Abstract
The cyclooxygenase-2 (COX2)-dependent inhibition of Leydig cell steroidogenesis has been demonstrated. To understand the mechanism for this effect of COX2, the present study examined the role of an enzyme downstream of COX2, namely thromboxane A synthase (TBXAS), in steroidogenesis. Inhibition of TBXAS activity with the inhibitor furegrelate induced a concentration-dependent increase in cAMP-induced steroidogenic acute regulatory (StAR) protein in MA-10 mouse Leydig cells. The increase in StAR protein occurred concomitantly with a significant increase in steroid hormone production. Similar results were obtained in StAR promoter activity assays and RT-PCR analyses of StAR mRNA levels, suggesting that inhibition of TBXAS activity enhanced StAR gene transcription. These observations were corroborated when TBXAS expression was specifically inhibited by RNA interference. Although the RNA interference reduced mRNA levels of TBXAS, it increased StAR mRNA levels, StAR protein, and steroidogenesis. Additional studies indicated that inhibition of TBXAS activity reduced DAX-1 protein, a repressor in StAR gene transcription. In the absence of cAMP, inhibition of TBXAS activity did not induce a significant increase in steroid hormone and StAR protein. However, addition of a low level of cAMP analogs dramatically increased steroidogenesis. Lastly, inhibition of protein kinase A activity essentially abolished the steroidogenic effect of the TBXAS inhibitor. Thus, the results from the present study suggest that a minimal level of protein kinase A activity is required for the steroidogenic effect of the TBXAS inhibitor and that inhibition of TBXAS activity or its expression increase the steroidogenic sensitivity of MA-10 mouse Leydig cells to cAMP stimulation.
IT IS WELL DOCUMENTED that trophic hormone stimulation of testicular Leydig cells induces cAMP formation followed by activation of protein kinase A (PKA) (1). PKA phosphorylates the transcription factors regulating gene expression of the steroidogenic acute regulatory (StAR) protein (2), which is critical for transfer of the substrate cholesterol to the inner mitochondrial membrane to initiate steroidogenesis (3,4,5). Studies in the recent two decades demonstrated that trophic hormones also induce arachidonic acid (AA) release (6,7,8,9), and AA metabolites transduce signals to the nucleus to regulate StAR gene expression (10,11). Both the PKA phosphorylation and the AA-mediated pathways are required for trophic hormone-induced signal transduction, with neither pathway alone being sufficient for StAR gene expression and steroid hormone production (12).
In the AA-mediated signaling pathway, AA is mainly catalyzed by three enzymes, lipoxygenase, epoxygenase, and cyclooxygenase, respectively, and metabolized to various products (13). It has been reported that cAMP stimulation of MA-10 Leydig cells significantly increased 5-lipoxygenase-generated AA metabolites 5-hydroperoxyeicosatetraenoic acid and 5-hydroxyeicosatetraenoic acid and also epoxygenase-produced metabolites 5,6-epoxy-8Z,11Z,14Z-eicosatrienoic acid, 8,9-epoxy-5Z,11Z,14Z-eicosatrienoic acid, and 11,12-epoxy-5Z,8Z,14Z-eicosatrienoic acid. Each of these AA metabolites significantly enhanced cAMP-stimulated StAR gene expression and steroid hormone production (14,15). In addition, inhibition of 5-lipoxygenase or epoxygenase activity depressed StAR gene expression and steroidogenesis in MA-10 cells (12). In contrast to the stimulatory effect of these two metabolic enzymes of AA, the third enzyme, cyclooxygenase-2 (COX2, an isoform of cyclooxygenase) acts to inhibit StAR gene expression. Inhibition of COX2 activity dramatically increased cAMP-stimulated StAR gene expression and steroidogenesis (16). Overexpression of COX2 in MA-10 cells significantly depressed StAR gene expression and steroid hormone production. Moreover, the age-related decline in testosterone biosynthesis is associated with an increase in COX2 expression in Leydig cells and was reversed by inhibition of its activity (17). The evidence provided by these studies demonstrated a COX2-dependent inhibition of cAMP-stimulated StAR gene expression and steroidogenesis in Leydig cells.
The mechanism responsible for the inhibitory effect produced by COX2 on steroidogenesis has not been well elucidated at this time. An earlier study reported an inhibitory effect of a COX2-generated AA metabolite, prostaglandin (PG) F2α, on LH-stimulated steroidogenesis in rat Leydig cells (18). Recently, it was reported that incubation of hamster Leydig cells with PGF2α resulted in decreases in human chorionic gonadotrophin (hCG)-stimulated StAR protein and testosterone production (19). Further studies revealed that PGF2α induced the expression of DAX-1 (dosage-sensitive sex reversal-adrenal hypoplasia gene on the X chromosome gene 1), a powerful transcriptional repressor of StAR gene transcription (20,21). PGF2α also induced another repressor, c-Fos, that binds to the StAR promoter and inhibits StAR gene expression (22). These studies suggested that the AA metabolites generated by COX2 and its downstream enzymes may be involved in the inhibitory effect on StAR gene expression. To understand the role of another enzyme downstream of COX2, thromboxane A synthase (TBXAS), in steroidogenesis, we inhibited its expression using RNA interference (RNAi) and also blocked its activity using an inhibitor. The results from the present study indicated that inhibition of TBXAS expression or activity enhanced cAMP-stimulated StAR gene expression and steroidogenesis in MA-10 mouse Leydig cells.
Materials and Methods
Reagents
N6,2-dibutyryladenosine cAMP (dbcAMP), H89, Waymouth’s MB/752 medium and 22(R)hydroxycholesterol were purchased from Sigma Chemical Co. (St. Louis, MO). TBXAS inhibitor furegrelate [sodium 5-(3-pyridinymethyl)-2-benzofurancarboxylic acid] and thromboxane (TBX) A2 receptor antagonist SQ29548 were purchased from Cayman (Ann Arbor, MI). Rabbit antiserum generated against StAR protein was a generous gift from Dr. W. L. Miller (University of California, San Francisco) (23). The monoclonal antibody against DAX-1 protein was a generous gift from Dr. P. Sassone-Corsi (Université Louis Pasteur, Strasbourg, France). Goat antirabbit IgG antibody conjugated with horseradish peroxidase was purchased from Biosource (Camarillo, CA). Horse serum was purchased from Invitrogen (Grand Island, NY). The dual-luciferase reporter assay system was purchased from Promega (Madison, WI). Other common chemicals used in this study were obtained from either Sigma or Fisher Chemicals (Pittsburgh, PA).
Cell culture
The MA-10 mouse Leydig tumor cell line was a generous gift from Dr. Mario Ascoli (Department of Pharmacology, University of Iowa, College of Medicine, Iowa City, IA). The cells were cultured in 12-well culture plates in Waymouth’s MB/752 medium containing 15% horse serum as previously described (24) in an incubator at 37 C and 5% CO2. Before each experiment, the medium was replaced with serum-free Waymouth’s medium.
RNAi
The small interference RNAs (siRNAs) were obtained from Ambion (Austin, TX) for RNAi of mouse TBXAS expression (Ambion siRNA ID 187220, corresponding to TBXAS DNA sequence 5′-CCCAAGATGGTAGCAGACA-3′) and for siRNA control (catalog item 4611). MA-10 cells at 0.2 × 106 cells per well in 12-well plates were cultured overnight. The siRNAs were introduced into the cells using the X-tremeGENE siRNA transfection reagent (Roche, Indianapolis, IN) following the manufacturer’s instructions. The transfected cells were cultured for 3 d before experiments.
Steroid production
MA-10 cells were cultured for 30 min in serum-free Waymouth’s medium in 12-well plates with or without the TBXAS inhibitor furegrelate (as described in the figure legends), and then 0.2 mm dbcAMP was added to the cultures for 6 h. The medium was collected at the end of each experiment and stored at −80 C. Progesterone concentrations in the medium were determined by RIA (25) and expressed as picograms per microgram cellular protein.
Enzyme immunoassays
MA-10 cells cultured in Waymouth’s medium in 12-well plates were treated with 0.8 mm furegrelate for 6 h. Then, the medium was collected. The concentrations of TBX B2 or PGF2α in the medium were determined using a chemiluminescence enzyme immunoassay kit or an enzyme immunoassay kit, respectively, following the manufacturer’s instructions (Assay Designs, Ann Arbor, MI), and expressed as picograms per milligram cellular protein.
Western blot analysis
StAR protein and DAX-1 protein in MA-10 cells were detected by Western blot analysis as described previously (26). The DAX-1- or StAR-specific bands were scanned and quantitated as integrated OD (IOD). The average IOD for each treatment was calculated and expressed as fold increase over control. Western blot analysis experiments were performed at least three times, and the results of one representative experiment are shown for each figure.
Transfection
MA-10 cells were cultured in 12-well plates (0.2 × 106 cells per well) overnight. The cells in each well were transfected with 0.5 μg DNA of the StAR promoter/luciferase plasmid PGL2/StAR expressing firefly luciferase driven by the −966-bp sequence of the StAR promoter (27). Transfections also included 6.0 ng of the pRL-SV40 vector DNA (a plasmid that constitutively expresses Renilla luciferase, a control reporter under the control of the SV40 promoter; Promega). Transfections were performed using FuGENE6 transfection reagent (Roche, Indianapolis, IN) following the manufacturer’s instructions. After 48 h in culture, the cells were used for additional experiments.
Luciferase assays
After the experiments, the cells were washed three times with ice-cold PBS and lysed with passive lysis buffer (Promega). The supernatants were used for luciferase assays using a dual luciferase reporter assay system following the manufacturer’s instructions (Promega). The relative light units (RLU, determined by dividing the reading from the PGL2/StAR promoter by the reading from Renilla luciferase) were measured using a TD-20/20 luminometer (Turner Designers, Sunnyvale, CA). StAR promoter activities are expressed as fold increases over the RLU of control.
RT-PCR
In experiments designed to determine StAR or TBXAS mRNA expression, cells were washed three times with cold PBS and used for total RNA purification using TRIzol reagent in accordance with the manufacturer’s instructions (Invitrogen, Carsbad, CA). The first-strand cDNA was synthesized from total RNA using the reverse transcription system (Promega). PCR for StAR was performed as previously described (28). PCR for TBXAS was performed with forward primer 5′-CCCTGTCCTCTTCTGAGTGC-3′ and reverse primer 5′-TGAAATCACCATGTCCAGATAC-3′ for 30 cycles of 94 C for 1.5 min, 56 C for 1.5 min, and 72 C for 2.5 min, followed by incubation at 72 C for 15 min. β-Actin was used as internal marker in PCR for StAR or TBXAS.
Statistical analysis
Each experiment was repeated at least three times. Statistical analysis of the data was performed with ANOVA followed by Tukey significant difference test using the GraphPad Prism 4 system (GraphPad Software, San Diego, CA). The data are shown as the mean ± se.
Results
Steroidogenesis and StAR protein expression
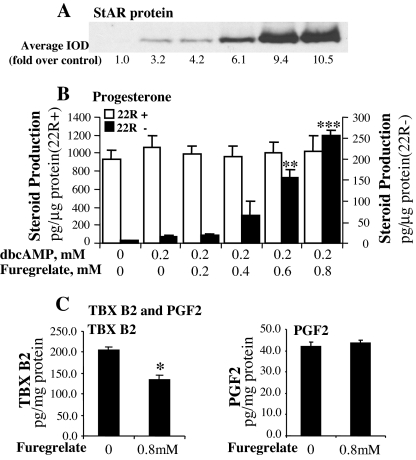
To determine the effects of TBXAS activity on StAR protein expression and steroidogenesis, MA-10 cells were incubated for 30 min in the medium containing a TBXAS inhibitor, furegrelate, and then 0.2 mm dbcAMP was added to the culture for 6 h. The incubation of MA-10 cells with the inhibitor significantly inhibited TBXAS activity but did not reduce PGF2α production (Fig. 1C). Similar results were obtained in previous studies that demonstrated the specificity of furegrelate in inhibition of TBXAS activity (29,30,31,32). The results in Fig. 1, A and B, show that although 0.2 mm dbcAMP alone did not induce a significant increase in steroidogenesis, inhibition of TBXAS activity markedly increased StAR protein expression in a concentration-dependent manner. The increase in StAR protein occurred concomitantly with a significant increase in steroid hormone production. When the concentration of furegrelate in the culture was increased to 0.8 mm, progesterone production by the cells was increased to 17-fold over that by the cells treated with 0.2 mm dbcAMP alone. To determine whether furegrelate affected the activities of steroidogenic enzymes in MA-10 cells, 22(R)hydroxycholesterol, a compound that can readily diffuse to the mitochondrial inner membrane in the absence of StAR protein, was added to each treatment. The RIA results indicated that in the presence of 22(R)hydroxycholesterol, there was no significant difference in steroid production among the treatments (Fig. 1B), indicating that the activities of steroidogenic enzymes were not affected by furegrelate.
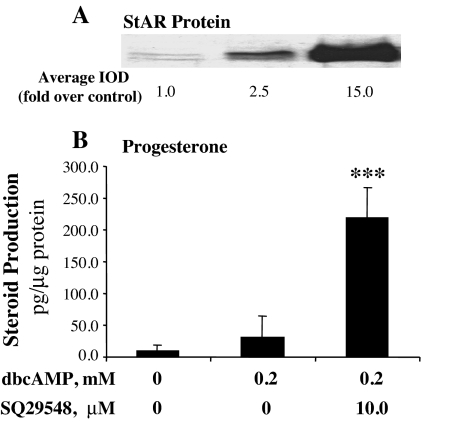
Figure 1.
Inhibition of TBXAS activity enhanced cAMP-induced steroidogenesis and StAR protein expression in MA-10 mouse Leydig cells. A, MA-10 cells were cultured in serum-free Waymouth’s medium with increasing concentrations of the TBX A synthase inhibitor furegrelate for 30 min, and then 0.2 mm dbcAMP was added to the culture for 6 h. The cells were collected, and 20 μg cell lysate protein was used for Western blot analysis of StAR protein. The StAR-specific bands were quantitated as IOD. The average IOD for each treatment was calculated and expressed as fold over control. B, MA-10 cells were cultured in the medium with or without 22(R)hydroxycholesterol (22R+ or 22R−) and treated as described above. Progesterone concentration in the medium was assessed by RIA and expressed as picograms per microgram cellular protein. **, P < 0.01, n = 4; ***, P < 0.001, n = 4; compared with 0.2 mm dbcAMP alone. C, MA-10 ells were culture in the medium containing 0.8 mm furegrelate for 6 h. The concentrations of TBX B2 and PGF2α in the medium were determined by EIA. *, P < 0.05, n = 4, compared with control.
StAR gene transcription
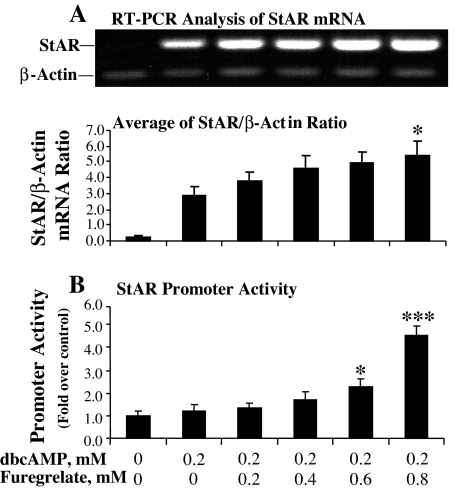
Luciferase assays of StAR promoter activity and RT-PCR analysis of StAR mRNA levels were performed to determine whether the TBXAS inhibitor affected StAR gene transcription. Similar to the results shown in Fig. 1, inhibition of TBXAS activity with increasing levels of furegrelate significantly increased StAR promoter activity, with the promoter activity being increased 4-fold as the concentration of furegrelate was increased from 0 to 0.8 mm in the culture with 0.2 mm dbcAMP (Fig. 2B). Paralleling the increase in StAR promoter activity, a concentration-dependent increase in StAR mRNA level was detected by RT-PCR analysis (Fig. 2A).
Figure 2.
Inhibition of TBXAS activity enhanced cAMP-induced StAR gene transcription in MA-10 mouse Leydig cells. MA-10 cells were cultured in serum-free Waymouth’s medium with increasing concentrations of the TBX A synthase inhibitor furegrelate for 30 min, and then 0.2 mm dbcAMP was added to the culture for 6 h. A, Cells were collected for total RNA isolation, and StAR mRNA was analyzed by RT-PCR with β-actin as an internal marker. The StAR- or β-actin-specific bands were quantitated as IOD and expressed as average of StAR/β-actin IOD ratio for each treatment. *, P < 0.05, n = 3, compared with 0.2 mm dbcAMP alone. B, MA-10 cells were transfected with a StAR promoter/luciferase plasmid (PGL2/StAR) and a pRLSV40 vector DNA, a plasmid that constitutively expresses Renilla luciferase. The cell lysate was used for luciferase assays using a dual luciferase reporter assay system. The RLU was determined by dividing the reading from the PGL2/StAR promoter by the reading from Renilla luciferase. The promoter activity was expressed as fold over RLU of control. *, P < 0.05, n = 3; ***, P < 0.001, n = 3; compared with 0.2 mm dbcAMP alone.
RNAi in TBXAS expression
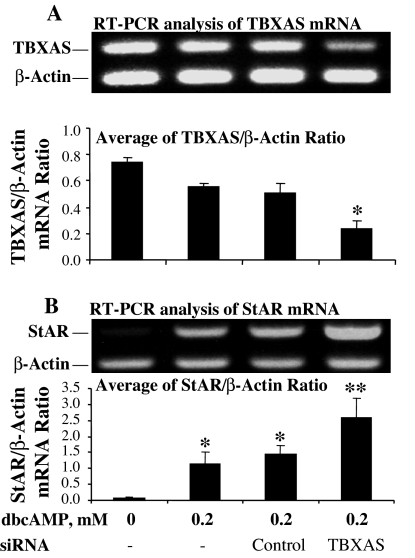
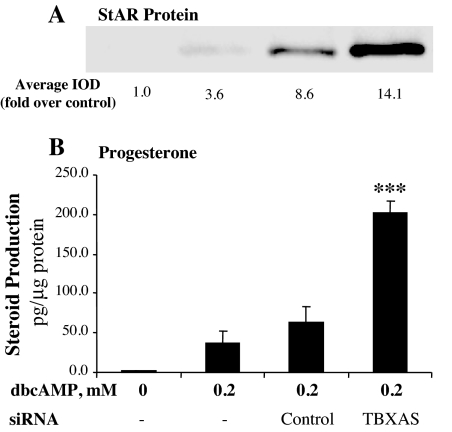
To verify the role of TBXAS in StAR gene expression and steroidogenesis, TBXAS expression was specifically inhibited using RNAi. Although the control siRNA did not affect TBXAS expression, transfection with TBXAS siRNA significantly reduced TBXAS mRNA levels in MA-10 cells (Fig. 3A). Although the transfection with control siRNA seemed to slightly increase StAR mRNA, StAR mRNA levels in MA-10 cells were increased significantly by TBXAS siRNA over that of the siRNA control (Fig. 3B). These observations were corroborated by the results from Western blot analyses of StAR protein expression and RIA for steroidogenesis by MA-10 cells. As shown in Fig. 4, inhibition of TBXAS expression by RNAi increased StAR protein and steroidogenesis, with progesterone production being significantly increased 3.2-fold over that of the siRNA control.
Figure 3.
RNAi of TBXAS expression increased cAMP-induced StAR mRNA levels in MA-10 mouse Leydig cells. MA-10 cells were transfected with siRNA corresponding to the TBXAS DNA sequence 5′-CCCAAGATGGTAGCAGACA-3′ or control siRNA using the X-tremeGENE siRNA transfection reagent. The cells were cultured for 3 d and then treated as indicated in the figure for 6 h. The cells were collected for total RNA isolation. TBXAS mRNA and StAR mRNA were analyzed by RT-PCR using β-actin as an internal marker. A, RT-PCR analysis of TBXAS mRNA levels. The TBXAS or β-actin-specific bands were quantitated as IOD and expressed as average of TBXAS/β-actin IOD ratio for each treatment. *, P < 0.05, n = 3, compared with siRNA control. B, RT-PCR analysis of StAR mRNA levels. The StAR- or β-actin-specific bands were quantitated as IOD and expressed as average of StAR/β-actin IOD ratio for each treatment. *, P < 0.05, n = 5, compared with the group without dbcAMP; **, P < 0.05, n = 5, compared with siRNA control.
Figure 4.
RNAi of TBXAS expression enhanced cAMP-induced steroidogenesis and StAR protein expression in MA-10 mouse Leydig cells. MA-10 cells were transfected with siRNA corresponding to the TBXAS DNA sequence 5′-CCCAAGATGGTAGCAGACA-3′ or control siRNA using the X-tremeGENE siRNA transfection reagent. The cells were cultured for 3 d and then treated as indicated in the figure for 6 h. A, Cells were collected, and 20 μg cell lysate protein was used for Western blot analysis of StAR protein. The StAR-specific bands were quantitated as IOD. The average IOD for each treatment was calculated and expressed as fold over control. B, Progesterone production in the culture medium was assessed by RIA and expressed as picograms per microgram cellular protein. ***, P < 0.001, n = 4, compared with siRNA control.
Blocking TBX A2-binding to its receptor enhanced steroidogenesis
To determine whether the TBXAS-generated AA metabolite TBX A2 was involved in the observed inhibitory effect, MA-10 cells were incubated with 10 μm SQ29548, a highly selective antagonist of TBX A2 receptor (33), for 30 min, and then 0.2 mm dbcAMP was added to the culture for 6 h. The results from the experiments indicated that although 0.2 mm dbcAMP alone did not induce a significant increase in steroidogenesis, coincubation of MA-10 cells with 10 μm of this receptor antagonist dramatically increased StAR protein expression (Fig. 5A). As StAR protein increased, steroid hormone production increased 8.5-fold over that of the cells treated with 0.2 mm dbcAMP alone (Fig. 5B).
Figure 5.
Blocking TBX A2 binding to its receptor enhanced cAMP-induced steroidogenesis in MA-10 mouse Leydig cells. MA-10 cells were cultured in serum-free Waymouth’s medium containing the TBX A2 receptor antagonist SQ29548 for 30 min, and then 0.2 mm dbcAMP was added to the culture for 6 h. A, Cells were collected, and 20 μg cell lysate protein was used for Western blot analysis of StAR protein. The StAR-specific bands were quantitated as IOD. The average IOD for each treatment was calculated and expressed as fold over control. B, Progesterone production in the culture medium was assessed by RIA and expressed as picograms per microgram cellular protein. ***, P < 0.001, n = 4, compared with 0.2 mm dbcAMP alone.
Synergistic interaction between cAMP and the TBXAS inhibitor in steroidogenesis
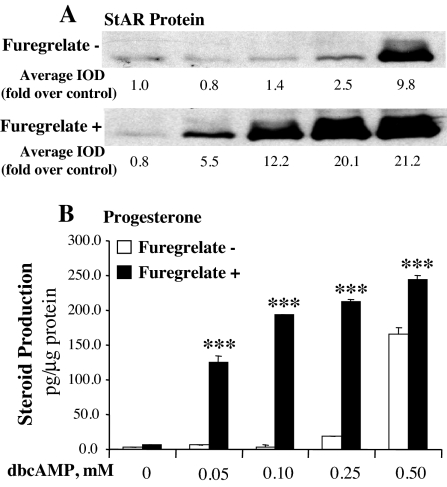
To further understand the role of TBXAS in steroidogenesis, MA-10 cells were incubated with or without 0.8 mm furegrelate for 30 min, and then increasing levels of dbcAMP were added to the culture for 6 h. In the absence of dbcAMP, inhibition of TBXAS activity with 0.8 mm furegrelate did not significantly increase StAR protein and steroidogenesis. However, addition of 0.05 mm dbcAMP to the MA-10 cell culture with the inhibitor dramatically increased steroid hormone production and StAR protein expression (Fig. 6). The steroidogenic effects of the TBXAS inhibitor were further enhanced by increasing concentrations of dbcAMP from 0.05 to 0.25 mm. The results shown in Fig. 6 illustrate that low levels of dbcAMP alone (0.05–0.1 mm) were unable to induce significant increases in StAR protein and steroid hormone. However, the effectiveness of such low levels of dbcAMP was dramatically enhanced by inhibition of TBXAS activity, with 0.1 mm dbcAMP inducing higher levels of StAR protein and steroidogenesis than those induced by 0.5 mm dbcAMP alone. In the presence of the TBXAS inhibitor, dbcAMP at 0.05, 0.1, and 0.25 mm increased progesterone productions by 25-, 44-, and 12-fold, respectively, over those stimulated with the same levels of dbcAMP alone (Fig. 6B). Also, StAR protein expression and steroid hormone production reached to the maximal levels at much lower levels of dbcAMP.
Figure 6.
Inhibition of TBXAS activity increased steroidogenic sensitivity of MA-10 mouse Leydig cells to cAMP stimulation. MA-10 cells were cultured for 30 min in serum-free Waymouth’s medium with or without 0.8 mm furegrelate, and then the increasing concentrations of dbcAMP was added to the culture for 6 h. A, Cells were collected, and 20 μg cell lysate protein was used for Western blot analysis of StAR protein. The StAR-specific bands were quantitated as IOD. The average IOD for each treatment was calculated and expressed as fold over control. B, Progesterone production in the culture medium was assessed by RIA and expressed as picograms per microgram cellular protein. ***, P < 0.001, n = 3, compared with the paired group stimulated with dbcAMP alone.
Essential role of PKA activity in the inhibitor- increased steroidogenesis
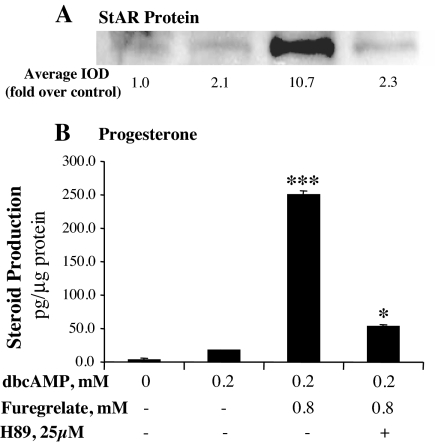
The above results and our previous observations encouraged us to determine the role of PKA activity in the increased steroidogenesis. PKA activity was inhibited using a PKA inhibitor, H89, in the culture of MA-10 cells treated with 0.2 mm dbcAMP and 0.8 mm furegrelate. Similar to the results described above, inhibition of TBXAS activity dramatically increased steroidogenesis. However, inhibition of PKA activity essentially abolished the furegrelate-increased StAR protein expression (Fig. 7A). Steroid hormone production was reduced to 21% of that induced by dbcAMP and the TBXAS inhibitor (Fig. 7B).
Figure 7.
PKA activity is essential for the stimulatory effects of the TBXAS inhibitor on steroidogenesis in MA-10 mouse Leydig cells. MA-10 cells were cultured for 30 min in serum-free Waymouth’s medium with 0.8 mm furegrelate and 25 μm of the PKA inhibitor H89, and then 0.2 mm dbcAMP was added to the culture for 6 h. A, Cells were collected, and 20 μg cell lysate protein was used for Western blot analysis of StAR protein. The StAR-specific bands were quantitated as IOD. The average IOD for each treatment was calculated and expressed as fold over control. B, Progesterone production in the culture medium was assessed by RIA and expressed as picograms per microgram cellular protein. *, P < 0.05, n = 3, compared with 0.2 mm dbcAMP alone; ***, P < 0.001, n = 3, compared with the group treated with H89.
DAX-1 expression
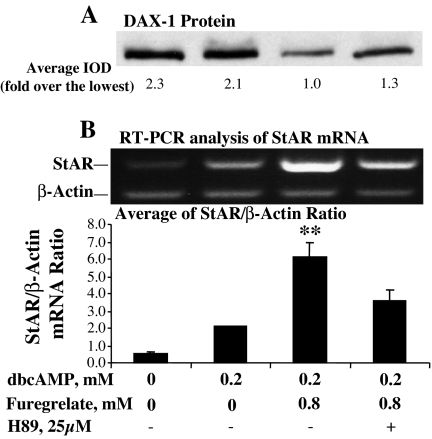
To further understand how TBXAS affects StAR gene transcription, the level of DAX-1 protein was analyzed. Results obtained from Western blot analyses indicated that inhibition of TBXAS activity markedly reduced DAX-1 protein (Fig. 8A). Also, StAR mRNA levels were analyzed by RT-PCR to determine the relationship between the reduced DAX-1 protein and StAR gene transcription. As shown in Fig. 8, the reduction in DAX-1 protein is associated with a significant increase in StAR mRNA levels.
Figure 8.
Inhibition of TBXAS activity reduced DAX-1 protein in MA-10 mouse Leydig cells. MA-10 cells were cultured for 30 min in serum-free Waymouth’s medium with 0.8 mm furegrelate and 25 μm of the PKA inhibitor H89, and then 0.2 mm dbcAMP was added to the culture for 6 h. A, Cells were collected, and 20 μg cell lysate protein was used for Western blot analysis of DAX-1 protein. The DAX-1-specific bands were quantitated as IOD. The average IOD for each treatment was calculated and expressed as fold over the lowest group. B, Cells were collected for total RNA isolation, and StAR mRNA was analyzed by RT-PCR with β-actin as an internal marker. The StAR- or β-actin-specific bands were quantitated as IOD and expressed as average of StAR/β-actin IOD ratio for each treatment. **, P < 0.01, n = 4, compared with the group treated with H89.
Discussion
Studies in recent years have demonstrated a COX2-dependent inhibition of StAR gene expression and steroidogenesis in testicular Leydig cells, but the mechanism responsible for the inhibitory effects of COX2 has not yet been elucidated. In COX2-mediated AA metabolism, AA is converted to PGH2, which is further metabolized to PGD2, PGE2, PGF2α, PGI2, and TBX A2 by the downstream enzymes, the prostaglandin synthases, and TBXAS. Following previous studies that reported an inhibitory effect of PGF2α on steroidogenesis (18,19), the present study suggests an inhibitory effect of TBXAS on StAR gene expression in MA-10 mouse Leydig cells.
This inhibitory effect of TBXAS on StAR gene expression was supported by several lines of experimentation in the present study. The results obtained from these experiments indicated that inhibition of TBXAS activity, using the specific inhibitor furegrelate, dramatically increased StAR promoter activity, StAR mRNA levels, StAR protein expression, and steroid hormone production in MA-10 Leydig cells. The inhibitory effect of TBXAS on steroidogenesis was corroborated by specific inhibition of TBXAS expression using TBXAS siRNA. The RNAi-reduced TBXAS expression was indicated by a significant decrease in TBXAS mRNA levels as detected by RT-PCR analysis. The results of the present study demonstrated that the RNAi-mediated inhibition of TBXAS expression significantly increased StAR mRNA levels, StAR protein, and steroid hormone production. The above observations were further strengthened when MA-10 cells were treated with a highly selective antagonist of TBX A2 receptor, with StAR protein and steroid production being dramatically increased by the receptor antagonist.
The manner in which TBXAS acts to inhibit StAR protein expression is unknown. The results from RT-PCR analysis of StAR mRNA levels and luciferase assays of StAR promoter activities indicated that the TBXAS inhibitor increased StAR protein by enhancing StAR gene transcription. Similar results were obtained when TBXAS expression was inhibited by RNAi, with StAR mRNA levels being increased as TBXAS mRNA levels in the cells were decreased. We attempted to find a possible explanation for the increase in StAR gene transcription elicited by the TBXAS inhibitor. Interestingly, inhibition of TBXAS activity markedly reduced DAX-1, a protein reported to bind to a hairpin structure in StAR promoter and inhibit StAR gene transcription (21). Although more studies are needed, these results suggested a possible effect of TBXAS activity on the level of DXA-1 protein in MA-10 cells.
Whereas inhibition of TBXAS activity dramatically increased cAMP-induced StAR protein expression and steroid hormone production, in the absence of cAMP or after inhibition of PKA activity, the TBXAS inhibitor alone was unable to induce significant increases in steroidogenesis and StAR protein. However, when TBXAS activity was inhibited, the steroidogenic sensitivity of MA-10 mouse Leydig cells was dramatically increased, with subthreshold levels of dbcAMP being able to induce maximal levels of StAR protein expression and steroidogenesis. This observation indicated that the inhibitor itself is not able to stimulate steroidogenesis, but rather reduced the threshold for cAMP-induced StAR gene expression. Similar observations were obtained in MA-10 cells treated with a COX2 inhibitor as previously reported (16). Results from the present and previous studies suggest that the combined activities of COX2 and TBXAS produce AA metabolites that control the threshold of trophic hormone-stimulated steroidogenesis by depressing StAR gene transcription.
Taken all together, these studies provide an opportunity for better understanding of the signal transduction in trophic hormone-stimulated steroidogenesis. The studies indicate that trophic hormone stimulation induces not only positive signals but also negative signals in StAR gene expression. In the AA-mediated signaling pathway, the positive signals are produced by lipoxygenase (15,34,35,36,37) and epoxygenase (14,38,39), whereas the negative signals are generated by COX2 and by the downstream enzymes PGF synthase (19,20,22) and TBXAS as described above. In the cAMP-PKA-phosphorylation pathway, PKA phosphorylates the transcription factors that enhance StAR gene transcription (2). On the other hand, PKA may also induce negative signals. For example, a previous study reported hCG-induced increase in COX2 expression in Leydig cells (19). This observation was corroborated by a recent study on LH- or cAMP-stimulated steroidogenesis, in which LH-induced rise in COX2 protein levels was nearly abolished by inhibition of PKA activity (40). These studies suggested that cAMP-PKA phosphorylation may be required for LH-induced COX2 expression. Therefore, the total effect of trophic hormone on StAR gene expression seems to depend on the levels and ratios of positive and negative signals in Leydig cells. Obviously, the LH-stimulated increase in StAR protein in that study was due to the increased positive signals that produced stronger steroidogenic effects than the increased negative signals (40), such as COX2-produced metabolites, which opposed it. It is possible that when LH stimulates StAR gene expression, it also induces feedback signaling, involving AA metabolites generated by COX2, PGF synthase, and TBXAS, to control StAR gene expression.
In summary, following previous studies that reported the inhibitory effects of AA metabolites produced by COX2-PGF synthase on steroidogenesis, the present study suggests that COX2 and TBXAS also act in concert to depress StAR gene transcription, possibly by being involved in the expression or stability of DAX-1, a StAR gene repressor.
Footnotes
This work was supported by National Institutes of Health (NIH) Grant AG025349 to X.J.W., NIH Grant AG028367 to P.G., NIH Grant HD-17481 and funds from the Robert A. Welch Foundation Grant B1-0028 to D.M.S., and a grant from the Lalor Foundation to S.R.K.
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 15, 2007
Abbreviations: AA, Arachidonic acid; COX2, cyclooxygenase 2; dbcAMP, N6,2-dibutyryladenosine cAMP; hCG, human chorionic gonadotropin; IOD, integrated OD; PG, prostaglandin; PKA, protein kinase A; RLU, relative light units; RNAi, RNA interference; siRNA, small interference RNA; StAR, steroidogenic acute regulatory; TBX, thromboxane; TBXAS, thromboxane A synthase.
References
- Cooke BA 1996 Transduction of the luteinizing hormone signal within the Leydig cell. In: Payne AH, Hardy MP, Russell LD, eds. The Leydig Cell. 1st ed. Vienna, IL: Cache River Press; 352–363 [Google Scholar]
- Reinhart AJ, Williams SC, Stocco DM 1999 Transcriptional regulation of the StAR gene. Mol Cell Endocrinol 151:161–169 [DOI] [PubMed] [Google Scholar]
- Clark BJ, Wells J, King SR, Stocco DM 1994 The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 269:28314–28322 [PubMed] [Google Scholar]
- Lin D, Sugawara T, Strauss 3rd JF, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL 1995 Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science 267:1828–1831 [DOI] [PubMed] [Google Scholar]
- Wang XJ, Liu Z, Eimerl S, Timberg R, Weiss AM, Orly J, Stocco DM 1998 Effect of truncated forms of the steroidogenic acute regulatory protein on intramitochondrial cholesterol transfer. Endocrinology 139:3903–3912 [DOI] [PubMed] [Google Scholar]
- Cooke BA, Dirami G, Chaudry L, Choi MS, Abayasekara DR, Phipp L 1991 Release of arachidonic acid and the effects of corticosteroids on steroidogenesis in rat testis Leydig cells. J Steroid Biochem Mol Biol 40:465–471 [DOI] [PubMed] [Google Scholar]
- Moraga PF, Llanos MN, Ronco AM 1997 Arachidonic acid release from rat Leydig cells depends on the presence of luteinizing hormone/human chorionic gonadotrophin receptors. J Endocrinol 154:201–209 [DOI] [PubMed] [Google Scholar]
- Cano F, Poderoso C, Cornejo Maciel F, Castilla R, Maloberti P, Castillo F, Neuman I, Paz C, Podesta EJ 2006 Protein tyrosine phosphatases regulate arachidonic acid release, StAR induction and steroidogenesis acting on a hormone-dependent arachidonic acid-preferring acyl-CoA synthetase. J Steroid Biochem Mol Biol 99:197–202 [DOI] [PubMed] [Google Scholar]
- Ronco AM, Moraga PF, Llanos MN 2002 Arachidonic acid release from rat Leydig cells: the involvement of G protein, phospholipase A2 and regulation of cAMP production. J Endocrinol 172:95–104 [DOI] [PubMed] [Google Scholar]
- Cooke BA 1999 Signal transduction involving cyclic AMP-dependent and cyclic AMP-independent mechanisms in the control of steroidogenesis. Mol Cell Endocrinol 151:25–35 [DOI] [PubMed] [Google Scholar]
- Wang XJ, Stocco DM 1999 Cyclic AMP and arachidonic acid: a tale of two pathways. Mol Cell Endocrinol 158:7–12 [DOI] [PubMed] [Google Scholar]
- Wang XJ, Walsh LP, Reinhart AJ, Stocco DM 2000 The role of arachidonic acid in steroidogenesis and steroidogenic acute regulatory (StAR) gene and protein expression. J Biol Chem 275:20204–20209 [DOI] [PubMed] [Google Scholar]
- Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB 1986 Arachidonic acid metabolism. Annu Rev Biochem 55:69–102 [DOI] [PubMed] [Google Scholar]
- Wang X, Shen CL, Dyson MT, Yin X, Schiffer RB, Grammas P, Stocco DM 2006 The involvement of epoxygenase metabolites of arachidonic acid in cAMP-stimulated steroidogenesis and steroidogenic acute regulatory protein gene expression. J Endocrinol 190:871–878 [DOI] [PubMed] [Google Scholar]
- Wang XJ, Dyson MT, Jo Y, Eubank DW, Stocco DM 2003 Involvement of 5-lipoxygenase metabolites of arachidonic acid in cyclic AMP-stimulated steroidogenesis and steroidogenic acute regulatory protein gene expression. J Steroid Biochem Mol Biol 85:159–166 [DOI] [PubMed] [Google Scholar]
- Wang XJ, Dyson MT, Jo Y, Stocco DM 2003 Inhibition of cyclooxygenase-2 activity enhances steroidogenesis and steroidogenic acute regulatory gene expression in MA-10 mouse Leydig cells. Endocrinology 144:3368–3375 [DOI] [PubMed] [Google Scholar]
- Wang XJ, Shen CL, Dyson MT, Eimerl S, Orly J, Hutson JC, Stocco DM 2005 Cyclooxygenase-2 regulation of the age-related decline in testosterone biosynthesis. Endocrinology 146:4202–4208 [DOI] [PubMed] [Google Scholar]
- Fuchs AR, Chantharaksri U 1981 Prostaglandin F2α regulation of LH-stimulated testosterone production in rat testis. Biol Reprod 25:492–501 [DOI] [PubMed] [Google Scholar]
- Frungieri MB, Gonzalez-Calvar SI, Parborell F, Albrecht M, Mayerhofer A, Calandra RS 2006 Cyclooxygenase-2 and prostaglandin F2α in Syrian hamster Leydig cells: inhibitory role on luteinizing hormone/human chorionic gonadotropin-stimulated testosterone production. Endocrinology 147:4476–4485 [DOI] [PubMed] [Google Scholar]
- Sandhoff TW, McLean MP 1999 Repression of the rat steroidogenic acute regulatory (StAR) protein gene by PGF2α is modulated by the negative transcription factor DAX-1. Endocrine 10:83–91 [DOI] [PubMed] [Google Scholar]
- Zazopoulos E, Lalli E, Stocco DM, Sassone-Corsi P 1997 DNA binding and transcriptional repression by DAX-1 blocks steroidogenesis. Nature 390:311–315 [DOI] [PubMed] [Google Scholar]
- Shea-Eaton W, Sandhoff TW, Lopez D, Hales DB, McLean MP 2002 Transcriptional repression of the rat steroidogenic acute regulatory (StAR) protein gene by the AP-1 family member c-Fos. Mol Cell Endocrinol 188:161–170 [DOI] [PubMed] [Google Scholar]
- Bose HS, Whittal RM, Baldwin MA, Miller WL 1999 The active form of the steroidogenic acute regulatory protein, StAR, appears to be a molten globule. Proc Natl Acad Sci USA 96:7250–7255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli M 1981 Characterization of several clonal lines of cultured Leydig tumor cells: gonadotropin receptors and steroidogenic responses. Endocrinology 108:88–95 [DOI] [PubMed] [Google Scholar]
- Resko JA, Norman RL, Niswender GD, Spies HG 1974 The relationship between progestins and gonadotropins during the late luteal phase of the menstrual cycle in rhesus monkeys. Endocrinology 94:128–135 [DOI] [PubMed] [Google Scholar]
- Townson DH, Wang XJ, Keyes PL, Kostyo JL, Stocco DM 1996 Expression of the steroidogenic acute regulatory protein in the corpus luteum of the rabbit: dependence upon the luteotropic hormone, estradiol-17β. Biol Reprod 55:868–874 [DOI] [PubMed] [Google Scholar]
- Caron KM, Ikeda Y, Soo SC, Stocco DM, Parker KL, Clark BJ 1997 Characterization of the promoter region of the mouse gene encoding the steroidogenic acute regulatory protein. Mol Endocrinol 11:138–147 [DOI] [PubMed] [Google Scholar]
- Rao RM, Jo Y, Leers-Sucheta S, Bose HS, Miller WL, Azhar S, Stocco DM 2003 Differential regulation of steroid hormone biosynthesis in R2C and MA-10 Leydig tumor cells: role of SR-B1-mediated selective cholesteryl ester transport. Biol Reprod 68:114–121 [DOI] [PubMed] [Google Scholar]
- Pestel S, Nath A, Jungermann K, Schieferdecker HL 2003 Inhibition of prostaglandin D2 clearance in rat hepatocytes by the thromboxane receptor antagonists daltroban and ifetroban and the thromboxane synthase inhibitor furegrelate. Biochem Pharmacol 66:643–652 [DOI] [PubMed] [Google Scholar]
- Jones AE, Watts JA, Debelak JP, Thornton LR, Younger JG, Kline JA 2003 Inhibition of prostaglandin synthesis during polystyrene microsphere-induced pulmonary embolism in the rat. Am J Physiol Lung Cell Mol Physiol 284:L1072–L1081 [DOI] [PubMed] [Google Scholar]
- Regal JF 1988 Role of arachidonate metabolites in C5a-induced bronchoconstriction. J Pharmacol Exp Ther 246:542–547 [PubMed] [Google Scholar]
- Gorman RR, Johnson RA, Spilman CH, Aiken JW 1983 Inhibition of platelet thromboxane A2 synthase activity by sodium 5-(3′-pyridinylmethyl)benzofuran-2-carboxylate. Prostaglandins 26:325–342 [DOI] [PubMed] [Google Scholar]
- Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, Lamontagne S, Rochette C, Sawyer N, Tremblay NM, Belley M, Gallant M, Dufresne C, Gareau Y, Ruel R, Juteau H, Labelle M, Ouimet N, Metters KM 2000 The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta 1483:285–293 [DOI] [PubMed] [Google Scholar]
- Dix CJ, Habberfield AD, Sullivan MH, Cooke BA 1985 Evidence for the involvement of lipoxygenase products in steroidogenesis. Biochem Soc Trans 13:60–63 [DOI] [PubMed] [Google Scholar]
- Mele PG, Dada LA, Paz C, Neuman I, Cymeryng CB, Mendez CF, Finkielstein CV, Cornejo Maciel F, Podesta EJ 1997 Involvement of arachidonic acid and the lipoxygenase pathway in mediating luteinizing hormone-induced testosterone synthesis in rat Leydig cells. Endocr Res 23:15–26 [DOI] [PubMed] [Google Scholar]
- Sullivan MH, Cooke BA 1985 Effects of calmodulin and lipoxygenase inhibitors on LH (lutropin)- and LHRH (luliberin)-agonist-stimulated steroidogenesis in rat Leydig cells. Biochem J 232:55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix CJ, Habberfield AD, Sullivan MH, Cooke BA 1984 Inhibition of steroid production in Leydig cells by non-steroidal anti-inflammatory and related compounds: evidence for the involvement of lipoxygenase products in steroidogenesis. Biochem J 219:529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zosmer A, Elder MG, Sullivan MH 1990 Production of intracellular arachidonic acid metabolites by human granulosa cells. Prostaglandins Leukot Essent Fatty Acids 41:265–267 [DOI] [PubMed] [Google Scholar]
- Zosmer A, Elder MG, Sullivan MH 2002 The production of progesterone and 5,6-epoxyeicosatrienoic acid by human granulosa cells. J Steroid Biochem Mol Biol 81:369–376 [DOI] [PubMed] [Google Scholar]
- Chen H, Luo L, Liu J, Zirkin BR 2007 Cyclooxygenases in rat Leydig cells: effects of luteinizing hormone and aging. Endocrinology 148:735–742 [DOI] [PubMed] [Google Scholar]