout mice (Fig. 2). The quantitative analysis of the ECL cell ultrastructure revealed that in SSTR2 knockout mice, the granules, but not the secretory vesicles and microvesicles, were increased greatly in terms of both number per cell profile and volume density (Fig. 3 and Table 4). The ratio of granules to secretory vesicles in terms of volume density was increased from 0.17 (wild-type mice) to 0.43 (knockout mice), whereas the ratio of microvesicles to secretory vesicles was decreased from 0.27 (wild-type mice) to 0.14 (knockout mice). Other parameters, including cellular, nuclear and cytoplasmic profiles, and volume density of lipofuscin bodies, were unchanged (Table 4).
Figure 2.
Density of epithelial cells immunostained for histamine (A), HDC (B), or VMAT2 (C) (number per mm length mucosa) in the oxyntic mucosa in wild-type (WT) and SSTR2 knockout (KO) mice. Mean ± sem (n = 6–9). *, P < 0.05. **, P < 0.01. NS, Not significant.
Figure 3.
Number of ECL-cell granules (A) and ultrastructure of ECL cells (B) in wild-type (WT) and SSTR2 knockout (KO) mice. Mean ± sem (n = 6–9). **, P < 0.01. Note that numerous secretory vesicles were observed in wild-type-ECL cells, whereas granules were predominant in knockout-ECL cells (indicated by arrows). Magnification, ×14,000.
Table 4.
Ultrastructural features of ECL cells in wild-type (WT) and SSTR2 knockout (KO) mice
| Cells examined (no.) | Cell profile area (μm2) | Nuclear profile area (μm2) | Nucleus (%) | Cytoplasm (μm2) | Lipofuscin bodies (%) | Granules no./cell (%) | Secretory vesicles no./cell (%) | Microvesicles no./cell (%) |
|---|---|---|---|---|---|---|---|---|
| WT-ECL cells (n = 11) | 161.7 ± 12.6 | 53.1 ± 7.6 | 35.1 ± 2.2 | 117.8 ± 15.0 | 1.2 ± 0.5 | 12.0 ± 2.2 (2.3 ± 0.4) | 61.0 ± 9.6 (13.5 ± 1.3) | 38.8 ± 7.4 (3.7 ± 0.7) |
| KO-ECL cells (n = 22) | 147.5 ± 12.3a | 42.2 ± 3.4a | 40.3 ± 2.5a | 105.3 ± 8.9a | 0.5 ± 0.2a | 29.6 ± 5.2 (7.2 ± 1.1)b | 68.5 ± 10.0 (16.9 ± 1.9)a | 31.2 ± 4.1 (2.4 ± 0.2)a |
Six mice of each strain were examined. Values are expressed as means ± sem. %, Volume density; no./cell, number per ECL-cell profile.
Not significant.
P < 0.01 vs. respective value in WT mice.
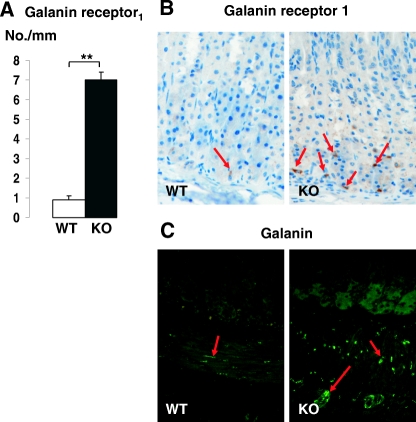
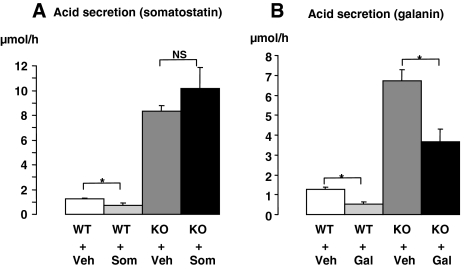
Immunostaining for the galanin receptor type 1 (GalR1) revealed a few immunoreactive cells (likely the ECL cells) in the oxyntic mucosa of wild-type mice and an increased number of immunoreactive cells (>7-fold; P < 0.05) in the oxyntic mucosa of SSTR2 knockout mice (Fig. 4, A and B). Moreover, galanin-immunoreactive fibers were abundant in the smooth muscle and intramural ganglia, and moderate in the oxyntic mucosa/submucosa of SSTR2 knockout mice (Fig. 4C). These findings are in line with the apparent low activity of the ECL cells since galanin has been shown to inhibit ECL cells (24,25). Indeed, galanin infusion, but not somatostatin, significantly inhibited acid secretion in SSTR2 knockout mice; whereas both peptides had a similar inhibitory effect in wild-type animals (Fig. 5) (4,26).
Figure 4.
A, Number of cells immunostained for GalR1 (number per mm) in the oxyntic mucosa. Representative transverse sections of the oxyntic mucosa showing GalR1 immunoreactivity (B) and galanin-immunoreactive nerve fibers (C) in wild-type (WT) and SSTR2 knockout (KO) mice. Magnification, ×200. Mean ± sem (n = 6–9). **, P < 0.01. Note that many immunoreactive receptor-rich cells were observed in knockout mice (indicated by arrows in B), whereas few cells were observed in wild-type mice (arrow in B). Arrows in C denote galanin-immunoreactive nerve fibers in the submucosa and muscular layers.
Figure 5.
Gastric acid output in wild-type (WT) and SSTR2 knockout (KO) mice in response to the iv infusion of somatostatin-14 (Som) (A) or galanin (Gal) (B) for 1 h. Controls in A and B received vehicle (Veh). Mean ± sem (n = 4–5). *, P < 0.05. NS, Not significant.
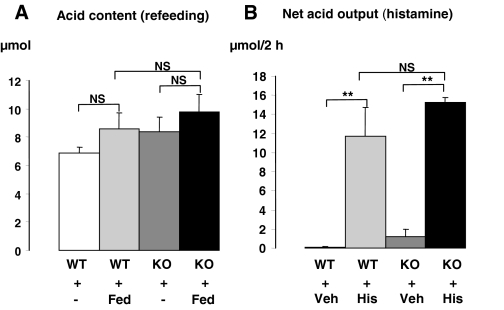
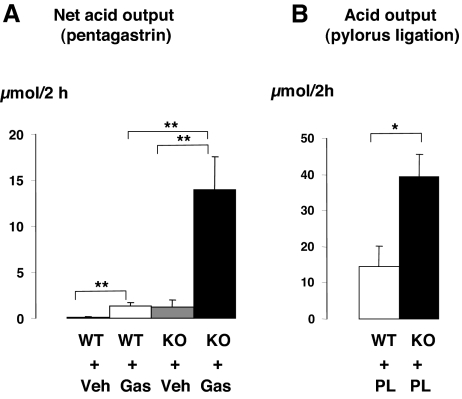
The number of parietal cells per oxyntic gland (i.e. H+,K+-ATPase immunoreactive cells) did not differ between wild-type and SSTR2 knockout mice (Table 3). Moreover, the ultrastructural appearance of the parietal cells with respect to tubulovesicles and canaliculi seemed to be normal in SSTR2 knockout mice (data not shown). The appearance of a normal structure of parietal cells was consistent with the similar secretory responses observed in wild-type and SSTR2 knockout mice in response to a meal or to histamine stimulation (Fig. 6). However, the net secretory responses to pentagastrin in urethane anesthetized mice or the acid output after pylorus ligation in conscious mice were 10- or 2.8-fold higher in SSTR2 knockout than in wild-type mice, respectively (Fig. 7).
Figure 6.
Gastric acid content after refeeding for 1 h (Fed) (A) and net acid secretory response in response to iv infusion of histamine for 2 h (His) (B) in wild-type (WT) and SSTR2 knockout (KO) mice. Controls in A were fasted throughout the experiment (−) and in B received vehicle (Veh). Mean ± sem (n = 3–5). **, P < 0.01. NS, Not significant.
Figure 7.
Net gastric acid output in wild-type (WT) and SSTR2 knockout (KO) mice in response to pentagastrin infusion for 2 h (Gas) (A) or to pylorus ligation for 2 h (PL) (B). Controls in A received vehicle (Veh). Mean ± sem (n = 3–5). *, P < 0.05. **, P < 0.01.
Discussion
In view of the well-known suppressive effects of somatostatin on gastrin cells, ECL cells, and parietal cells, it was expected that the lack of a functional somatostatin-SSTR2 inhibitory pathway would result in hypergastrinemia, hyperactivity of ECL cells, and hypersecretion of gastric acid. However, that was not the case, as shown previously (3,4,14,15) and corroborated here.
It is generally believed that the circulating levels of gastrin are determined by the ratio of gastrin cells and somatostatin cells in the antrum. In the SSTR2 knockout mice, the ratio of gastrin and somatostatin cells was more than 2-fold higher compared with wild-type mice. However, this was not associated with hypergastrinemia. In fact, the SSTR2 knockout mice were normogastrinemic (15), which is in line with the unchanged gastric acid secretory response to a normal meal in conscious mutant mice, as shown in the present study. A similar phenotype was observed in gastrin and CCK double knockout mice (27). Thus, the role of somatostatin in the regulation of gastrin release needs to be explored further.
In SSTR2 knockout mice, the number of endocrine cells in the oxyntic mucosa (with ECL cells constituting the predominant cell type) was unchanged compared with wild-type animals, as revealed by immunohistochemistry with antibodies against chromogranin A or pancreastatin. It should be noted that the chromogranin A-immunoreactive cells were more numerous than the pancreastatin-immunoreactive cells, probably suggesting that pancreastatin immunostaining demonstrates predominantly the ECL cells population. Indeed, we have shown that circulating pancreastatin is a marker for ECL cells (28,29,30). The functional state of the ECL cells is reflected by their HDC activity. Activation of the ECL cells is accompanied by a series of ultrastructural alterations, leading to exocytosis of histamine (18,31,32,33). Histamine is formed by decarboxylation of histidine in the cytosol and taken up by the granules via VMAT2. Accumulation of histamine in the granules results in the formation of secretory vesicles, which are released through exocytosis. The process of exocytosis is coupled with endocytosis, resulting in the generation of microvesicles (18). In the present study, ECL cells were analyzed by quantitative immunohistochemistry and electron microscopy. Histamine immunostaining revealed a trend toward a reduced number of cells, and HDC immunostaining showed a marked reduction in the number of ECL cells in SSTR2 knockout mice compared with wild-type controls. It has been demonstrated that there is a positive correlation between the number of HDC-immunoreactive ECL cells and HDC activity (31,34). This is because the polyclonal antibody used is raised against the carboxyl-terminal fragment of the HDC molecule (amino acids 603–615, 64 kDa) that is the active form of the enzyme (35,36). Furthermore, the reduced VMAT2 immunoreactivity of the ECL cells was associated with the impaired transformation of granules to secretory vesicles, leading to accumulation of granules in the cytoplasm of ECL cells in SSTR2 knockout mice. In addition, the exocytosis rate of the secretory vesicles seemed to be inhibited in SSTR2 knockout mice, as reflected by the reduced ratio of microvesicles to secretory vesicles.
Studies on isolated ECL cells have shown that ECL cells can be inhibited not only by somatostatin, via SSTR2, but also by galanin, via GalR1 (24,25). It has been reported that GalR1 occur on the surface of ECL cells, and on myenteric and submucous neurons in the stomach, supporting the idea that the GalR1 contributes to galanin-induced inhibition of gastric acid secretion by suppressing histamine release from the ECL cells (37). In the present study, GalR1 in the oxyntic mucosa were found to be expressed at a low level in wild-type mice and at a high level in SSTR2 knockout mice. Apparently, there was a coexistence of high expression of Gal1 receptor and low expression of HDC and VMAT2 in the mutant mice, suggesting a reverse association between HDC-immunoreactive ECL cells and GalR1 immunoreactivity. Moreover, the increased GalR1 expression in the knockout mice was likely accompanied with an increase in the density of galanin-immunoreactive nerve fibers in the stomach. Together, we may suggest that a shift from somatostatin-dependent inhibition of acid secretion to a galanin-dependent inhibition occurs in these mutant mice. Supporting this hypothesis, iv infusion of galanin significantly inhibited acid secretion in SSTR2 knockout mice, whereas that of somatostatin had no effect. In addition, somatostatin-containing cells (D cells) in the oxyntic mucosa are known to play a paracrine inhibitory role by releasing somatostatin on ECL and parietal cells. Therefore, the slightly increased somatostatin cell density observed in the corpus of SSTR2 knockout mice might be attributed to a compensatory mechanism due to the lack of functional SSTR2 on ECL and parietal cells.
Although the ECL cells seemed inactive in SSTR2 knockout mice, the parietal cells were capable of producing acid in response to a meal or to stimulation with histamine. Moreover, in the mutant mice, parietal cells had an enhanced secretory response to exogenous gastrin or to vagal stimulation (evoked by pylorus ligation). The acid secretory response elicited by vagal excitation is known to be independent of the ECL cells (38). Thus, the enhanced acid output suggests that signaling pathways depending on gastrin receptors and M3 receptors on the parietal cells might be up-regulated in SSTR2 knockout mice. Although it seems to be debatable whether gastrin is able to stimulate directly the parietal cell (39), there is evidence to indicate that parietal cells express the gastrin/CCK2 receptor (40,41,42). However, the physiological significance of these receptors has been hard to prove because isolated parietal cells respond poorly to gastrin (43,44,45). It has been difficult to assess the relative contribution of gastrin receptors that are located on ECL cells vs. parietal cells to secretory responses in vivo. In the present study, we were able to show that the parietal cells responded to pentagastrin with a more than 10-fold greater acid output in SSTR2 knockout mice compared with wild-type mice, suggesting that gastrin affects the parietal cells directly. We may also suggest that there is probably a “cross-talk” between gastrin receptors and SSTR2 in the parietal cell. In fact, studies in isolated parietal cells have suggested that the SSTR2 is coupled to Gi trimeric protein that inhibits the gastrin receptor-coupled Gq pathway that activates phospholipase C to induce an increase in inositol trisphosphate, causing a release of intracellular calcium upon stimulation (45,46,47). We suggest that in wild-type mice, SSTR2 exerts an inhibitory restraint over the gastrin receptor on parietal cells, maintaining these receptors in a silent state. In the absence of SSTR2, the efficiency of the gastrin-induced response will be amplified, because of the lack of inhibitory restraint, resulting in enhanced secretory responses.
Parietal cells, but not ECL cells, are known to express the M3 receptor (48,49). The M3 receptor, like the gastrin receptor, is known to be coupled to the Gq protein (50). Conceivably, upon removal of the inhibitory restraint by SSTR2, the M3 receptor-activated pathways will be up-regulated. Indeed, supporting this hypothesis, the SSTR2 knockout mice produced a significant 2.5-fold higher acid in response to vagal stimulation evoked by pylorus ligation than their wild-type counterparts. However, the lack of SSTR2 did not enhance histamine-induced acid secretion, which is in line with the results of a previous study, showing that exogenous somatostatin only partially inhibited histamine-stimulated gastric acid secretion in mice (51). In fact, it is known that histamine H2 receptors are coupled to both the Gq protein and Gs protein that activates adenylate cyclase and increases intracellular cAMP (45,52,53), a signaling pathway independent of SSTR2. Therefore, it may be speculated that multiple intracellular mechanisms will interact (i.e. receptor cross talk through various transduction pathways), determining which signals will induce acid secretion.
Numerous reports have indicated that somatostatin and its analogs (mainly octreotide) inhibit cell growth and proliferation, an observation that has promoted their use as antiproliferating drugs (54). Several somatostatin receptor subtypes have induced cell cycle arrest in various tumor cells lines through different mechanisms (55,56,57). Because the gastric epithelium is rich in SSTR2 and undergoes continuous cell multiplication and differentiation (8), it is tempting to speculate that somatostatin plays a role in these processes. However, SSTR2 knockout mice did not display any alteration with respect to the development, proliferation, and differentiation of the gastric mucosa in general and of endocrine cells in particular, suggesting that the presence of SSTR2 is not necessary for the development and maintenance of the stomach architecture. Alternatively, compensatory mechanisms may negate the gene deletion effect.
Together, these results suggest that mice lacking SSTR2 are characterized by: 1) a shift in the regulation of the ECL cells from a paracrine somatostatin-SSTR2-dependent pathway to a neurocrine-dependent pathway, with galanin-GalR1 pathway being a strong candidate; and 2) a shift in the stimulatory regulation of the parietal cells from the gastrin-ECL cell axis to an enhancement of the direct action of gastrin and vagal pathways. As a consequence of these functional adjustments, basal gastric acid secretion and the postprandial secretory response in SSTR2 knockout mice are similar to wild-type mice. This illustrates the fact that the control mechanisms behind gastric acid secretion display a high degree of plasticity, as previously demonstrated in gastrin and CCK double knockout mice that lack both gastrin-histamine and CCK-somatostatin regulatory pathways (27,58).
Acknowledgments
We thank Dr. J. H. Schaeffer (Merck Research Laboratories, Drammen, Norway) for the generous donation of the somatostatin receptor type 2 knockout breeders and Professor Rolf Håkanson (University of Lund, Lund, Sweden) for his useful comments on the manuscript.
Footnotes
This work was supported by grants from the Norwegian Research Council and Gene Therapy Projects (to D.C.), the St. Olav’s Hospital Cancer Research Foundation (Trondheim, Norway) (to C.-M.Z.), the Conselleria de Cultura Educació i Ciència-Generalitat Valenciana, GV-23-1-4 (to V.M.), and the National Institute of Diabetes and Digestive and Kidney Diseases, DK-30110, DK 41301 and Veterans Administration Senior Scientist Award (to Y.T.). L.P. was supported by a fellowship from the San Pablo Centro de Estudios Universitarios Foundation.
Disclosure Statement: The authors have nothing to declare.
First Published Online November 1, 2007
Abbreviations: CCK, Cholecystokinin; EC, enterochromaffin; ECL, enterochromaffin-like; Gal1, galanin receptor type 1; GalR1, Gal1 receptor; HDC, histidine decarboxylase; PCNA, proliferating cell nuclear antigen; SSTR2, somatostatin receptor type 2; VMAT2, vesicle monoamine transport type 2.
References
- Schubert ML 2005 Gastric secretion. Curr Opin Gastroenterol 21:636–643 [PubMed] [Google Scholar]
- Zeng N, Sachs G 2005 Neuronal regulation of gastric endocrine cells. In: Taché Y, Goto Y, Ohning G, Yamada T, eds. Gut-brain peptides in the new millennium. Los Angeles: CURE Foundation; 55–68 [Google Scholar]
- Piqueras L, Taché Y, Martinez V 2004 Peripheral PACAP inhibits gastric acid secretion through somatostatin release in mice. Br J Pharmacol 142:67–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piqueras L, Taché Y, Martinez V 2004 Galanin inhibits gastric acid secretion through a somatostatin-independent mechanism in mice. Peptides 25:1287–1295 [DOI] [PubMed] [Google Scholar]
- Chen D, Aihara T, Zhao CM, Håkanson R, Okabe S 2006 Differentiation of the gastric mucosa. I. Role of histamine in control of function and integrity of oxyntic mucosa: understanding gastric physiology through disruption of targeted genes. Am J Physiol Gastrointest Liver Physiol 291:G539–G544 [DOI] [PubMed] [Google Scholar]
- Lindström E, Chen D, Norlén P, Andersson K, Håkanson R 2001 Control of gastric acid secretion: the gastrin-ECL cell-parietal cell axis. Comp Biochem Physiol A Mol Integr Physiol 128:505–514 [DOI] [PubMed] [Google Scholar]
- Sternini C, Wong H, Wu SV, de Giorgio R, Yang M, Reeve Jr J, Brecha NC, Walsh JH 1997 Somatostatin 2A receptor is expressed by enteric neurons, and by interstitial cells of Cajal and enterochromaffin-like cells of the gastrointestinal tract. J Comp Neurol 386:396–408 [PubMed] [Google Scholar]
- Schindler M, Humphrey PP 1999 Differential distribution of somatostatin sst2 receptor splice variants in rat gastric mucosa. Cell Tissue Res 297:163–168 [DOI] [PubMed] [Google Scholar]
- Allen JP, Canty AJ, Schulz S, Humphrey PP, Emson PC, Young HM 2002 Identification of cells expressing somatostatin receptor 2 in the gastrointestinal tract of Sstr2 knockout/lacZ knockin mice. J Comp Neurol 454:329–340 [DOI] [PubMed] [Google Scholar]
- Prinz C, Sachs G, Walsh JH, Coy DH, Wu SV 1994 The somatostatin receptor subtype on rat enterochromaffin like cells. Gastroenterology 107:1067–1074 [DOI] [PubMed] [Google Scholar]
- Wyatt MA, Jarvie E, Feniuk W, Humphrey PP 1996 Somatostatin sst2 receptor-mediated inhibition of parietal cell function in rat isolated gastric mucosa. Br J Pharmacol 119:905–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurang K, Wang J, Lloyd KC 1997 Somatostatin inhibition of acid and histamine release by activation of somatostatin receptor subtype 2 receptors in rats. J Pharmacol Exp Ther 281:245–252 [PubMed] [Google Scholar]
- Fykse V, Coy DH, Waldum HL, Sandvik AK 2005 Somatostatin-receptor 2 (sst2)-mediated effects of endogenous somatostatin on exocrine and endocrine secretion of the rat stomach. Br J Pharmacol 144:416–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piqueras L, Martinez V 2004 Role of somatostatin receptors on gastric acid secretion in wild-type and somatostatin receptor type 2 knockout mice. Naunyn Schmiedebergs Arch Pharmacol 370:510–20 [DOI] [PubMed] [Google Scholar]
- Martinez V, Curi AP, Torkian B, Schaeffer JM, Wilkinson HA, Walsh JH, Taché Y 1998 High basal gastric acid secretion in somatostatin receptor subtype 2 knockout mice. Gastroenterology 114:1125–1132 [DOI] [PubMed] [Google Scholar]
- Chen D, Friis-Hansen L, Håkanson R, Zhao CM 2005 Genetic dissection of the signaling pathways that control gastric acid secretion. Inflammopharmacology 13:201–207 [DOI] [PubMed] [Google Scholar]
- Zheng H, Bailey A, Jiang MH, Honda K, Chen HY, Trumbauer ME, Van der Ploeg LH, Schaeffer JM, Leng G, Smith RG 1997 Somatostatin receptor subtype 2 knockout mice are refractory to growth hormone-negative feedback on arcuate neurons. Mol Endocrinol 11:1709–1717 [DOI] [PubMed] [Google Scholar]
- Zhao CM, Chen D, Lintunen M, Panula P, Håkanson R 1999 Secretory organelles in ECL cells of the rat stomach: an immunohistochemical and electron-microscopic study. Cell Tissue Res 298:457–470 [DOI] [PubMed] [Google Scholar]
- Chen D, Zhao C-M, Andersson K, Sundler F, Håkanson R 1996 Ultrastructure of enterochromaffin-like cells in rat stomach: effects of alpha-fluoromethylhistidine-evoked histamine depletion and hypergastrinemia. Cell Tissue Res 283:469–478 [DOI] [PubMed] [Google Scholar]
- Weibel ER 1969 Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol 26:235–302 [DOI] [PubMed] [Google Scholar]
- Weibel ER, Bolender RP 1973 Stereological techniques for electron microscopic morphometry. In: Hayat MA, ed. Principles and techniques of electron microscopy: biological applications. New York: Van Nostrand Reinhold; 237–296 [Google Scholar]
- Helander HF, Hirschowitz BI 1974 Quantitative ultrastructural studies on inhibited and partly stimulated gastric parietal cells. Gastroenterology 67:447–452 [PubMed] [Google Scholar]
- Ito S, Schofield GC 1974 Studies on the depletion and accumulation of microvilli; and changes in tubulovesicular compartment of mouse parietal cells in relation to gastric acid secretion. J Cell Biol 63:364–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström E, Björkqvist M, Boketoft A, Chen D, Zhao CM, Kimura K, Håkanson R 1997 Neurohormonal regulation of histamine and pancreastatin secretion from isolated rat stomach ECL cells. Regul Pept 71:73–86 [DOI] [PubMed] [Google Scholar]
- Zeng N, Kang T, Wen Y, Wong H, Walsh J, Sachs G 1998 Galanin inhibition of enterochromaffin-like cell function. Gastroenterology 115:330–339 [DOI] [PubMed] [Google Scholar]
- Kato S, Korolkiewicz R, Rekowski P, Szyk A, Sugawa Y, Takeuchi K 1998 Inhibition of gastric acid secretion by galanin in rats. Relation to endogenous histamine release. Regul Pept 74:53–59 [DOI] [PubMed] [Google Scholar]
- Chen D, Zhao CM, Håkanson R, Samuelson LC, Rehfeld JF, Friis-Hansen L 2004 Altered control of gastric acid secretion in gastrin-cholecystokinin double mutant mice. Gastroenterology 126: 476–487 [DOI] [PubMed] [Google Scholar]
- Chen D, Monstein HJ, Nylander AG, Zhao CM, Sundler F, Håkanson R 1994 Acute responses of rat stomach enterochromaffin like cells to gastrin: secretory activation and adaptation. Gastroenterology 107:18–27 [DOI] [PubMed] [Google Scholar]
- Håkanson R, Ding XQ, Norlen P, Chen D 1995 Circulating pancreastatin is a marker for the enterochromaffin-like cells of the rat stomach. Gastroenterology 108:1445–1452 [DOI] [PubMed] [Google Scholar]
- Kimura K, Chen D, Lindstrom E, Zhao CM, Håkanson R 1997 Evidence that rat stomach ECL cells represent the main source of circulating pancreastatin. Regul Pept 68:177–180 [DOI] [PubMed] [Google Scholar]
- Torbergsen K, Wiksen H, Johansen K, Rahimipoor S, Falkmer UG, Zhao CM 2005 Immunoreactivity of gastric ECL and A-like cells in fasted and fed rats and mice. Biotech Histochem 80:21–30 [DOI] [PubMed] [Google Scholar]
- Chen D, Zhao CM, Norlén P, Björkqvist M, Ding XQ, Kitano M, Håkanson R 2000 Effect of cholecystokinin-2 receptor blockade on rat stomach ECL cells. A histochemical, electron-microscopic and chemical study. Cell Tissue Res 299:81–95 [DOI] [PubMed] [Google Scholar]
- Zhao CM, Chen D, Dornonville de la Cour C, Lindqvist A, Persson L, Håkanson R 2004 Histamine and histidine decarboxylase are hallmark features of ECL cells but not G cells in rat stomach. Regul Pept 118:61–66 [DOI] [PubMed] [Google Scholar]
- Ohning GV, Song M, Wong HC, Wu SV, Walsh JH 1998 Immunolocalization of gastrin-dependent histidine decarboxylase activity in rat gastric mucosa during feeding. Am J Physiol 275(4 Pt 1):G660–G667 [DOI] [PubMed] [Google Scholar]
- Dartsch C, Chen D, Persson L 1998 Multiple forms of rat stomach histidine decarboxylase may reflect posttranslational activation of the enzyme. Regul Pept 77:33–41 [DOI] [PubMed] [Google Scholar]
- Dartsch C, Chen D, Håkanson R, Persson L 1999 Histidine decarboxylase in rat stomach ECL cells: relationship between enzyme activity and different molecular forms. Regul Pept 81:41–48 [DOI] [PubMed] [Google Scholar]
- Pham T, Guerrini S, Wong H, Reeve Jr J, Sternini C 2002 Distribution of galanin receptor 1 immunoreactivity in the rat stomach and small intestine. J Comp Neurol 450:292–302 [DOI] [PubMed] [Google Scholar]
- Zhao CM, Chen D, Monstein HJ, Ding XQ, Sundler F, Håkanson R 1996 Rat stomach enterochromaffin-like cells are not stimulated by pylorus ligation. A biochemical and ultrastructural study. Scand J Gastroenterol 31:31–37 [DOI] [PubMed] [Google Scholar]
- Bakke I, Qvigstad G, Sandvik AK, Waldum HL 2001 The CCK-2 receptor is located on the ECL cell, but not on the parietal cell. Scand J Gastroenterol 36:1128–1133 [DOI] [PubMed] [Google Scholar]
- Kopin AS, Lee YM, McBride EW, Miller LJ, Lu M, Lin HY, Kolakowski Jr LF, Beinborn M 1992 Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc Natl Acad Sci USA 89:3605–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz F, Goke MN, Otte JM, Schrader H, Reimann B, Kruse ML, Siegel EG, Peters J, Herzig KH, Folsch UR, Schmidt WE 2001 Cellular expression of CCK-A and CCK-B/gastrin receptors in human gastric mucosa. Regul Pept 102:101–110 [DOI] [PubMed] [Google Scholar]
- Tømmerås K, Bakke I, Sandvik AK, Larsson E, Waldum HL 2002 Rat parietal cells express CCK(2) receptor mRNA: gene expression analysis of single cells isolated by laser-assisted microdissection. Biochem Biophys Res Commun 297:335–340 [DOI] [PubMed] [Google Scholar]
- Cabero JL, Li ZQ, Mardh S 1991 Gastrin potentiates histamine-stimulated aminopyrine accumulation in isolated rat parietal cells. Am J Physiol 261(4 Pt 1):G621–G627 [DOI] [PubMed] [Google Scholar]
- Cabero JL, Li ZQ, Mardh S 1993 Gastrin action on aminopyrine accumulation in isolated pig parietal cells requires cAMP. Biochim Biophys Acta 1177:245–252 [DOI] [PubMed] [Google Scholar]
- Athmann C, Zeng N, Scott DR, Sachs G 2000 Regulation of parietal cell calcium signaling in gastric glands. Am J Physiol Gastrointest Liver Physiol 279:G1048–G1058 [DOI] [PubMed] [Google Scholar]
- Wank SA 1995 Cholecystokinin receptors. Am J Physiol 269(5 Pt 1):G628–G646 [DOI] [PubMed] [Google Scholar]
- Wank SA 1998 G protein-coupled receptors in gastrointestinal physiology. I. CCK receptors: an exemplary family. Am J Physiol 274(4 Pt 1):G607–G613 [DOI] [PubMed] [Google Scholar]
- Lindström E, Håkanson R 2001 Neurohormonal regulation of secretion from isolated rat stomach ECL cells: a critical reappraisal. Regul Pept 97:169–180 [DOI] [PubMed] [Google Scholar]
- Aihara T, Fujishita T, Kanatani K, Furutani K, Nakamura E, Taketo MM, Matsui M, Chen D, Okabe S 2003 Impaired gastric secretion and lack of trophic responses to hypergastrinemia in M3 muscarinic receptor knockout mice. Gastroenterology 125: 1774–1784 [DOI] [PubMed] [Google Scholar]
- Chiba T, Fisher SK, Agranoff BW, Yamada T 1989 Autoregulation of muscarinic and gastrin receptors on gastric parietal cells. Am J Physiol 256(2 Pt 1):G356–G363 [DOI] [PubMed] [Google Scholar]
- Piqueras L, Taché Y, Martinez V 2003 Somatostatin receptor type 2 mediates bombesin-induced inhibition of gastric acid secretion in mice. J Physiol 549(Pt 3):889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SJ 1991 Histamine receptors and interactions between second messenger transduction systems. Agents Actions Suppl 33:145–159 [DOI] [PubMed] [Google Scholar]
- Wellner-Kienitz MC, Bender K, Meyer T, Pott L 2003 Coupling to Gs and G(q/11) of histamine H2 receptors heterologously expressed in adult rat atrial myocytes. Biochim Biophys Acta 1642:67–77 [DOI] [PubMed] [Google Scholar]
- Martinez V, Taché Y 2004 Somatostatin. In: Johnson LR, ed. Encyclopedia of gastroenterology. Boston: Academic Press; 426–433 [Google Scholar]
- Buscail L, Delesque N, Esteve JP, Saint-Laurent N, Prats H, Clerc P, Robberecht P, Bell GI, Liebow C, Schally AV, Vaysse N, Susini C 1994 Stimulation of tyrosine phosphatase and inhibition of cell proliferation by somatostatin analogues: mediation by human somatostatin receptor subtypes SSTR1 and SSTR2. Proc Natl Acad Sci USA 91:2315–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscail L, Esteve JP, Saint-Laurent N, Bertrand V, Reisine T, O’Carroll AM, Bell GI, Schally AV, Vaysse N, Susini C 1995 Inhibition of cell proliferation by the somatostatin analogue RC-160 is mediated by somatostatin receptor subtypes SSTR2 and SSTR5 through different mechanisms. Proc Natl Acad Sci USA 92:1580–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel YC 1999 Somatostatin and its receptor family. Front Neuroendocrinol 20:157–198 [DOI] [PubMed] [Google Scholar]
- Schmidt WE, Schmitz F 2004 Genetic dissection of the secretory machinery in the stomach. Gastroenterology 126:606–609 [DOI] [PubMed] [Google Scholar]