Abstract
T lymphocytes mature in the thymus to become functional T cells. Studies with chimeric mice and T cell receptor (TCR) transgenic (tg) mice have indicated that the major histocompatibility gene complex (MHC) of thymic radio-resistant (presumed to be epithelial) cells positively select the MHC-restricted T cell repertoire. Surprisingly, mice without a thymus reconstituted with an MHC-incompatible thymus generate effector T cells which are, in general, specific for the host and not for the thymic MHC. The present study reanalyzed this longstanding paradox in nude mice that were reconstituted with an MHC-incompatible thymus plus or minus immunologically defective bone marrow-derived cells or in nude mice expressing a transgenic T cell receptor. A pathway of thymus-dependent but thymic MHC-independent T cell maturation is revealed where expansion of the antiviral T cell repertoire depends on the MHC of bone marrow-derived cells. These results indicate an alternative, if not a general, pathway of T cell maturation and selection: the thymus may function essentially as an organ promoting T cell receptor expression; T cell specificity, however, reflects repertoire expansion plus cell survival and effector T cell induction driven by the MHC of bone marrow-derived cells. Therefore pure thymus defects can be efficiently reconstituted by allo- and xenogeneic thymic grafts.
Evidence for a key role of the major histocompatibility gene complex (MHC) of the radio-resistant part of the thymus in positively selecting MHC-restricted specificities of T cells has been obtained from two types of experiments. First, thymectomized and lethally irradiated H-2a/b F1 recipient mice reconstituted with lymphohemopoietic H-2a/b stem cells, or thymus-deficient H-2a/b F1 nude mice, both grafted with an H-2a thymus, generated T cells restricted virtually exclusively to the thymic H-2a (reviewed in refs. 1–4). The collective evidence from many experiments with irradiation chimeras [except for some double irradiation chimeras (5, 6)], and reconstituted nude mice (3, 7–12) rendered the possibility unlikely, but not completely impossible, that lymphohemopoietic cells contributed importantly to positive selection of T cells in the thymus. Second, T cell receptor (TCR) transgenic (tg) precursor T cells were shown to mature only in a thymus with the corresponding MHC (13–16). Nevertheless, these two sets of results were discussed and questioned (17–19) (reviewed in refs. 2, 4) from the very beginning because positive selection was rarely absolute. Key questions raised were whether the results with chimeras reflected (i) true positive selection by the thymic epithelial MHC determining the TCR repertoire generated independently of the immunizing antigen, (ii) “suppressive mechanisms” caused by allogeneic combinations of bone marrow-derived cells, thymus grafts, and irradiated or nude hosts used, (iii) specificity determined by the immunizing antigen alone as presented by bone marrow-derived cells, or (iv) combinations of the above possibilities. The above summarized experimental results were in fact in contrast to variable findings with H-2-incompatible irradiation chimeras (17, 20, 21) or with H-2a lymphocytes acutely depleted of anti-H-2b alloreactive T cells (18) and some experiments with irradiation bone marrow chimeras that implied either a variable role of antigen-presenting cells in positive selection (5, 11) or in expansion/survival of selected T cells (22–25), or at a level of induction, only without a need for positive selection (19) (reviewed in ref. 4). Additionally, some early experiments with chimeras suggested that sometimes the antigen-presenting cells of the chimeric host played a limiting role in induction of mature T cells (17, 19, 20, 26, 27). These latter experiments were partially based, however, on complicated protocols that require adoptive transfers (20) or multiple restimulations in vitro [and undefined multiple minor histocompatibility antigens (17)] or on assays that used hapten-specific (and often crossreactive) T cell responses (9); most experiments used few titrations so that the relevance of these data has remained unclear. On the other hand, experiments that used intrathymic transfer of cell lines (28–30) or adenovirus-induced expression of new MHC antigens in the thymus (31) suggested that perhaps positive selection by MHC expressed on thymic epithelial cells alone was possible. Surprisingly, studies with nude mice reconstituted with completely histoincompatible thymic grafts contradicted the decisive role of thymic epithelial cells in positive selection. Nude H-2d mice reconstituted with an incompatible H-2b thymus graft developed effector T cells exclusively restricted to the host H-2d (32). A possible key difference between thymically reconstituted nude vs. radiation bone marrow chimeras may have been that nude mice expressed the same MHC on all cells except for the allogeneic MHC on thymic epithelial and stromal cells. In contrast, irradiation bone marrow and thymically reconstituted chimeras may possess low unknown numbers of radioresistent peripheral lymphohemopoietic cells (33), follicular dendritic cells (34), lymphocytes, and other somatic cells that could still somehow be involved in shaping the T cell repertoire (reviewed in refs. 1, 2, 4).
The present studies took advantage of new mutant mice, to make a fresh attempt at examining the role of the MHC of bone marrow-derived cells in selecting T cell repertoires in thymus-deficient mice reconstituted with an allogeneic thymus. The experiments presented here confirm a key role of the thymic epithelial cells in T cell repertoire maturation. This step, at least under the conditions tested in nude mice, can be thymic MHC-independent and can be fulfilled by xenogeneic thymus grafts. The results reveal a key role of bone marrow-derived cells in T cell repertoire selection/expansion, in addition to their accepted role in T cell induction.
MATERIALS AND METHODS
Experimental Animals.
All mice, except where noted, were bred at the Institute for Experimental Animals, University of Zürich, and kept under specific pathogen-free conditions and in sterile Laminar flow conditions after reconstitution. The locally bred nude ICR are positive for H-2Lq and negative for H-2b. Nude BALB/c (H-2d), nude C57BL/6 (H-2b), and nude F1 were purchased from BRL, Füllinsdorf, Switzerland; nude CBA (H-2k) were purchased from CRC, London. SCID (H-2d) mice were from Iffa Credo, Orléans, France; breeding pairs of SCID (H-2k) on a C3H background had been obtained from the Jackson Laboratory; RAG 10/0 mice were from S. Tonegawa, Massachusetts Institute of Technology, Boston; MHC class I + II0/0 mice were a gift from D. Mathis and C. Benoist, Strasbourg, France, and were bred locally. RAG 10/0 (H-2k and H-2d) were a gift from the Basel Institute of Immunology, Basel, Switzerland. The TCR tg line 318 has been described elsewhere (35).
Cell Lines, Dendritic Cells, Cr-Release Assay, and Virus.
MC57G (H-2b), L929 (H-2k), EL-4 (H-2b), and P815 (H-2d) cells (from the American Type Culture Collection) have been used widely. The Cr-release assay and lymphocytic choriomeningitis virus (LCMV) (WE strain) have been previously described (12, 35).
Reconstitution.
Recipient nude mice were irradiated with 4.5 Gy 1 day before reconstitution. Thymi of 14- to 15-day-old fetal donors or thymic fragments of adult mice were transplanted under the kidney capsule under general anesthesia; fetal liver cells or adult spleen cells plus bone marrow cells (2–5 × 107) were transfused i.v. on the same day (32, 36).
Flow Cytometric Analysis.
Peripheral blood or splenic cells were stained with the following antibodies and were analyzed in a FACScan (Becton Dickinson) (12, 35): anti-CD4FITC (cat. 01064D), anti-CD8 FITC (cat. 031113), anti-CD8 biotin (cat. 01042D), anti-Vα2 PE (cat. 01655A), FITC-anti-Vβ8 1.2 (cat. 01342c), TRI-anti-Vβ8 1.2 (cat. 01342c), anti-Db biotin (cat. 06112D), anti-Dd biotin (cat. 06132D), and anti-Dq biotin (cat. 06162) were from PharMingen; anti-q (30–5-7 was a gift of U. Koszinowski and H. Hengel, Maximilian University of Munich, Germany).
RESULTS
Characterization of the Experimental Model.
Nude mice of H-2q, H-2b, H-2d, H-2k or of H-2b/d F1 type were reconstituted with thymic grafts of various allogeneic H-2 types alone or together with immunologically inert lymphohemopoietic cells from genetically T and B cell-defective thymus donor mice (i.e., from SCID or RAG-10/0 mice). Several of these nude mice were examined at 14–16 days of fetal life or as young adults before and after 3–5 months of reconstitution for histological evidence of thymus rudiments; they were always tiny and were devoid of lymphocytes (reviewed in refs. 8, 37) (36, 38).
Initially, some of the original key experiments were repeated: (H-2b xH-2d) F1 nude mice (irradiated with 4.5 Gy) were reconstituted with H-2b RAG-10/0 or H-2d SCID or H-2d RAG-10/0 thymic grafts from day 14 to 15 fetal donors (or thymus fragments of adult mice with identical results, not shown). After 12–16 weeks, these reconstituted mice were immunized with LCMV [2 × 102 plaque-forming units (pfu) i.v.]. In a primary cytotoxic T lymphocyte (CTL) assay 8 days later, the specific cytotoxic T cell response was found to be restricted to the thymic H-2 (Table 1, Exp. 1), and virus had been eliminated from spleen and liver (results not shown). These data confirmed earlier results (reviewed in refs. 1, 36) and, because immunodeficient donors were used, established unequivocally that possibly contaminating T or B cells from the graft could not have skewed H-2 restriction via H-2-specific “suppressive mechanisms” (reviewed in refs. 1, 2, 4, 16).
Table 1.
H-2 restriction of nude T cells after reconstitution with thymus grafts without or together with immunodefective bone marrow-derived cells
| Exp. | Recipient* | Donor
|
Specific lysis of targets, (%)
|
||||
|---|---|---|---|---|---|---|---|
| Thymus | Bone marrow- derived cells | MC57 (H-2b) | P185 (H-2d) | ||||
| LCMV | Uninf. | p118 | Uninf. | ||||
| 1 | Nude (H-2b × H-2d) | H-2b | None | 84 | 4 | 15 | 6 |
| (C57BL/6 × BALB/c) F1 | RAG0/0 | 74 | 3 | 5 | 7 | ||
| H-2d | None | 19 | 10 | 64 | 6 | ||
| SCID | 11 | 1 | 70 | 3 | |||
| H-2d | None | 2 | 2 | 56 | 1 | ||
| RAG0/0 | <1 | <1 | 46 | 2 | |||
| MC57 (H-2b) | L929 (H-2k) | ||||||
| 2 | Nude (H-2b) C57BL/6 (>95%) | H-2k | None (<8%) | 69 | 7 | 14 | 15 |
| RAG0/0 | 52 | 5 | 11 | 12 | |||
| 35 | 1 | 7 | 7 | ||||
| 24 | 2 | 5 | 4 | ||||
| Nude (H-2b) C57BL/6 (>95%) | H-2k | None | 99 | 14 | 19 | 25 | |
| RAG0/0 | 77 | 7 | 12 | 14 | |||
| 42 | 1 | 4 | 14 | ||||
| 32 | 3 | 3 | 17 | ||||
| Nude (H-2b) C57BL/6 (60%) | H-2k | H-2k (40%) | 23 | 35 | 70 | 19 | |
| RAG0/0 | RAG0/0 | 14 | 11 | 74 | 22 | ||
| 7 | 5 | 69 | 14 | ||||
| 9 | 2 | 44 | 8 | ||||
| Nude (H-2b) C57BL/6 (50%) | H-2k | H-2k (50%) | 68 | 11 | 71 | 35 | |
| RAG0/0 | RAG0/0 | 49 | 5 | 67 | 24 | ||
| 27 | 3 | 60 | 16 | ||||
| 12 | 1 | 44 | 12 | ||||
| Controls: | |||||||
| C3H (H-2k) | 16 | 12 | 76 | 30 | |||
| C57BL/6 (H-2b) | 97 | 9 | 32 | 27 | |||
The data shown are representative for three comparable experiments for Exp. 1, a total of four to eight mice each tested in Exp. 2 and 3. uninf., uninfected.
Mice were infected with 102 pfu of LCMV; 7 or 8 days later, CTL activity was tested in a 5-h assay on the indicated targets at spleen cell-to-target cell ratios of 90, 30, 10, or 3:1 [except for Exp. 1 and controls, where only 90 and 30 or 90 are shown (top to bottom)]. Bold values are significant above relevant controls (p < 0.1). Numbers in parentheses are % of lymphocytes of the indicated H-2.
Reconstitution of Nude Mice with Completely MHC-Incompatible Thymus Grafts Plus or Minus Immunodefective Bone Marrow-Derived Cells.
In a second series of experiments, nude H-2b mice treated with an H-2 incompatible H-2k- thymic graft under the kidney capsule alone generated host H-2b-restricted but not thymic H-2k-restricted effector T cells (Table 1, Exp. 2). Virus was below detectable levels in spleens and livers (not shown). Spleen cells from unprimed chimeras were also transferred to irradiated (8.5 Gy H-2b × H-2k) F1 recipients that then were infected with LCMV or to H-2k nude recipients. Eight days later, only chimeric donor, but not reconstituting thymic H-2-restricted CTL activity, was measured (data not shown). These data confirmed our earlier results (32) and indicated that the observed lack of thymic H-2-restricted effector T cell activity is not caused simply by absence of appropriate antigenic-specific stimulation.
In parallel experiments, we attempted to imitate the postulated possibility that irradiation bone marrow chimeras may contain low levels of surviving lymphohemopoietic cells that could influence positive selection (see above). Therefore, nude mice were reconstituted with a thymic graft plus bone marrow- derived cells from H-2-incompatible and immune-defective SCID or RAG-10/0 donors. The thymic grafts exhibited conventional thymic cortex and medulla when examined histologically (not shown). Surprisingly, and in contrast to thymus-only reconstituted nude mice, nude H-2b mice given a thymus plus T and B cell-defective bone marrow-derived cells from H-2k RAG-10/0 mice mounted a response specific for both host and donor H-2; sometimes the response was biased for donor H-2k (Table 1, Exp. 2). As a control, we tested several nude mice reconstituted with bone-marrow cells and/or spleen cells from allogeneic SCID or RAG 10/0 mice alone without a thymus graft; as expected, these mice failed to generate antiviral T cell responses (not shown). These results were confirmed in additional experiments where nude ICR (H-2q) mice were reconstituted with H-2b RAG-10/0 thymi with or without the addition of immunodeficient H-2b RAG-10/0 bone marrow, fetal liver, or spleen cells. These latter mice were infected with LCMV and tested for primary and secondary cytotoxic T cell activity against H-2b or H-2q targets infected with LCMV or pulsed with the corresponding T cell epitope peptides (12). Again, nude host H-2q-restricted but no donor H-2b-restricted primary and secondary CTLs were generated in thymus-only reconstituted mice, but both host- and donor-restricted T cell responses were mounted if the reconstitution included immunodefective lymphohemopoietic cells (Table 2, Exp. 3). These experiments indicated that mice bearing a fetal thymic graft alone or a thymic plus lymphohemopoietic graft were tolerant to the allogeneic thymic MHC as tested by CTL responses. Together with contrasting earlier results (32, 39), but not others (reviewed in ref. 40), this question needs further evaluation. Corresponding results were obtained with H-2b nude mice reconstituted with H-2d SCID or BALB/c (H-2d) nude mice with a RAG 10/0 (H-2b) allogeneic thymic graft plus or minus T plus B cell-defective bone marrow-derived cells (not shown). Taken together, the presented results document a decisive role of the MHC of lymphohemopoietic but not of the thymic MHC in selection of the general and specific T cell repertoire in nude mice. The results were obtained with several H-2 haplotype combinations; therefore, the findings seem generally valid, and it is unlikely that unrecognized crossreactivities or complementation phenomena could explain the results.
Table 2.
H-2 restriction of nude T cells after reconstitution with thymus grafts without or together with immunodefective bone marrow-derived cells
| Exp. | Recipient* | Donor
|
Specific lysis of targets, (%)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary response
|
Secondary response after restimulation with
|
||||||||||
| C57BL/6 (H-2b)
|
ICR (H-2q)
|
||||||||||
| MC57 (H-2b)
|
DBA1 (H-2q)
|
MC57 (H-2b)
|
DBA1 (H-2q)
|
||||||||
| Thymus | Bone marrow- derived cells | LCMV | Uninf. | p118 | Uninf. | LCMV | Uninf. | p118 | Uninf. | ||
| 3 | Nude (H-2q) | H-2b | None | <1 | 23 | 80 | <1 | 9 | <1 | 88 | 27 |
| ICR (>95%) | RAG0/0 | <1 | 16 | 55 | <1 | 5 | <1 | 90 | 11 | ||
| 3 | 1 | 35 | <1 | 3 | <1 | 68 | 3 | ||||
| <1 | <1 | 13 | <1 | 3 | <1 | 41 | <1 | ||||
| Nude (H-2q) | H-2b | H-2b | 70 | <1 | 80 | <1 | 92 | <1 | 86 | 26 | |
| ICR (70%) | RAG0/0 | RAG0/0 (30%) | 45 | 2 | 80 | 2 | 67 | <1 | 71 | 6 | |
| 38 | <1 | 58 | <1 | 40 | <1 | 49 | 3 | ||||
| 17 | <1 | 34 | <1 | 18 | <1 | 25 | 3 | ||||
| Controls: | |||||||||||
| ICR (H-2q) | 6 | 2 | 69 | <1 | † | 82 | 105 | 27 | |||
| C57BL/6 (H-2b) | 81 | 12 | 27 | 24 | 100 | 30 | † | 96 | |||
The data shown are representative for three comparable experiments for Exp. 1, a total of four to eight mice each tested in Exp. 2 and 3; uninf., uninfected.
Mice were infected with 102 pfu of LCMV; 7 or 8 days later, CTL activity was tested in a 5-h assay on the indicated targets at spleen cell-to-target cell ratios of 90, 30, 10, or 3:1 (top to bottom). Secondary restimulation in vitro for Exp. 3 was for 5 days with infected indicated irradiated splenocytes as stimulator cells. Cultures were used undiluted or diluted 3-, 9-, or 27-fold (top to bottom) and assayed in the standard 51Cr release assay for 5 h. Bold values are significant above relevant controls (p < 0.1). Numbers in parentheses are % of lymphocytes of the indicated H-2.
Alloreactive CTL activity lysed infected and uninfected targets comparably.
Reconstitution with MHC Class I + II0/0 and Rat Thymic Grafts.
Nude C57BL/6 (H-2b) mice were reconstituted with d16 fetal thymi from MHC class I + II0/0 H-2b or from near-term Lewis rat donors (Table 3). These chimeras mounted a good primary anti-LCMV CTL response 12–16 weeks after reconstitution. In the same series of experiments, we also tested some ICR nude (H-2q) mice reconstituted with fetal MHC-Class I0/0 (β2-microglobulin0/0) thymi; these thymic chimeras also mounted a primary H-2b-restricted conventional antiviral CTL response 8 days after LCMV infection (not shown). This is in contrast to MHC-class I0/0 mice, which when infected as adults fail to mount an efficient CD8+ T cell response (25, 41).
Table 3.
The capacity of MHC-deficient murine thymi or rat thymi to reconstitute CD8+ T cell maturation and effector function in nude mice
| Recipient | Donor Thymus | Specific lysis of target cells, (%)
|
|
|---|---|---|---|
| MC57 (H-2b)
| |||
| LCMV | Uninf. | ||
| Nude (H-2b) | MHC | 72 | <1 |
| C57BL/6 | Class I0/0 | 74 | <1 |
| Class II0/0 | 50 | <1 | |
| (H-2b) | 47 | <1 | |
| Nude (H-2b) | Lewis | 80 | 7 |
| C57BL/6 | (Rat)0 | 54 | 3 |
| 31 | 2 | ||
| 19 | 1 | ||
| Nude (H-2b) | None | 5 | 3 |
| Control: | |||
| C57BL/6 | None | 85 | 6 |
Control values for LCMV-immune but H-2 incompatible effector CTLs or normal spleen cells were below 10% on peptide loaded or infected or normal targets. One example of two to three tested similar thymically reconstituted nude mice infected with 102 pfu of LCMV (8 days previously) is shown. Effector-to-target cell ratios were 90, 30, 10, 3:1 or 90:1. Bold values represent significant values above various controls (p < 0.1).
uninf., uninfected.
T Cell Maturation in Nude Mice Expressing a tg TCR.
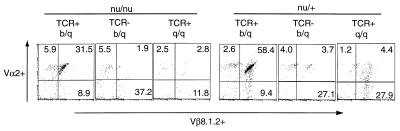
To analyze further the role of thymus-independent peripheral selection of the T cell repertoire, a second experimental approach was used. If the thymus was essentially an organ promoting TCR rearrangement and expression independent of thymic MHC, highly frequent T cells with an already rearranged tg TCR+ may expand in the absence of a thymus, but may depend on the fitting MHC on lymphohemopoietic cells. To test this possibility, we crossed mice transgenic for a TCR specific for LCMV-GP p33 plus Db [line 318 TCR Vα2, Vβ8.2, H-2b (15)] to nude (H-2q) mice and screened for nude tg TCR+ offspring that expressed H-2b/q or H-2q/q. We first examined euthymic H-2q/q that possessed the tg TCR as revealed by PCR typing (15); these mice failed to express the 318 TCR above background level when analyzed by FACS with a Vα2-specific antibody (Fig. 1). LCMV-immune splenocytes of euthymic tg TCR+ H-2q/q mice failed to lyse p33 + Db targets, but lysed p118 + H2q targets (legend to Table 4). In contrast, euthymic H-2b/q mice possessing the tg TCR were positive by PCR typing and expressed Vα2 tg TCR+ T cells at the expected level of about 50% of CD8+ T cells (Fig. 1); their LCMV-immune spleen cells lysed p33 + Db targets efficiently (legend to Table 4). These results showed that the 318 TCR was neither positively nor negatively selected in ICR (H-2q) mice (Fig. 1). When blood lymphocytes of nude mice that were positive for the tg TCR by PCR were tested by FACS for Vα2+ Vβ8 1.2+ tg TCR CD8+ T cells (Fig. 1) or were analyzed for Vα2+ CD4− CD8+ cells (Table 4), selective expansion of tg TCR+ CD8+ T cells was evident in H-2b/q but not in H-2q/q tg TCR+ nude mice (Table 4; Fig. 1); in TCR+ H-2b/q, nude mice numbers of CD4− 8+ T cells were increased at least 10 times (Table 4; Fig. 1). As expected from earlier reports on (possibly oligoclonal) T cells in nude mice expressing CD8 and/or TCR-Vα or Vβ (refs. 42, 43, but note that relevant background values for fetally thymectomized mice are not available), the total number of CD8+ T cells and tg TCR+ T cells in nu/nu mice was about 10- to 100-fold lower, respectively, than in thymus-competent mice (Table 4). After infection with LCMV, all nude mice had comparable total spleen cell numbers within a factor of 2–3. H-2b/q tg TCR+ nude mice generated a potent cytotoxic tg T cell response specific for p33 + Db and no response to p118 + Lq (Table 4). In contrast, H-2q/q nude mice that were typed positive for the tg TCR by PCR did not exhibit measurable primary LCMV plus Db or Lq specific cytotoxic T cell activity (Table 4). Day 7-immune CD4− CD8+ T cells of tg TCR+ nude H-2b/q mice mostly expressed the tg TCR (i.e., >90%, Table 3, 12.8/13.4; 8.7/9.4); the proportion had increased at least 10-fold and therefore tg TCR+ T cells were overall at least 100-fold, but probably considerably more numerous than in the various control nude mice (Table 4 and Fig. 1). These tg TCR+ CD8+ T cells exhibited levels of CD8 and TCR expression within normal ranges. Thus the tg 318 TCR matured in nude mice expanded (although less than in thymus competent controls) independent of a thymus but dependent on the MHC of bone marrow-derived cells.
Figure 1.
Positive selection of tg TCR+ T cells in nude H-2b/q but not nude H-2q/q mice. Expression of the tg TCR318 (Db + LCMV-GP33–41, Vα2+, Vβ8 1.2+) on CD8+ T cells in (ICR nu/nu H-2q × TCR318 H-2b) × ICR nu/nu H-2q offspring. Mice that were nu/nu or nu/+ were typed by PCR as tg TCR+ or tg TCR−. Peripheral blood lymphocytes of 8- to 15-wk-old mice were gated on CD8+ to determine percentage of the Vα2+ Vβ8 1.2+ cells (Upper Right).
Table 4.
Maturation of tg TCR+ CD8+ T cells in nude mice: Dependence on host MHC
| Specific lysis of targets (%)
|
||||||
|---|---|---|---|---|---|---|
| CD4−CD8+ (%)†
|
MC57 H-2b
|
DBA1 H-2b
|
||||
| CD4−CD8+V α 2+(%)
|
p33
|
Nor.
|
p118
|
Nor.
|
||
| Mouse* | Naive blood | d7 LCMV spleen | ||||
| nu/nu | <0.1 | 0.5 | 9 | 5 | 9 | <1 |
| TCR 318+ | <0.1 | 0.1 | 3 | 9 | 7 | <1 |
| H-2q/q | <1 | 2 | 3 | <1 | ||
| 1 | 1 | 2 | <1 | |||
| nu/nu | <0.1 | 0.6 | 9 | 10 | 5 | <1 |
| TCR 318+ | <0.1 | 0.1 | 7 | 10 | 7 | <1 |
| H-2q/q | 6 | 5 | 4 | <1 | ||
| 5 | 7 | 5 | <1 | |||
| nu/nu | 0.9 | 13.4 | 65 | 16 | 10 | 10 |
| TCR 318+ | 0.7 | 12.8 | 55 | 14 | 14 | 6 |
| H-2b/q | 23 | 7 | 10 | <1 | ||
| 14 | 6 | 7 | <1 | |||
| nu/nu | 1.5 | 9.4 | 73 | 17 | 16 | 18 |
| TCR 318+ | 1.1 | 8.7 | 47 | 15 | 18 | 10 |
| H-2b/q | 25 | 6 | 10 | 6 | ||
| 9 | 1 | 2 | <1 | |||
| Controls: | ||||||
| ICR | 8.0 | 18.2 | 16 | 15 | 65 | <1 |
| H-2q/q | 0.5 | 0.6 | 10 | 7 | 70 | <1 |
| C57BL/6 | 12.2 | 50.4 | 100 | 10 | 15 | 15 |
| H-2b/b | 1.1 | 4.5 | 93 | 10 | 16 | 18 |
| TCR 318 | 15.4 | 28.4 | 44 | 12 | 14 | 15 |
| H-2b/b | 8 | 18 | 24 | 4 | 9 | 2 |
Significant values are bold. Nor., normal targets.
Mice were infected i.v. with 102 pfu of LCMV-WE and CTL activity tested in a standard 51Cr release assay. Spontaneous release was <15% on MC57 and <20% on DBA 1 targets; the % lysis figures shown are for 90, 30, 10, and 3:1 or 90, 30:1 target cells from top to bottom.
The results are expressed as % of living nucleated cells and are representative of 8 H-2q/q and 10 H-2b/q nude tg TCR+ mice tested. LCMV immune spleen cells from euthymic nu/+TCR 318 H-2q/q mice lysed p 33 MC57 targets tested at 5, 1, 1, or 1% vs. normal MC57 6, 4, 1, or 1%, p118 DBA1 targets to 91, 92, 67, 42% vs. normal DBA1 targets 11, 18, 17, or 16%. Thus there is no relevant positive selection of functional tgTCR on H-2q (see also Fig. 1).
DISCUSSION
Together, the two series of experiments with nude mice question the exclusive role of thymic epithelial cells in shaping the TCR repertoire and document an important role of bone marrow-derived cells in this process. The results indicate that, under the usual very low T cell precursor frequency conditions in nude mice, specific effector T cells can mature only dependent on a grafted thymus, which apparently functions largely as a differentiation and TCR-gene rearrangement-promoting organ for T cells. In contrast, specific TCR tg precursor T cells with an already rearranged tg TCR, which are at least 104–105 times more frequent than other precursor T cells, are able to mature in the absence of a thymus. Expansion of these TCR-matured or tg TCR+ precursor T cells seems to be largely guided by MHC-expressing lymphohemopoietic cells (22–24). Earlier findings of limited alloreactivity found particularly in aged nude mice (44) probably reflect similar thymus-independent expansion mechanisms where the relatively high frequency of alloreactive or hapten-specific T cells may become detectable. Together, these data are compatible with several earlier findings suggesting that alloreactivity derives largely from crossreactivity of self-MHC-restricted T cells (reviewed in refs. 14, 45, 46), and they could perhaps suggest that TCR interact with MHC peptide complexes in a more random fashion than has been suggested by available crystallographic analyses so far (reviewed in ref. 47).
The present experiments with nude mice reconstituted with a histoincompatible or even xenogeneic thymic graft established that the MHC of the thymic epithelium is not really limiting the TCR repertoire that is shaped by the MHC of bone marrow-derived cells. Nevertheless, attempts at dissecting the relative importance of bone marrow-derived cells in repertoire selection/expansion vs. survival-promotion vs. induction of effector T cells have not been successful so far. We have obtained only negative evidence that the induction step alone is limiting (32). If antigen-specific induction by antigen-presenting cells expressing the thymic MHC alone had limited demonstration of thymus-allogeneically restricted nude recipient T cells, one should have expected that this specificity is revealed after adoptive immunization experiments. The negative results therefore indicate that there is an antigen-independent general selection/expansion/survival mechanism (22, 24, 25, 48) yielding increased relative precursor frequencies of CTL corresponding to the MHC of bone marrow-derived cells. Whether this reflects crossreactivity between MHC alleles or even xenogeneic and host MHC or suggests that TCR–MHC interactions represent a continuous spectrum of specificities from which effector T cells are induced (see also proposal in ref. 4) remains open. In the absence of direct and clearer results on an exclusive role of thymic epithelial cells in TCR repertoire selection, the meaning of the term “positive selection” may have to be changed to reflect general selection/expansion/survival and, as stated above, to also include induction. A clear dissection of these interrelated steps remains to be done, but may be difficult to obtain.
In conclusion, the new experimental mutant mice discovered and developed during the past 20 years made it possible to take a fresh look at the role of the radio-resistant thymus, i.e., thymus epithelial cells, vs. that of lymphohemopoietic cells in T cell repertoire selection. Together with earlier experimental evidence from thymus and bone marrow chimeras, this study indicates that MHC-restricted T cell maturation and expansion is most efficient if thymus and T cell precursors and bone marrow-derived cells all share the same MHC. Although the thymus is critically important for T cell maturation resulting in TCR expression, apparently independently of thymic MHC, our results reveal an alternative, if not perhaps a general, pathway of T cell repertoire selection/expansion driven by the MHC of bone marrow-derived cells. Therefore, positive and negative selection, although most efficiently done in the thymus, may not depend on distinct cell types and may not represent two separable steps, but could result from one single affinity/avidity-driven event in any organized lymphoid tissue. Accordingly positive but also negative selection usually takes place in the thymus not by absolute necessity, but because all three events, i.e., thymus-enhanced TCR expression, T cell expansion, and T cell deletion, usually coincide, i.e., are very close with respect to timing and localization (reviewed in refs. 3, 16, 49–51). Our results have obvious medical implications because they help to explain that MHC incompatible and even xenogeneic thymus transplants may offer thymus-deficient patients functional reconstitution of protective host MHC restricted T cells (7, 8, 21, 32, 52).
Acknowledgments
We thank Dr. D. Mathis and C. Benoist for MHC I+II0/0 mice, U. Koszinowski and H. Hengel for anti-H-2q antibody, and E. Wagner, Basel Institute for Immunology, for Rag0/0 mice. This work was supported by the Swiss National Science Foundation and the Kanton Zürich.
ABBREVIATIONS
- MHC
major histocompatibility gene complex
- TCR
T cell receptor
- tg
transgenic
- LCMV
lymphocytic choriomeningitis virus
- CTL
cytotoxic T lymphocyte
- pfu
plaque-forming units
References
- 1.Moller G. Immunol Rev. 1978;42:3–270. [Google Scholar]
- 2.Singer A. J Immunol. 1988;140:2481–2483. [PubMed] [Google Scholar]
- 3.Möller G. Immunol Rev. 1993;135:5–240. [Google Scholar]
- 4.Matzinger P. Immunol Rev. 1993;135:81–117. doi: 10.1111/j.1600-065x.1993.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 5.Longo D L, Schwartz R H. Nature (London) 1980;287:44–46. doi: 10.1038/287044a0. [DOI] [PubMed] [Google Scholar]
- 6.Longo D L, Kruisbeek A M, Davis M L, Matis L A. Proc Natl Acad Sci USA. 1985;82:5900–5904. doi: 10.1073/pnas.82.17.5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Law L W. Nature (London) 1966;210:1118. doi: 10.1038/2101118a0. [DOI] [PubMed] [Google Scholar]
- 8.Kindred B. Prog Allergy. 1979;26:137–238. [PubMed] [Google Scholar]
- 9.Bradley S M, Kruisbeek A M, Singer A. J Exp Med. 1982;156:1650–1664. doi: 10.1084/jem.156.6.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruisbeek A M, Sharrow S O, Singer A. J Immunol. 1983;130:1027–1032. [PubMed] [Google Scholar]
- 11.Bix M, Raulet D. Nature (London) 1992;359:330–333. doi: 10.1038/359330a0. [DOI] [PubMed] [Google Scholar]
- 12.Speiser D E, Stubi U, Zinkernagel R M. Nature (London) 1992;355:170–172. doi: 10.1038/355170a0. [DOI] [PubMed] [Google Scholar]
- 13.Kisielow P, Teh H S, Bluthmann H, von Boehmer H. Nature (London) 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 14.Sha W C, Nelson C A, Newberry R D, Kranz D M, Russell J H, Loh D Y. Nature (London) 1988;336:73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- 15.Pircher H, Bürki K, Lang R, Hengartner H, Zinkernagel R M. Nature (London) 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 16.von Boehmer H. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]
- 17.Matzinger P, Mirkwood G. J Exp Med. 1978;148:84–92. doi: 10.1084/jem.148.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doherty P C, Bennink J C. J Exp Med. 1979;149:150–157. doi: 10.1084/jem.149.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner H, Rollinghoff M, Rodt H, Thierfelder S. Eur J Immunol. 1980;10:521–525. doi: 10.1002/eji.1830100707. [DOI] [PubMed] [Google Scholar]
- 20.Zinkernagel R M, Callahan G N, Althage A, Cooper S, Streilein J W, Klein J. J Exp Med. 1978;147:897–911. doi: 10.1084/jem.147.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onoe K, Fernandes G, Good R A. J Exp Med. 1980;151:115–132. doi: 10.1084/jem.151.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rocha B, von Boehmer H. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 23.Kisielow P, Miazek A. J Exp Med. 1995;181:1975–1984. doi: 10.1084/jem.181.6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirberg J, Berns A, von Boehmer H. J Exp Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nesic D, Vukmanovic S. J Immunol. 1998;160:3705–3712. [PubMed] [Google Scholar]
- 26.Doherty P, Walsh F S. Neurosci Lett. 1989;96:1–6. doi: 10.1016/0304-3940(89)90233-4. [DOI] [PubMed] [Google Scholar]
- 27.Zinkernagel R M, Sado T, Althage A, Kamisaku H. Eur J Immunol. 1984;14:14–23. doi: 10.1002/eji.1830140104. [DOI] [PubMed] [Google Scholar]
- 28.Vukmanovic S, Grandea A G, III, Faas S J, Knowles B B, Bevan M J. Nature (London) 1992;359:729–732. doi: 10.1038/359729a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hugo P, Kappler J W, Godfrey D I, Marrack P C. Nature (London) 1992;360:679–682. doi: 10.1038/360679a0. [DOI] [PubMed] [Google Scholar]
- 30.Pawlowski T, Elliott J D, Loh D Y, Staerz U D. Nature (London) 1993;364:642–645. doi: 10.1038/364642a0. [DOI] [PubMed] [Google Scholar]
- 31.Nakano N, Rooke R, Benoist C, Mathis D. Science. 1997;275:678–683. doi: 10.1126/science.275.5300.678. [DOI] [PubMed] [Google Scholar]
- 32.Zinkernagel R M, Althage A, Waterfield E, Kindred B, Welsh R M, Callahan G, Pincetl P. J Exp Med. 1980;151:376–399. doi: 10.1084/jem.151.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz S I, Tamaki K, Sachs D H. Nature (London) 1979;282:324–326. doi: 10.1038/282324a0. [DOI] [PubMed] [Google Scholar]
- 34.Humphrey J H, Grennan D, Sundaram V. Eur J Immunol. 1984;14:859–864. doi: 10.1002/eji.1830140916. [DOI] [PubMed] [Google Scholar]
- 35.Kyburz D, Aichele P, Speiser D E, Hengartner H, Zinkernagel R M, Pircher H. Eur J Immunol. 1993;23:1956–1962. doi: 10.1002/eji.1830230834. [DOI] [PubMed] [Google Scholar]
- 36.Zinkernagel R M, Althage A, Callahan G. J Exp Med. 1979;150:693–697. doi: 10.1084/jem.150.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zinkernagel R M. Immunol Rev. 1978;42:224–270. doi: 10.1111/j.1600-065x.1978.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 38.Salaun J, Bandeira A, Khazaal I, Calman F, Coltey M, Coutinho A, Le Douarin N M. Science. 1990;247:1471–1474. doi: 10.1126/science.247.4949.1471. [DOI] [PubMed] [Google Scholar]
- 39.von Boehmer H, Schubiger K. Eur J Immunol. 1984;14:1048–1052. doi: 10.1002/eji.1830141116. [DOI] [PubMed] [Google Scholar]
- 40.Le Douarin N, Corbel C, Bandeira A, Thomas-Vaslin V, Modigliani Y, Coutinho A, Salaun J. Immunol Rev. 1996;149:35–53. doi: 10.1111/j.1600-065x.1996.tb00898.x. [DOI] [PubMed] [Google Scholar]
- 41.Muller D, Koller B H, Whitton J L, LaPan K E, Brigman K K, Frelinger J A. Science. 1992;255:1576–1578. doi: 10.1126/science.1347959. [DOI] [PubMed] [Google Scholar]
- 42.MacDonald H R, Lees R K, Bron C, Sordat B, Miescher G. J Exp Med. 1987;166:195–209. doi: 10.1084/jem.166.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rocha B. Eur J Immunol. 1990;20:919–925. doi: 10.1002/eji.1830200430. [DOI] [PubMed] [Google Scholar]
- 44.Hunig T, Bevan M J. J Exp Med. 1980;152:688–702. doi: 10.1084/jem.152.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherman L A, Chattopadhyay S. Annu Rev Immunol. 1993;11:385–402. doi: 10.1146/annurev.iy.11.040193.002125. [DOI] [PubMed] [Google Scholar]
- 46.Obst R, Munz C, Stevanovic S, Rammensee H G. Eur J Immunol. 1998;28:2432–2443. doi: 10.1002/(SICI)1521-4141(199808)28:08<2432::AID-IMMU2432>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 47.Garboczi D N, Biddison W E. Immunity. 1999;10:1–7. doi: 10.1016/s1074-7613(00)80001-1. [DOI] [PubMed] [Google Scholar]
- 48.Kisielow P, von Boehmer H. Adv Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]
- 49.Fink P J, Bevan M J. Adv Immunol. 1995;59:99–133. doi: 10.1016/s0065-2776(08)60630-6. [DOI] [PubMed] [Google Scholar]
- 50.Pennisi E. Science. 1996;271:1665–1667. doi: 10.1126/science.271.5256.1665. [DOI] [PubMed] [Google Scholar]
- 51.Bevan M J. Immunity. 1997;7:175–178. doi: 10.1016/s1074-7613(00)80520-8. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Y, Fishman J A, Sergio J J, Oliveros J L, Pearson D A, Szot G L, Wilkinson R A, Arn J S, Sachs D H, Sykes M. J Immunol. 1997;158:1641–1649. [PubMed] [Google Scholar]