Abstract
Ghrelin and the ghrelin receptor (GH secretagogue receptor, GHS-R), are believed to have important roles in energy homeostasis. We describe results from the first studies to be conducted in congenic (N10) adult ghrelin−/− and Ghsr−/− mice under conditions of both positive (high-fat diet) and negative (caloric restriction) energy balance. In contrast to results from young N2 mutant mice, changes in body weight and energy expenditure are not clearly distinguishable across genotypes. Although respiratory quotient was lower in mice fed a high-fat diet, no differences were evident between littermate wild-type and null genotypes. With normal chow, a modest decrease trend in respiratory quotient was detected in ghrelin−/− mice but not in Ghsr−/− mice. Under caloric restriction, the weight loss of ghrelin−/− and Ghsr−/− mice was identical to wild-type littermates, but blood glucose levels were significantly lower. We conclude that adult congenic ghrelin−/− and Ghsr−/− mice are not resistant to diet-induced obesity but under conditions of negative energy balance show impairment in maintaining glucose homeostasis. These results support our hypothesis that the primary metabolic function of ghrelin in adult mice is to modulate glucose sensing and insulin sensitivity, rather than directly regulate energy intake and energy expenditure.
GHRELIN HAS BEEN considered one of the most promising antiobesity targets; however, its physiological role in energy homeostasis is controversial. Pharmacological treatment of rats and mice with ghrelin stimulates food intake, increases body weight, and induces fat deposition (1,2,3). Circulating ghrelin is highest before feeding and declines immediately after nutrient ingestion, but levels are paradoxically low in obese humans and rodents (4,5). In humans, a link between ghrelin and obesity was made after the observation that in obese subjects who underwent gastric bypass surgery, ghrelin production declined in parallel with sustained weight loss and reduced appetite (6,7). However, gastric bypass was also reported to have no effect on ghrelin levels, but an inverse relationship between ghrelin and insulin was observed. This suggests that changes in ghrelin production reflect a new state of energy balance (8).
We have shown that Ghsr−/− mice are refractory to the stimulatory effects of ghrelin on GH release and appetite, confirming that the GH secretagogue receptor (GHS-R) is a physiologically relevant ghrelin receptor (9). Despite this, ghrelin−/− mice and Ghsr−/− mice have normal growth rates and normal appetites under conditions of standard laboratory housing (9,10). Wortley et al. (11) made similar observations in their independently generated ghrelin−/− mice.
To appropriately interpret results derived from metabolic studies in gene-ablated mice, genetic background must be carefully considered. Clear differences have been observed in feeding and body weight in 129Sv and C57BL/6 mice (12,13); indeed, the 129Sv mouse is far more resistant to diet-induced obesity (DIO) than the C57BL/6 mouse (14,15). These differences are important considerations because conventionally, embryonic stem cells derived from 129Sv mice are targeted for gene ablation, and the modified embryonic stem cells are injected into the blastocyst of C57BL/6 mice to produce chimeric mice. When the chimeras are bred to produce wild-type (WT) (+/+) or null (−/−) mice, the genetic background is unevenly distributed so that the null genotype has more traits of the 129Sv (e.g. leanness) than the WT littermates; therefore, to make realistic comparisons, it is essential to backcross the mice to establish a pure background.
To minimize selective genetic traits, we backcrossed the ghrelin−/− and Ghsr−/− mice for 10 generations with C57BL/6J mice, and the phenotypes of adult congenic mutant mice were compared with WT mice under conditions of positive and negative energy balance. Positive energy balance was induced by 35% high-fat (HF) diet feeding, and negative energy balance was induced by 50% caloric restriction (CR). Ablation of ghrelin signaling also did not prevent DIO in these congenic adult mice. We did detect a modest decrease in respiratory quotient (RQ) in these adult ghrelin−/− mice compared with WT mice fed a normal diet, but no differences were observed when adult mice were fed a HF diet.
Materials and Methods
Animals and data analysis
All experiments were conducted on adult male WT, ghrelin−/−, and Ghsr−/− mice. Mice were kept in a standard housing facility and singly housed 1 wk before and during the experiments. All procedures used in animal experiments were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine. Data are presented in the figures as mean ± sem, except in Fig. 2, where to simplify the figures, only the mean value is displayed. The number of mice per group is indicated by n. Significant differences between the groups were evaluated by ANOVA tests (two-way ANOVA or repeated-measures ANOVA) using SigmaStat 3.0 software. Statistical significance was considered as P < 0.05.
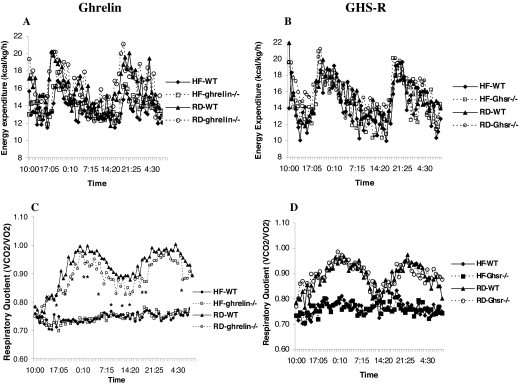
Figure 2.
Calorimetry parameters of adult ghrelin−/− and Ghsr−/− mice fed RD and HF diets. The 16-wk-old WT and ghrelin−/− and Ghsr−/− male mice were fed regular chow or 35% HF diet for 10 wk, and then the calorimetry studies were performed. The results are represented as the mean values of eight mice. *, Time points where P < 0.05 comparing RD-fed WT vs. ghrelin−/− mice. A and C, Energy expenditure (A) and RQ (C) in ghrelin−/− mice; B and D, energy expenditure (B) and RQ (D) in Ghsr−/− mice. The lines on the abscissas represent time points where data were collected. The light cycle was 0600–1800 h.
Hormone assays
Blood was collected from the tail vein. Mice were fasted for 24 h, and blood was drawn from the tail, collected in EDTA-containing tubes, and kept at 4 C during processing. Samples were centrifuged at 3000 rpm for 30 min, and hydrochloric acid and phenylmethylsulfonyl fluoride were immediately added to the plasma. Samples were aliquoted into polypropylene vials and stored at −80 C until assayed. Total plasma ghrelin levels were measured by RIA using kits purchased from Linco Research (St. Charles, MO). This RIA kit uses a polyclonal antibody raised in rabbits and applies it against both octanoylated and des-octanoylated ghrelin and [125I]ghrelin as the tracer. The lower limit of detection was 80 pg/ml, and the intraassay coefficient of variation was 4%. Active ghrelin levels were measured by another commercially available RIA kit from Linco Research. This RIA kit uses an antibody raised in guinea pigs and applies it against octanoylated ghrelin and 125I-octanoylated ghrelin as the tracer. This assay has been found to be highly specific for active ghrelin, with less than 0.1% cross-reactivity for des-octanoyl ghrelin, and no cross-reactivity with ghrelin 14–28, motilin-related peptide, leptin, insulin, glucagon, or glucagon-like peptide 7–36. The lower detection limit was 10 pg/ml. The intraassay coefficient of variation was 5.3%. Blood glucose values were determined by One-Touch Ultra glucometer (Lifescan, Milpitas, CA). Plasma assays were carried out using the manufacturer’s protocols: insulin, using rat insulin RIA kit (Linco Research), and IGF-I, using rat IGF-I RIA kit (Diagnostic Systems Laboratories, Inc., Webster, TX).
Body weight and food intake under different diets
The experimental mice were individually caged, provided with ad libitum access to water, and fed with either regular diet (RD) from PicoLab Rodent Diet (Oakville, Ontario, Canada) or HF diets from Harlan Teklad (Madison, WI). The percentage of macronutrients provided is based on weight, and the calorie equivalents are listed in parentheses. RD, diet 5053, has 9% fat (12% by calories), 20% protein, and 40% carbohydrate. The 35% HF diet, TD 0217, has 35.1% fat (62.2% by calories), 22.9% protein, and 24.2% carbohydrate. The 36% HF diet, TD12331, has 35.8% fat (58% by calories), 23% protein, and 35.5% carbohydrate. The 75% high-carbohydrate diet is TD 00199.
Indirect calorimetry
Metabolic parameters were obtained by using an Oxymax (Columbus Instruments, Columbus, OH) open-circuit indirect calorimetry system. Briefly, oxygen consumption (VO2) (milliliters per kilogram per hour) and carbon dioxide production (VCO2) ((milliliters per kilogram per hour) by each animal were measured for a 48-h period. VO2 and RQ (ratio of VCO2/VO2) were then calculated. Energy expenditure (or heat) was calculated as the product of the calorific value of oxygen (3.815 + 1.232 × RQ) and the volume of O2 consumed. The light cycle was 0600–1800 h.
Evaluation of body weight and food intake during fasting and refeeding
The mice were weighed, and chow was removed. Forty-eight hours later, the animals were weighed again and then given a weighed amount of chow. Body weights were measured at 24 and 48 h after the start of the fast and 1, 2, 3, and 5 d after refeeding. The food intake was measured at 1, 2, 4, and 6 h and 1, 2, 3, and 5 d after refeeding.
CR
RD (diet 5053) was used during the CR protocol. All experimental mice were individually caged for 12 d before the start of CR, and average daily food consumption during these 12 d was calculated for each group. To determine whether the ghrelin−/− and Ghsr−/− mice could maintain blood glucose levels in an environment of chronic negative energy balance, mice were provided 50% of the average daily amount of food consumed ad libitum and fed daily at 1500 h. Body weight and blood glucose were measured before feeding, at 2- or 4-d intervals.
Results
Genetic background of ghrelin- and Ghsr- null mice
The null mice were initially generated on a 129Sv and C57BL/6J background. To reduce the impact of genetic heterogeneity on metabolic phenotype, the ghrelin−/− and Ghsr−/− mice were backcrossed with C57BL/6J mice for 10 generations. To determine whether both N10 null mice were congenic, the mice were analyzed for 110 microsatellite markers (Charles River Laboratory, Wilmington, MA). Besides the particular markers associated with gene deletion, all other markers were 100% identical to those characteristics of C57BL/6J mice, indicating that the N10 null mice are congenic (99.9% identical to C57BL/6J).
Phenotype of ghrelin- and Ghsr-null mice under positive energy balance
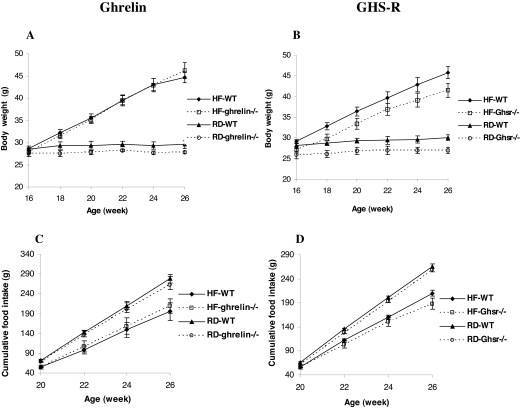
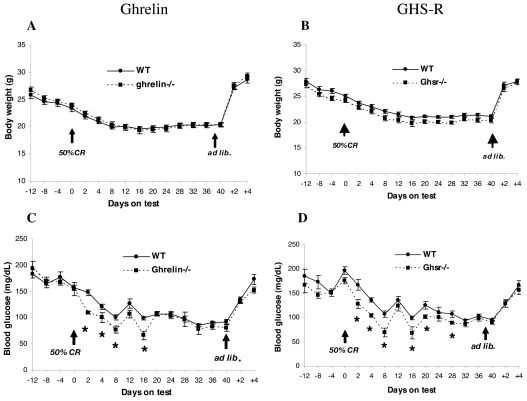
To investigate whether ghrelin and/or GHS-R might play a role in DIO, 16-wk-old adult male WT, ghrelin−/−, and Ghsr−/− mice were fed a 35% HF diet or RD for 10 wk; body weight and food intake were monitored biweekly (Fig. 1, A and B). Body weights of mice fed the HF diet were significantly higher than those fed RD (P < 0.05), although the body weights of Ghsr−/− mice were slightly lower than those of WT, relative weight gain was not significantly different between WT, ghrelin−/−, and Ghsr−/− mice. Consistent with our previous reports (9,10), regardless of diet, the body weights of ghrelin−/− mice were the same as their WT littermates, whereas the body weights of Ghsr−/− mice remained slightly lower (P < 0.05). Irrespective of genotype, biweekly food intake was greater in mice fed RD and less in mice fed the HF diet (P < 0.05, Fig. 1, C and D). We also analyzed whole-body composition by PXImus densitometer and found no significant differences between WT and null mice fed any of the test diets (data not shown). Collectively, these data suggest that ablation of ghrelin or the Ghsr fails to prevent DIO in adult mice of C57BL/6J background.
Figure 1.
Body weight (A and B) and cumulative food intake (C and D) of adult ghrelin- and Ghsr−/− mice fed 35% HF diet and RD. Body weight and food intake were measured biweekly from 16 to 26 wk of age in ghrelin−/− and Ghsr−/− mice and their WT littermates (n = 8). The data are presented as mean ± sem. Body weight, P < 0.05 WT vs. Ghsr−/− with both HF and RD.
Metabolic profile of mice fed HF diet and RD
The core body temperature of WT, ghrelin−/−, and Ghsr−/− mice was identical under ad libitum fed or 24- to 48-h fasted conditions (data not shown), suggesting that ghrelin/GHS-R signaling had no major effect on metabolic rate. Tschop et al. (3) reported that peripheral daily administration of ghrelin caused weight gain by reduced fat utilization as manifested by an increase in RQ. Wortley et al. (11) reported a reduced RQ in their mixed background N2 ghrelin−/− mice fed a 45% HF diet. To determine whether genetic background might influence metabolic fuel preference, we examined the metabolic characteristics of adult congenic ghrelin−/− and Ghsr−/− mice. Sixteen-week-old male mice were fed a 35% HF diet or RD for 10 wk, and then metabolic parameters were measured. We found no significant differences in energy expenditure (Fig. 2, A and B) between ghrelin−/− and Ghsr−/− mice compared with their WT littermates. RQ was significantly reduced in mice fed the HF diet, but the effects were indistinguishable between null mice and their WT littermates (Fig. 2, C and D). Interestingly, there was a modest but clearly decreased trend in the RQ of ghrelin−/− mice fed a RD. Indeed, the difference was statistically significant between WT and ghrelin−/− mice at a number of time points (as indicated by P < 0.05 from repeated-measures ANOVA, Fig. 2C); however, such a trend was absent in Ghsr−/− mice fed the same diet.
Total and active ghrelin levels in mice fed different diets
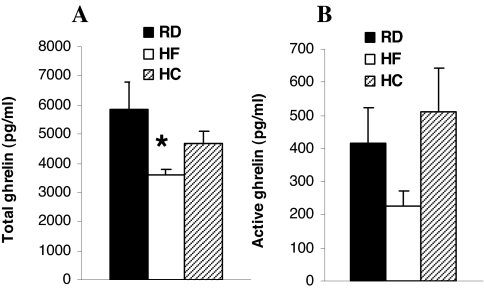
HF and high-carbohydrate diets influence total ghrelin concentrations differently (16). Active ghrelin (the octanoylated peptide) represents less than 10% of the total ghrelin peptide. To address whether composition of the diet plays a role in regulating active ghrelin concentrations, plasma levels of total ghrelin and active ghrelin were measured in WT mice fed different diets. Compared with mice fed normal and high-carbohydrate diets, total ghrelin was significantly lower in mice fed the HF diet (Fig. 3A, P < 0.05). Active ghrelin was also slightly reduced with HF feeding, but the difference did not reach statistical significance (Fig. 3B, P = 0.057).
Figure 3.
Total (A) and active (B) plasma ghrelin concentrations in 24-h-fasted WT male mice. The 16-wk-old mice were fed RD, 35% HF diet, or 75% high-carbohydrate (HC) diet for 10 wk. Plasma was collected at 26 wk of age (n = 8). *, P < 0.05.
Does deletion of Ghsr in DIO mice prevent weight gain after weight loss?
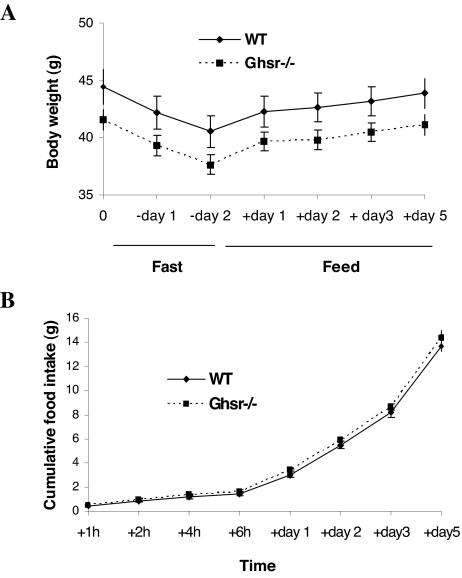
To address the question of whether the GHS-R has a role in adaptive response to weight loss, Ghsr−/− and WT control male mice were fed a HF diet for 10 wk, fasted for 48 h, then re-fed with HF diet. Body weight decreased and increased identically in both genotypes during the fasting and refeeding phases, respectively (Fig. 4A). This suggests that Ghsr ablation neither inhibits weight loss in obese mice (fasting phase) nor compromises weight gain after weight loss (refeeding phase). Food intake during the refeeding phase was also identical in the WT and null genotypes (Fig. 4B). Hence, the absence of the Ghsr in obese mice does not prevent weight gain after weight loss or fasting-induced hyperphagia.
Figure 4.
Changes in body weight and food intake during fasting and refeeding. The 14-wk-old Ghsr−/− (N10) male mice were fed a 36% HF diet for 10 wk. At 24 wk of age, the mice were fasted for 48 h and then allowed free access to food. Body weight (A) and food intake (B) at different intervals were measured (n = 9). P > 0.05, WT vs. Ghsr−/− mice, for body weights and food intake.
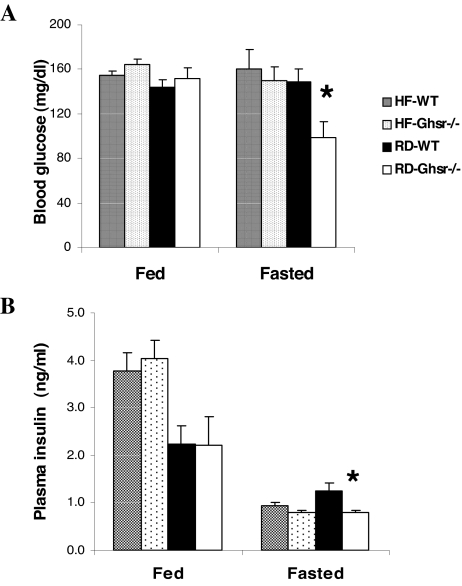
Glucose and insulin levels in Ghsr−/− mice fed regular and HF diets
An inverse association between ghrelin and insulin has been reported in humans (8). To address whether the ghrelin/GHS-R pathway is an essential regulator of glucose homeostasis according to diet, we compared the effects of HF feeding on glucose regulation in WT and Ghsr−/− mice. Although glucose levels were unaffected by HF feeding (Fig. 5A), insulin levels were increased in both genotypes (Fig. 5B). In WT and Ghsr−/− mice fed HF diet, fasting (18 h) had no effect on glucose but reduced plasma insulin levels identically in WT and Ghsr−/− mice. In contrast, in mice fed RD, fasting produced a marked reduction in blood glucose and plasma insulin in Ghsr−/−mice but not WT mice (Fig. 5B, P < 0.05). Plasma leptin levels were also compared. Leptin was 4-fold higher in mice fed the HF diet compared with those on RD irrespective of whether mice were of WT or mutant genotype (data not shown).
Figure 5.
Fed and fasted (18 h) blood glucose (A) and plasma insulin (B) of mice after 10 wk of 36% HF diet or RD feeding. Mice were 24 wk old when blood samples were collected (n = 9). *, P < 0.05 WT vs. Ghsr−/− for fasted glucose and insulin of mice fed RD.
Ghrelin- and Ghsr-null mice under conditions of negative energy balance
To study ghrelin’s role under conditions of 50% CR, body weight changes and blood glucose concentrations of individually caged 10-wk-old male WT, ghrelin−/−, and Ghsr−/− mice were compared (Fig. 6). Average daily food intake was measured for 12 d, and the mice were calorie restricted by feeding them 50% of their average daily intake for 40 d. Weight loss during CR was identical in WT and null mutant genotypes. Glucose levels fell in WT and in both null genotypes, but the drop in blood glucose concentrations was significantly greater in ghrelin−/− and Ghsr−/− mice than in their WT littermates (P < 0.05), reaching a nadir after 16 d. Interestingly, glucose levels in all genotypes were identical after 28 d of CR. Upon restoring ad libitum feeding on d 40, glucose levels returned to pre-CR levels, and body weight was restored.
Figure 6.
Body weight (A) and blood glucose (B) of 10-wk-old ghrelin−/− and Ghsr−/− mice and their littermate controls (WT) during 50% CR. The experimental mice were individually caged, and food intake was measured from −12 d to −4 d, and average food consumption was calculated. Beginning on d 0, a calculated amount of food representing 50% CR was given to the mice daily at 1500 h for 40 d; then the mice were switched back to ad libitum feeding. For ghrelin−/− and WT, n = 9; for Ghsr−/− and WT, n = 7. Blood glucose was measured by One Touch Ultra glucometer. *, P < 0.05, WT vs. ghrelin−/− and Ghsr−/−.
Discussion
When WT and null mice were fed either RD or HF diet, body weights of ghrelin−/− mice were no different from that of their WT littermates (Fig. 1A); however, body weights of Ghsr−/− mice were always modestly lower than WT littermates (Fig. 1B), consistent with their reduced IGF-I levels (9). IGF-I levels are not significantly lower in ghrelin−/− mice, as shown in our unpublished data and as reported by Wortley et al. (17). The lower IGF-I level in Ghsr−/− mice suggests that reduced body weight is likely due to the lack of GHS-R regulation of the GH/IGF-I pathway (18,19).
Because the DIO-prone C57BL/6 mice generally exhibit increased body weight and adiposity after 3 months of age (12), we subjected young adult (16-wk-old) mice to HF diet and measured metabolic parameters at 26 wk. Our results of mature N10 congenic ghrelin−/− and Ghsr−/− mice show that ablation of the ghrelin/GHS-R signal does not prevent DIO (Fig. 1). Wortley et al. (17) and Zigman et al. (20) showed that mixed-background N2 ghrelin−/− and Ghsr−/− mice are resistant to DIO when HF diets were fed immediately after weaning. The discrepancy could be due to the differences in genetic background of the mice used. The mice we used had been backcrossed with C57BL/6J mice for 10 generations to reach a congenic state, 99.9% identical to C57BL/6J, whereas Wortley et al. (17) and Zigman et al. (20) used N2 mice that are mixed background of C57BL/6 and 129Sv (75% C57BL/6 and 25% 129Sv). Because the 129Sv mouse strain has a lean phenotype, whereas C57BL/6 is highly susceptible to DIO, it is possible that the lean trait of the 129Sv background present in their null mice contributed to the DIO-resistant phenotype.
Besides genetic background, the other difference between our studies and those of Wortley et al. (17) and Zigman et al. (20) is that they fed their mice HF diet immediately after weaning, whereas our mice were raised on RD and then fed HF diet as adults. Perhaps during the early postweaning period, ghrelin modifies the development of neural circuitry in hypothalamic areas that control food intake. Hence, the lack of a ghrelin signal in the developing orexigenic circuits might cause immature animals to be resistant to the HF diet. As the animals age, the central circuits may develop compensatory pathways so that adult mutant genotypes become responsive to HF feeding (21). It is possible that as mice reach adulthood, they develop compensatory pathways to adjust for the loss of a ghrelin/GHS-R signal. There is other evidence supporting the idea that ghrelin’s effect on energy homeostasis is age dependent. Acute ghrelin injection increases feeding in fast-growing young (130 g) but not adult (370 g) rats (22). Vaccination to neutralize ghrelin in rats initially reduced weight gain (23), but the effect on appetite was transient and failed to show a long-term reduction on body weight (24). These results suggest that young, but not adult animals are responsive to regulation of ghrelin signaling. These collective results indicate that the metabolic effects of ghrelin are dependent upon age and genetic background.
Even though overwhelming pharmacological evidence suggests that ghrelin is an orexigenic factor (25,26), direct evidence supporting a role for ghrelin as an obesity hormone in mammals is highly debatable. In rats selectively bred to develop DIO, both ghrelin and GHS-R expression are reduced in the hypothalamus (27). Furthermore, when ghrelin and GHS-R expression were studied in seasonal mammals (e.g. bears) that experience marked changes in body weight and fat mass, the seasonal changes are not accompanied by changes in either ghrelin or GHS-R (28). We considered that perhaps an intricate balance between ghrelin and leptin regulates appetite and body composition. However, we found that the leptin-deficient ob/ob mouse bred onto the ghrelin−/− background has the same hyperphagic and obese phenotype as the ob/ob mouse, indicating that ghrelin unopposed by leptin does not play a dominant role in orexigenic regulation and fat deposition (29). Together, these data suggest that the ghrelin/GHS-R signaling pathway is a modulator rather than a dominant regulator of energy homeostasis.
Studies comparing RQ and energy expenditure in WT, ghrelin−/−, and Ghsr−/− mice have produced results that are at variance. Comparing with RD feeding, a marked reduction in RQ was observed in all genotypes (WT, ghrelin−/−, and Ghsr−/− mice) that were fed a HF diet; however, no differences were observed between genotypes. In comparing adult WT and congenic ghrelin−/− fed RD, the latter exhibit a modest decrease in RQ (Fig. 2C), which is consistent with the report that under RD, exogenous ghrelin increases RQ (3). However, the lack of reduction in RQ with the HF diet contrasts with the report generated from 14- to 16-wk-old mixed background (N2) ghrelin−/− mice, where HF feeding reduced RQ greater in ghrelin−/− mice than in WT control mice without affecting energy expenditure (11). It is noteworthy that when the HF feeding was initiated immediately after weaning, the authors failed to detect a difference in RQ between their ghrelin−/− mice and WT controls, but instead they detected increased energy expenditure in ghrelin−/− mice (17). When Ghsr−/− mice of mixed background (N2) were fed HF diet immediately after weaning, Zigman et al. (20) reported a reduced RQ in Ghsr−/− mice compared with WT mice with no difference in energy expenditure. The discrepancy of RQ data between our studies and the reports by other investigators could be due to the differences in genetic background and/or age of the mice.
It has been reported that macronutrient type regulates total ghrelin levels. Administration of fat suppresses circulating ghrelin levels much less than carbohydrates or proteins (16). Our data show that in contrast to a normal or high-carbohydrate diet, a HF diet reduced plasma ghrelin levels, and HF feeding proportionately reduced the concentrations of ghrelin and desacyl-ghrelin (Fig. 3). Because total ghrelin concentrations are lower in obese humans and rodents (30,31), we postulate that the decrease in ghrelin levels induced by the HF diet is related to DIO. It was reported that octanoate attenuates adipogenesis in 3T3-L1 preadipocytes (32), which suggests that the octanoyl group of ghrelin plays a role in diet-induced adipogenesis. However, our data showed that the ratio of ghrelin and desacyl ghrelin were comparable regardless of the diets.
Studies in patients subjected to stomach bypass surgery showed a correlation between low ghrelin level and sustained weight loss (6,7); therefore, it was speculated that ghrelin ablation prevents weight gain after weight loss. However, our results show that deletion of Ghsr does not prevent DIO and does not prevent weight gain after fasting-induced weight loss or fasting-induced hyperphagia (Fig. 4). Our data suggest that sustained weight loss after stomach bypass surgery may not be regulated by ghrelin.
Under fasting conditions, RD-fed Ghsr−/− mice exhibit lower glucose and insulin levels than RD-fed WT mice, suggesting that GHS-R pays a role in glucose homeostasis and Ghsr deletion increases insulin sensitivity (Fig. 5). However, these differences are obscured in HF-fed mice, suggesting that the effects of GHS-R on glucose homeostasis are associated with the energy state of the animals.
How does ablation of ghrelin signaling increase insulin sensitivity? Phosphoinositide (PI) 3-kinase is involved in the insulin-mediated effects of glucose uptake, lipid deposition, and adiponectin secretion in adipocytes and genetic disruption of the PI 3-kinase subunit p85α increases insulin sensitivity. Adipose tissue plays a critical role in the antagonistic effects of GH on insulin actions on carbohydrate and lipid metabolism. GH regulates p85α expression and PI 3-kinase activity in white adipose tissue (33). Ghrelin activation of the GHS-R stimulates GH release (9); therefore, the amplitude of episodic GH is expected to be lower in Ghsr−/− mice. Because GH secretion is episodic with peaks approximately every 3 h, direct GH measurement requires sequential blood sampling and fluid replacement at 10-min intervals for up to 12 h, which is impractical in mice. However, consistent with reduced amplitude of GH release, serum IGF-I is lower in Ghsr−/− than in WT controls. GH, as a lipolytic hormone, is known to increase insulin resistance (34); therefore, increased insulin sensitivity exhibited by Ghsr−/− mice might be a consequence of lower GH levels.
The ghrelin−/− and Ghsr−/− mice had lower blood glucose levels than their WT littermates when they were subjected to 50% CR (Fig. 6). Hence, ghrelin and the GHS-R appear to be involved in providing a counterregulatory glucose response during negative energy balance. Plasma ghrelin concentration is inversely correlated with body weight and body fat; CR is associated with increased plasma ghrelin concentration (35). Under CR conditions, fatty acids are mobilized and oxidized to potentially produce octanoic acid resulting in octanoylation of desacyl-ghrelin. The greater glucose levels of WT mice during the early phases of the CR may be driven by this increase in ghrelin. The fact that the difference in glucose is less pronounced during prolonged CR may be explained by depletion of the supply of free fatty acids and induction of ketosis.
Using glucose tolerance tests and hyperinsulinemic-euglycemic clamp studies, we demonstrated that ghrelin−/− mice have improved glucose tolerance and increased insulin sensitivity (29). In agreement with Dezaki et al. (36), we showed that ghrelin inhibits glucose-stimulated insulin release (29). Therefore, we speculate that the inhibitory effect of ghrelin on insulin release provides tonic regulation of pancreatic β-cells and restrains insulin secretory activity during food deprivation (37), which is consistent with the greater reductions in blood glucose levels observed during CR in ghrelin−/− and Ghsr−/− mice (Fig. 6).
In conclusion, ghrelin and the GHS-R appear to be nonessential regulators of appetite in adult mice. Deletion of ghrelin or the Ghsr in these adult C57BL/6J background mice does not prevent DIO or prevent weight gain after weight loss. The collective data suggest that ghrelin’s effects on metabolic fuel preference are transient and may not have a significant effect throughout the lifespan. Perhaps adult C57BL/6J ghrelin−/− and Ghsr−/− mice are subject to metabolic adaptations especially in regard to energy intake and expenditure. The degree and type of adaptation may be age and background dependent. Nevertheless, the ghrelin/GHS-R pathway, at least in highly selective backcross bred male mice, appears to play an important role in glucose homeostasis by regulating insulin sensitivity and glucose sensing, particularly under conditions of negative energy balance.
Acknowledgments
We thank Mr. Firoz A. Vohra at the U.S. Department of Agriculture/Agricultural Research Service Children’s Nutrition Research Center and Department of Pediatrics for his participation in calorimetry studies, Dr. Mark Asnicar for his insightful intellectual input throughout the studies, Linda M. Hicks and Salmaan A. Jawaid for their technical assistance with animal care and genotyping, and Michael R. Honig and Edith A. Gibson for editorial assistance.
Footnotes
We gratefully acknowledge the support of the National Institutes of Health Grants RO1AG18895 and RO1AG19230 (RGS) and the support of the Ted Nash Long Life Foundation.
Disclosure Summary: Y.S. and N.F.B have nothing to disclose. J.M.G. is the principal investigator for a trial of a ghrelin agonist in cancer cachexia. R.G.S. is a paid advisory board member for Elixir Pharmaceuticals and Znomics, Inc.
First Published Online November 15, 2007
Abbreviations: CR, Caloric restriction; DIO, diet-induced obesity; GHS-R, GH secretagogue receptor; HF, high fat; PI, phosphoinositide; RD, regular diet; RQ, respiratory quotient; VCO2, carbon dioxide production; VO2, oxygen consumption; WT, wild type.
References
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K 1999 Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S 2001 A role for ghrelin in the central regulation of feeding. Nature 409:194–198 [DOI] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML 2000 Ghrelin induces adiposity in rodents. Nature 407:908–913 [DOI] [PubMed] [Google Scholar]
- Ariyasu H, Takaya K, Hosoda H, Iwakura H, Ebihara K, Mori K, Ogawa Y, Hosoda K, Akamizu T, Kojima M, Kangawa K, Nakao K 2002 Delayed short-term secretory regulation of ghrelin in obese animals: evidenced by a specific RIA for the active form of ghrelin. Endocrinology 143:3341–3350 [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Masuzaki H, Hosoda K, Aizawa-Abe M, Suga J, Suda M, Ebihara K, Iwai H, Matsuoka N, Satoh N, Odaka H, Kasuga H, Fujisawa Y, Inoue G, Nishimura H, Yoshimasa Y, Nakao K 1999 Increased glucose metabolism and insulin sensitivity in transgenic skinny mice overexpressing leptin. Diabetes 48:1822–1829 [DOI] [PubMed] [Google Scholar]
- Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ 2002 Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346:1623–1630 [DOI] [PubMed] [Google Scholar]
- Leonetti F, Silecchia G, Iacobellis G, Ribaudo MC, Zappaterreno A, Tiberti C, Iannucci CV, Perrotta N, Bacci V, Basso MS, Basso N, Di Mario U 2003 Different plasma ghrelin levels after laparoscopic gastric bypass and adjustable gastric banding in morbid obese subjects. J Clin Endocrinol Metab 88:4227–4231 [DOI] [PubMed] [Google Scholar]
- Holdstock C, Engstrom BE, Ohrvall M, Lind L, Sundbom M, Karlsson FA 2003 Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab 88:3177–3183 [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang P, Zheng H, Smith RG 2004 Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA 101:4679–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Ahmed S, Smith RG 2003 Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol 23:7973–7981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW 2004 Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci USA 101:8227–8232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK 2001 Nutrient preference and diet-induced adiposity in C57BL/6ByJ and 129P3/J mice. Physiol Behav 72:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Alleva AM 1990 Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr 51:963–969 [DOI] [PubMed] [Google Scholar]
- West DB, Boozer CN, Moody DL, Atkinson RL 1992 Dietary obesity in nine inbred mouse strains. Am J Physiol 262:R1025–R1032 [DOI] [PubMed] [Google Scholar]
- Taylor BA, Phillips SJ 1996 Detection of obesity QTLs on mouse chromosomes 1 and 7 by selective DNA pooling. Genomics 34:389–398 [DOI] [PubMed] [Google Scholar]
- Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE 2005 Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology 146:845–850 [DOI] [PubMed] [Google Scholar]
- Wortley KE, Del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW 2005 Absence of ghrelin protects against early-onset obesity. J Clin Invest 115:3573–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman IM, Bach MA, Van Cauter E, Farmer M, Krupa DA, Taylor AM, Schilling LM, Cole KY, Skiles EH, Pezzoli SS, Hartman ML, Veldhuis JD, Gormley GJ, Thorner MO 1996 Stimulation of the growth hormone (GH)-insulin-like growth factor-I axis by daily oral administration of a GH secretagogue (MK-0677) in healthy elderly subjects. J Clin Endocrinol Metab 81:4249–4257 [DOI] [PubMed] [Google Scholar]
- Smith RG, Van der Ploeg LH, Howard AD, Feighner SD, Cheng K, Hickey GJ, Wyvratt Jr MJ, Fisher MH, Nargund RP, Patchett AA 1997 Peptidomimetic regulation of growth hormone secretion. Endocr Rev 18:621–645 [DOI] [PubMed] [Google Scholar]
- Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK 2005 Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 115:3564–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove KL, Cowley MA 2005 Is ghrelin a signal for the development of metabolic systems? J Clin Invest 115:3393–3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilg S, Lutz TA 2006 The orexigenic effect of peripheral ghrelin differs between rats of different age and with different baseline food intake, and it may in part be mediated by the area postrema. Physiol Behav 87:353–359 [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Iwasaki S, Moss JA, Chang J, Otsuji J, Inoue K, Meijler MM, Janda KD 2006 Vaccination against weight gain. Proc Natl Acad Sci USA 103:13226–13231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, Xu J, Chinookoswong N, Veniant-Ellison M, Gregg C, Liu S, Chen D, Brankow D, Lindberg R, Gu W, Immunononeutralization of peripheral acyl-ghrelin with a monoclonal antibody in diet-induced obese mice metabolic disorders. Program of the 88th Annual Meeting of The Endocrine Society, Boston, MA, 2006 [Google Scholar]
- Muccioli G, Tschop M, Papotti M, Deghenghi R, Heiman M, Ghigo E 2002 Neuroendocrine and peripheral activities of ghrelin: implications in metabolism and obesity. Eur J Pharmacol 440:235–254 [DOI] [PubMed] [Google Scholar]
- Cummings DE, Overduin J, Foster-Schubert KE 2005 Roles for ghrelin in the regulation of appetite and body weight. Curr Opin Endocrinol Diabetes 12:72–79 [DOI] [PubMed] [Google Scholar]
- Levin BE, Dunn-Meynell AA, Ricci MR, Cummings DE 2003 Abnormalities of leptin and ghrelin regulation in obesity-prone juvenile rats. Am J Physiol 285:E949–E957 [DOI] [PubMed] [Google Scholar]
- Tups A, Helwig M, Khorooshi RM, Archer ZA, Klingenspor M, Mercer JG 2004 Circulating ghrelin levels and central ghrelin receptor expression are elevated in response to food deprivation in a seasonal mammal (Phodopus sungorus). J Neuroendocrinol 16:922–928 [DOI] [PubMed] [Google Scholar]
- Sun Y, Asnicar M, Saha PK, Chan L, Smith RG 2006 Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell metabolism 3:379–386 [DOI] [PubMed] [Google Scholar]
- Marzullo P, Verti B, Savia G, Walker GE, Guzzaloni G, Tagliaferri M, Di Blasio A, Liuzzi A 2004 The relationship between active ghrelin levels and human obesity involves alterations in resting energy expenditure. J Clin Endocrinol Metab 89:936–939 [DOI] [PubMed] [Google Scholar]
- Beck B, Musse N, Stricker-Krongrad A 2002 Ghrelin, macronutrient intake and dietary preferences in Long-Evans rats. Biochem Biophys Res Commun 292:1031–1035 [DOI] [PubMed] [Google Scholar]
- Han J, Farmer SR, Kirkland JL, Corkey BE, Yoon R, Pirtskhalava T, Ido Y, Guo W 2002 Octanoate attenuates adipogenesis in 3T3-L1 preadipocytes. J Nutr 132:904–910 [DOI] [PubMed] [Google Scholar]
- del Rincon JP, Iida K, Gaylinn BD, McCurdy CE, Leitner JW, Barbour LA, Kopchick JJ, Friedman JE, Draznin B, Thorner MO 2007 Growth hormone regulation of p85α expression and phosphoinositide 3-kinase activity in adipose tissue: mechanism for growth hormone-mediated insulin resistance. Diabetes 56:1638–1646 [DOI] [PubMed] [Google Scholar]
- Segerlantz M, Bramnert M, Manhem P, Laurila E, Groop LC 2001 Inhibition of the rise in FFA by acipimox partially prevents GH-induced insulin resistance in GH-deficient adults. J Clin Endocrinol Metab 86:5813–5818 [DOI] [PubMed] [Google Scholar]
- Barazzoni R, Zanetti M, Biolo G, Guarnieri G 2005 Metabolic effects of ghrelin and its potential implications in uremia. J Ren Nutr 15:111–115 [DOI] [PubMed] [Google Scholar]
- Dezaki K, Hosoda H, Kakei M, Hashiguchi S, Watanabe M, Kangawa K, Yada T 2004 Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in β-cells: implication in the glycemic control in rodents. Diabetes 53:3142–3151 [DOI] [PubMed] [Google Scholar]
- Egido EM, Rodriguez-Gallardo J, Silvestre RA, Marco J 2002 Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur J Endocrinol 146:241–244 [DOI] [PubMed] [Google Scholar]