Abstract
Chronic kidney disease (CKD) is associated with an increase in inflammatory cytokines and can result in cachexia with loss of muscle and fat stores. We previously demonstrated the efficacy of treating a model of cancer cachexia with ghrelin and a ghrelin receptor agonist. Currently, we examine a surgical model of CKD in rats, resulting in uremia and decreased accrual of lean body mass. Treatment with ghrelin and two ghrelin receptor agonists (BIM-28125 and BIM-28131) resulted in increased food intake and an improvement in lean body mass accrual that was related in part to a decrease in muscle protein degradation as assessed by muscle levels of the 14-kDa actin fragment resulting from cleaved actomyosin. Additionally, there was a decrease in circulating inflammatory cytokines in nephrectomized animals treated with ghrelin relative to saline treatment. Ghrelin-treated animals also had a decrease in the expression of IL-1 receptor in the brainstem and a decrease in expression of prohormone convertase-2, an enzyme involved in the processing of proopiomelanocortin to the anorexigenic peptide α-MSH. We conclude that ghrelin treatment in uremia results in improved lean mass accrual in part due to suppressed muscle proteolysis and possibly related to antiinflammatory effects.
PATIENTS WITH CHRONIC kidney disease (CKD) often exhibit symptoms of anorexia, loss of lean body mass, and increased energy expenditure, matching the syndrome of cachexia seen in other chronic diseases such as cancer, chronic heart failure, and AIDS (1,2). In addition, patients with CKD-induced uremia frequently exhibit a dangerous decrease in protein nutritional status, including decreased levels of albumin and prealbumin as well as catabolism of actinomycin in muscle tissue via caspase-3 and the ubiquitin-proteasome pathway (3,4,5,6,7,8). As with other diseases associated with cachexia, these catabolic processes seen in CKD are linked to systemic inflammatory changes, including increases in acute-phase reactants such as C-reactive protein and inflammatory cytokines (9,10,11,12,13,14,15). Indeed, this inflammation is felt to be in the causative pathway of the production of cachexia (16,17,18,19).
CKD is similar to other diseases associated with cachexia in that the symptoms of cachexia in CKD are associated with a decrease in quality of life and an increase in mortality (20,21,22,23,24,25,26). Unfortunately, no single agent has emerged as a beneficial treatment for cachexia (27). One potential treatment for cachexia in multiple disease states is the use of the orexigenic hormone ghrelin (28,29). Although ghrelin levels are already elevated above normal in cachexia-associated disease states such as cancer, heart failure, and CKD, administration of supraphysiological doses of ghrelin has been shown to increase food intake in human subjects with cancer and heart failure (30,31,32,33,34).
We have previously delivered ghrelin and ghrelin agonists in a rat model of cancer cachexia, demonstrating improved lean body mass retention and increases in gene expression of agouti-related peptide (AgRP) and neuropeptide Y(NPY), two orexigenic neuropeptides produced in the arcuate nucleus of the hypothalamus, an important appetite-regulating center (35).
In addition to its effects on appetite regulation, ghrelin has been shown to have antiinflammatory properties. The GH secretagogue (GHS)-1a receptor is expressed on lymphocytes, and administration of the GHS-1a agonist GHRP-2 in the setting of a mouse model of arthritis resulted in a decrease in IL-6 levels and reduced signs of joint inflammation (36). Also, macrophages that have been pretreated with ghrelin show reduced IL-6 production in response to inflammatory stimuli. In our rat model of cancer cachexia, we showed a decrease in expression of the IL-1 receptor in the hypothalamus and the brainstem after ghrelin treatment (35).
In the present experiments, we evaluated the efficacy of ghrelin and two ghrelin-receptor agonists in a rat model of surgically induced CKD. In these experiments, we demonstrated increased food intake, lean body mass retention, decreased systemic inflammation, and a decreased muscle catabolism, further establishing a role for ghrelin in the treatment of cachexia.
Materials and Methods
Compounds
BIM-28131, BIM-28125, and human ghrelin were provided by IPSEN (Milford, MA) (37,38,39). BIM-28131 and BIM-28125 are peptide analogs that bind to the known ghrelin receptor (GHS-1a) with sub-nanomolar affinity (Ki, 0.42 ± 0.063 and 0.48 ± 0.16 nm, respectively). In this regard, synthetic analogs show about three times greater affinity than native ghrelin (Ki, 1.12 ± 0.17 nm). BIM-28131 and BIM-28125 are also more potent (EC50, 0.71 ± 0.09 and 0.98 + 0.08 nm, respectively) in activating the GHS-1a receptor than native ghrelin (EC50, 4.2 ± 1.2 nm) as assessed in vitro by calcium mobilization. BIM-28131 and BIM-28125 have greater enzymatic stability in plasma than native ghrelin (t1/2 rat plasma was 24 vs. 1.9 h for ghrelin) and, when injected iv, are observed to have a 10-fold greater circulating half-life as compared with native ghrelin.
Experimental animals
Studies were approved by the Institutional Animal Care and Use Committee of the Oregon Health and Science University and conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. F344/NTacfBR male rats (Taconic Farms, Inc., Germantown, NY) were housed two per cage, fed rat chow (Diet 5001; Purina Mills, Inc., St. Louis, MO) and acclimated for at least 3 d before use.
Nephrectomy procedure
The five sixths nephrectomy was performed in two stages. For stage 1, the animals were anesthetized with standard rat cocktail and placed prone in a clean environment. A 1-cm posterior incision was made on the right flank through which the right kidney was located. For animals undergoing nephrectomy, the renal capsule was removed and the upper and lower one third of the kidney was transected and the resultant wound cauterized, leaving the middle one third of the kidney with the renal artery and vein intact. For animals receiving a sham operation, the renal capsule was opened up and cauterized to simulate the manipulations performed in the nephrectomy. The external surgical wounds were then closed via suture and allowed to recover in individual housing.
Nine days after the initial surgery, the animals were again anesthetized and placed prone in the surgical area. This time a left 1-cm incision was performed and the left kidney isolated. For animals undergoing nephrectomy, the renal capsule was removed and the vasculature was tied off with suture. The vascular bundle was then transected distal to the suture and the entire kidney removed. Any residual bleeding was cauterized. For animals in the sham group, the renal capsule was removed and cauterized. The external surgical wounds were closed with suture. Although still under anesthesia, the osmotic mini-pumps were placed in all animals. A 1-cm incision was made in the midline overlying the posterior thorax at the level of the forelimbs, and the underlying sc space was opened via blunt dissection. The osmotic minipumps were placed inside and the incision closed with suture. This procedure marked d 0 of treatment.
Compound administration
A continuous sterile infusion of BIM-28125, BIM-28131, human ghrelin, or saline was administered at a rate of 0.5 μl/h for 14 d sc using Alzet mini-osmotic pumps (model 2002; Durect Corp., Cupertino, CA). The day before implantation of the pumps, the mean body weight of each group was determined. To calculate the concentration required for the treatment group, we considered the molecular weight of each compound, the dose (150 nmol/kg·d), and the pump delivery rate. Each compound was dissolved in vehicle solution (2% inactivated rat serum saline, 5% Tween 80) sonicated, and filtered through a 0.2-μl syringe filter.
Body composition
Body composition was determined 2 d before the second stage of the nephrectomy or sham operation under anesthesia by dual-energy x-ray absorptiometry (DEXA, Discovery A-QDR Series; Hologic Corp., Waltham, MA) and on d 14 of compound treatment before euthanasia with CO2.
Tissue collection
After euthanasia, blood, brain, stomach, and muscle were collected. A subset of the rats had their hypothalami removed, preserved in RNAlater solution (Applied Biosystems, Inc. Foster City, CA), and stored at −70 C for extraction of RNA and RT-PCR analysis. A subset of the rats had their hypothalami and brainstems removed, preserved in RNAlater solution, and stored at −70 C for extraction of RNA and RT-PCR analysis. Hypothalamic blocks were dissected by making coronal transections at the optic chiasm and at the intersection between the hypothalamus and the mammillary bodies and sagittal transections along the optic tracts. Cortex was then removed at the level of the corpus callosum. Brainstem blocks were dissected by removal of the cerebellum and coronal transections at the rostral border of the pons and at the spinal cord.
Cytokine measurement
Rat serum samples were tested simultaneously for cytokines IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, and TNF-α using a Bio-Plex rat cytokine 9-Plex assay (Bio-Rad Laboratories, Hercules, CA). The assay was run according to the manufacturer’s instructions. In brief, the premixed standards were reconstituted in 0.5 ml of a Bio-Plex human serum standard diluent, generating a stock concentration of 50,000 pg/ml for each cytokine. The standard stock was serially diluted in the Bio-Plex rat serum standard diluent to generate eight points for the standard curve. The assay was performed in a 96-well filtration plate supplied with the assay kit. Premixed beads (50 μl) coated with target capture antibodies were transferred to each well of the filtration plate and washed twice with Bio-Plex wash buffer. The samples were diluted 1:4 in the Bio-Plex serum sample diluent. Premixed standards or diluted samples (50 μl) were added to each well containing washed beads. The plate was shaken and incubated at room temperature for 30 min at low speed (300 rpm). After incubation and washing, premixed biotin-conjugated detection antibodies were added to each well. Then the plate was incubated for 30 min with shaker at low speed (300 rpm). After incubation and washing, streptavidin and phycoerythrin was added to each well. The incubation was terminated after shaking for 10 min at room temperature. After washing, the beads were resuspended in 125 μl Bio-Plex assay buffer. Beads were read on the Bio-Plex suspension array system (Bio-Rad), and the data were analyzed using Bio-Plex Manager software version 3.0 with 5PL curve fitting.
RNA preparation and RT-PCR
Total RNA was extracted using QIAGEN RNeasy kits (QIAGEN, Inc., Valencia, CA), and DNA was removed from total RNA using RNase-free DNase (QIAGEN). RT reactions were prepared using a TaqMan reverse transcription kit (Applied Biosystems, Inc., Foster City, CA). For each reaction, cDNA synthesis was prepared using 500 ng RNA in a reaction containing 4 μl 10× RT buffer, 9 μl 25 mm MgCl2, 8 μl 10 mm dNTPs, 1.5 μl 50 μm random hexamers, 1 μl RNase inhibitor, 1.5 μl MulitScribe reverse transcriptase, and enough nuclease-free water to make 40 μl. RT reactions were performed on an Eppendorf Mastercycler (Eppendorf AG, Hamburg, Germany) programmed for 25 C for 10 min, 37 C for 1 h, and 95 C for 5 min. Samples were diluted with 40 μl nuclease-free water stored at 4 C until RT-PCR was performed.
RT-PCR was performed on an ABI 7300 real-time PCR system using rat-specific primer probe sets obtained from Applied Biosystems. The GenBank acquisition numbers corresponding to these primer probe sets was as follows: AgRP, XM 574228.2; NPY, NM 012614.1; proopiomelanocortin (POMC), NM 139326.2; prohormone convertase-2 (PC-2), NM 012746.1; IL-1β, NM 031512.1; and IL-1RI, NM 013123.2. Each RT-PCR contained 10 μl TaqMan Universal PCR Master Mix, 1 μl Assays-on-Demand Gene Expression Assay Mix, 4 μl nuclease-free water, and 5 μl cDNA. Samples and endogenous controls (Eukaryotic 18S rRNA) were run in duplicate to assure repeatability. Auto cycle threshold values were calculated using 7300 RQ Study Software version 1.3 and verified. Gene expression values are expressed fold change relative to sham mean.
GH and IGF-I assays
Serum collected at the time of euthanasia was tested for GH levels by RIA (National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA). A separate aliquot underwent ethanol/HCl extraction to remove binding proteins and was tested for IGF-I levels, using rat IGF-I enzyme immunoassay (DSL-2900; Diagnostic Systems Laboratories, Webster, TX). The standard curve of the assay was performed in accordance with the manufacturer’s provided samples.
Muscle proteolysis
Hind-limb muscles were collected at the time of euthanasia and snap frozen at −70 C before being tested for the presence of the 14-kDa actin fragment, an indicator of muscle protein degradation as described (5,40,41,42). Briefly, the 14-kDa actin fragment was detected in the insoluble fraction of gastrocnemius muscles. Muscles were homogenized in PBS containing proteinase inhibitor cocktail (Roche, Indianapolis, IN) and centrifuged, and the pellet was dissolved in 2× SDS loading buffer. The mixture was boiled for 20 min and centrifuged at top speed for 5 min. Proteins in the supernatant were separated by 15% SDS-PAGE, and the 14-kDa actin fragment was detected by immunoblot analysis using antibodies that detect the carboxy terminus of actin (Sigma Chemical Co., St. Louis, MO). We did not collect muscles from rats treated with BIM-28125 and had only a partial set of muscles from animals treated with BIM-28131. Consequently, we examined only muscles from the nephrectomized (Neph)/saline, Neph/ghrelin, and sham/saline groups to assess for the presence of increased protein breakdown.
Statistics
One-way ANOVA was used to determine differences among the nephrectomy and sham groups, using a Bonferoni post hoc test; P < 0.05 was considered significant. In the case of the serum cytokines, each individual set of data was considered by ANOVA, and the composite of all of the cytokine levels together was considered after normalizing all levels to the sham/saline group, using a mixed-model analysis for a trend for cytokines values among the different groups. This model takes into account the propensity of some animals to have higher or lower cytokine levels across all of the different cytokines and accounts for within-sample variability.
Results
Production of CKD
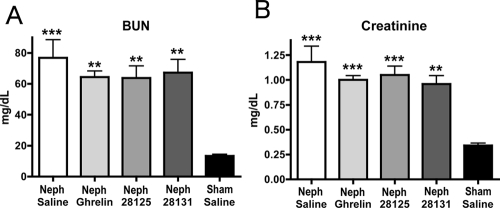
Subtotal nephrectomy produced CKD in these rats. There was a significant 5-fold increase in blood urea nitrogen (BUN) [Neph/saline 76.75 ± 11.81 mg/dl, n = 8 (P < 0.001 vs. sham/saline); Neph/ghrelin 64.36 ± 3.88 mg/dl, n = 11 (P < 0.01 vs. sham/saline); Neph/BIM-28125 63.13 ± 7.89 mg/dl, n = 10 (P < 0.01 vs. sham/saline); Neph/BIM-28131 67.13 ± 8.57 mg/dl, n = 8 (P < 0.01 vs. sham/saline); sham/saline 13.40 ± 1.03 mg/dl, n = 5] and a 4-fold increase in serum creatinine (Neph/saline 1.18 ± 0.16 mg/dl, n = 8 (P < 0.001 vs. sham/saline); Neph/ghrelin 1.00 ± 0.043 mg/dl, n = 11 (P < 0.001 vs. sham/saline); Neph/BIM-28125 1.05 ± 0.089 mg/dl, n = 10 (P < 0.001 vs. sham/saline); Neph/BIM-28131 0.96 ± 0.084 mg/dl, n = 8 (P < 0.01 vs. sham/saline); sham/saline 0.24 ± 0.024 mg/dl, n = 5] (Fig. 1, A and B). There was no significant difference between the Neph/ghrelin group and the Neph/saline group.
Figure 1.
BUN and creatinine. BUN (A) and creatinine (B) at final euthanasia for Neph animals treated with saline, ghrelin, BIM-28125, or BIM-28131 and animals that received a sham surgery and were treated with saline. All treatment compounds were given at a dose of 150 nmol/kg·d; both the compounds and saline were delivered by osmotic mini-pump for 14 d. Significance is shown compared with sham/saline: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Body weights and food intake
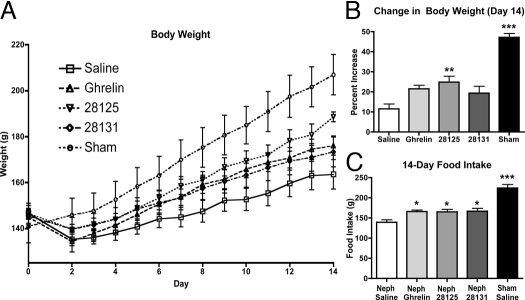
After subtotal nephrectomy, rats receiving ghrelin, BIM-28125, and BIM-28131 exhibited steady improvement in weight, although the weight gain vs. Neph animals receiving saline was significant only for the BIM-28125-treated groups. Sham-operated rats exhibited an improvement in weight relative to all groups [Neph/saline 11.53 ± 2.51 g, n = 8; Neph/ghrelin 21.58 ± 1.75 g, n = 11; Neph/BIM-28125 24.93 ± 2.80 g, n = 10 (P < 0.01 vs. Neph/saline); Neph/BIM-28131 19.40 ± 3.31 g, n = 8; sham/saline 47.17 ± 2.01 g, n = 5 (P < 0.001 vs. all other groups)] (Fig. 2, A and B).
Figure 2.
Body weight changes and food intake. Body weight time course (A), final percent change from baseline (B), and total food intake (C) over 14 d of treatment for Neph animals treated with saline, ghrelin, BIM-28125, or BIM-28131 and animals who received a sham surgery and were treated with saline. Significance is shown compared with Neph/saline: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Treatment with ghrelin and ghrelin-receptor agonist after subtotal nephrectomy significantly improved food intake compared with the Neph/saline group [Neph/saline 139.08 ± 6.25 g, n = 8; Neph/ghrelin 165.74 ± 4.33 g, n = 11 (P < 0.05 vs. Neph/saline); Neph/BIM-28125 165.34 ± 5.72 g, n = 10 (P < 0.05 vs. Neph/saline); Neph/BIM-28131 166.64 ± 7.52 g, n = 8 (P < 0.05 vs. Neph/saline); sham/saline 224.44 ± 8.52 g, n = 5 (P < 0.001 vs. all other groups)] (Fig. 2C). The sham-surgery group had a significant increase in food intake relative to the all of the nephrectomy groups.
DEXA
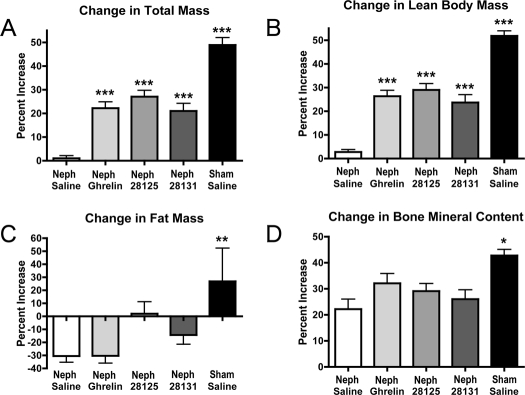
After subtotal nephrectomy, CKD rats receiving saline had no increase in body mass as measured by DEXA, but CKD rats receiving ghrelin, BIM-28125, or BIM-28131 exhibited a significant increase in body mass [Neph/saline +0.98 ± 1.16%, n = 8; Neph/ghrelin +22.21 ± 2.65%, n = 11 (P < 0.001 vs. Neph/saline); Neph/BIM-28125 +27.07 ± 2.63%, n = 10 (P < 0.001 vs. Neph/saline); Neph/BIM-28131 +20.97 ± 3.22%, n = 8 (P < 0.001 vs. Neph/saline); sham/saline +48.85 ± 3.09%, n = 5 (P < 0.001 vs. Neph/saline)] (Fig. 3A). Sham-operated rats had a significant increase in body mass relative to each of the nephrectomy groups (P < 0.05).
Figure 3.
Body composition changes. Changes in total body mass (A), lean body mass (B), fat mass (C), and bone mineral content (D) as determined by DEXA before and after 14 d of treatment for Neph animals treated with saline, ghrelin, BIM-28125, or BIM-28131 and animals who received a sham surgery and were treated with saline. Significance is shown compared with Neph/saline: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
There was a significant increase in lean body mass for CKD rats receiving ghrelin, BIM-28125, and BIM-28131 and the sham-operated rats. There was no increase in lean body mass of the Neph/saline-treated rats. CKD rats receiving ghrelin, BIM-28125, or BIM-28131 exhibited an increase in lean body mass over that seen in the Neph/saline-treated rats, as did sham-operated rats relative to all groups [Neph/saline +2.79 ± 0.98%, n = 8; Neph/ghrelin +26.30 ± 2.50%, n = 11 (P < 0.001 vs. Neph/saline); Neph/BIM-28125 +28.96 ± 2.65%, n = 10 (P < 0.001 vs. Neph/saline); Neph/BIM-28131 +23.62 ± 3.34%, n = 8 (P < 0.001 vs. Neph/saline); sham/saline +51.79 ± 2.14%, n = 5 (P < 0.001 vs. Neph/saline)] (Fig. 3B).
CKD rats receiving saline or ghrelin has a significant decrease in their fat mass, whereas those receiving BIM-28125 and BIM-28131 and the sham group exhibited no significant change in fat mass. There was no significant difference in the change in fat mass in the CKD groups relative to each other, although there was a significant increase in the sham-operated animals relative to the Neph/saline and Neph/ghrelin groups (Neph/saline −30.48 ± 4.82%, n = 8; Neph/ghrelin −30.40 ± 5.57%, n = 11; Neph/BIM 28125 +2.01 ± 9.12%, n = 10; Neph/BIM 28131 −14.28 ± 7.20%, n = 8; sham/saline +26.83 ± 25.52%, n = 5) (Fig. 3C).
There was a significant increase in bone mineral content in all groups relative to baseline values; among the CKD groups, however, there was no significant difference among their changes in bone mineral content. Sham-operated rats exhibited an increase in bone mineral content relative to the Neph/saline group [Neph/saline +22.17 ± 3.81%, n = 8; Neph/ghrelin +32.05 ± 3.78%, n = 11; Neph/BIM-28125 +29.11 ± 2.90%, n = 10; Neph/BIM-28131 +26.00 ± 3.56%, n = 8; sham/saline +42.78 ± 2.32, n = 5 (P < 0.05 vs. Neph/saline)] (Fig. 3D).
Cytokines
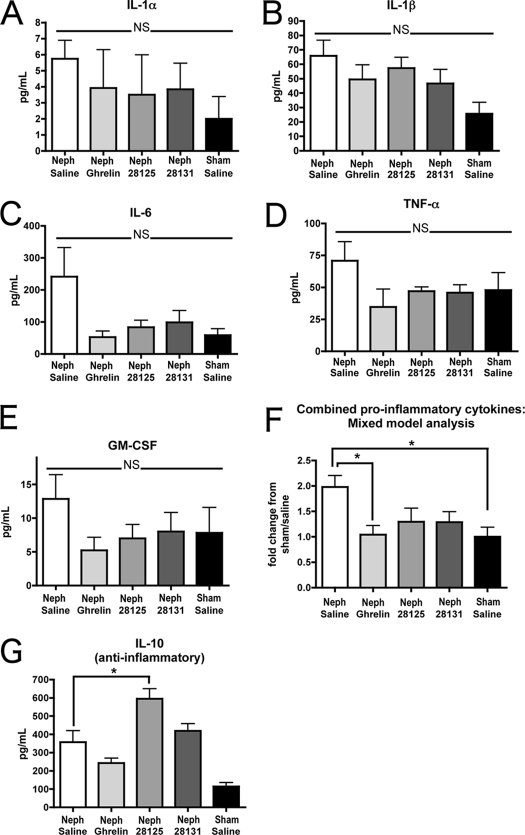
Among the proinflammatory cytokines measured, trends were found in all measured cytokines, but there was no significant difference in any single cytokine within the different treatment groups (IL-1α: Neph/saline 5.75 ± 1.14 pg/ml, n = 10; Neph/ghrelin 3.92 ± 2.39 pg/ml, n = 11; Neph/BIM-28125 3.50 ± 2.49 pg/ml, n = 9; Neph/BIM-28131 3.84 ± 1.62 pg/ml, n = 11; sham/saline 2.00 ± 1.39 pg/ml, n = 5; IL-1β: Neph/saline 65.81 ± 10.84 pg/ml, n = 19; Neph/ghrelin 49.52 ± 10.03 pg/ml, n = 22; Neph/BIM-28125 57.37 ± 7.48 pg/ml, n = 9; Neph/BIM-28131 46.58 ± 9.87 pg/ml, n = 11; sham/saline 25.72 ± 7.89 pg/ml, n = 10; IL-6: Neph/saline 241.00 ± 90.87 pg/ml, n = 20; Neph/ghrelin 52.42 ± 18.68 pg/ml, n = 22; Neph/BIM-28125 83.65 ± 21.71 pg/ml, n = 9; Neph/BIM-28131 98.41 ± 36.66 pg/ml, n = 11; sham/saline 58.37 ± 20.05 pg/ml, n = 10; GM-CSF: Neph/saline 12.85 ± 3.60 pg/ml, n = 19; Neph/ghrelin 5.24 ± 1.93 pg/ml, n = 22; Neph/BIM-28125 7.01 ± 2.05 pg/ml, n = 9; Neph/BIM-28131 8.01 ± 2.83 pg/ml, n = 11; sham/saline 7.82 ± 3.78 pg/ml, n = 10; TNF-α: Neph/saline 70.84 ± 14.90 pg/ml, n = 19; Neph/ghrelin 34.71 ± 14.00 pg/ml, n = 22; Neph/BIM-28125 47.04 ± 3.36 pg/ml, n = 9; Neph/BIM-28131 45.81 ± 6.23 pg/ml, n = 11; sham/saline 47.83 ± 13.73 pg/ml, n = 10) (Fig. 4, A–E). However, when we examined the results using a mixed-model ANOVA to detect significant trends among the proinflammatory cytokines for each treatment group, we found a significant decrease in proinflammatory cytokines in ghrelin-treated CKD rats and sham-operated rats relative to the Neph/saline group (P < 0.05) (Fig. 4F).
Figure 4.
Serum cytokine levels. Serum was collected at the time of euthanasia for Neph animals receiving saline (n = 24) ghrelin (n = 26), or BIM-28131 as well as for sham-treated animals (n = 6). Serum was tested using ELISA for inflammatory cytokine levels as follows: A, IL-1α; B, IL-1β; C, IL-6; D, GM-CSF; E, TNF- α. F, Results for each of the proinflammatory cytokines were then normalized to the sham/saline average and combined. These data were analyzed using a mixed-model ANOVA, a statistical approach that includes a random effect to account for the clustering of data within each group. G, ELISA analysis of IL-10, an antiinflammatory cytokine. Significance is shown compared with Neph/saline: *, P < 0.05.
For the antiinflammatory cytokine IL-10, there was a significant increase found in CKD rats receiving BIM-28125 or BIM-28131 relative to the Neph/saline group [Neph/saline 357.27 ± 62.38 pg/ml, n = 19 (P < 0.01 vs. Sham/saline); Neph/ghrelin 242.41 ± 27.51 pg/ml, n = 22; Neph/BIM-28125 594.83 ± 55.22 pg/ml, n = 9 (P < 0.05 vs. Neph/saline, P < 0.001 vs. Neph/ghrelin, P < 0.001 vs. sham/saline); Neph/BIM-28131 419.26 ± 38.71 pg/ml, n = 11 (P < 0.01 vs. sham/saline); sham/saline 113.99 ± 20.92 pg/ml, n = 10] (Fig. 4G).
Gene expression
Appetite-regulating neuropeptides.
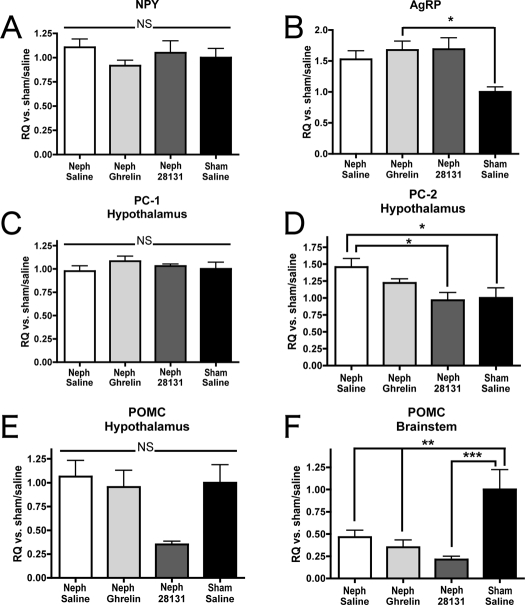
Neph animals receiving ghrelin exhibited an increase in expression of the orexigenic neuropeptide AgRP relative to the sham group; however, this was not significantly increased over Neph/saline levels [results given as fold increase: Neph/saline 1.53 ± 0.54, n = 16; Neph/ghrelin 1.68 ± 0.62, n = 19 (P < 0.05 vs. sham/saline); BIM-28131 1.69 ± 0.52, n = 8; sham/saline 1.00 ± 0.25, n = 10] (Fig. 5B). There was no significant difference in NPY expression among any of the groups (Neph/saline 1.11 ± 0.35, n = 17; Neph/ghrelin 0.92 ± 0.24, n = 19; Neph/BIM-28131 0.97 ± 0.33, n = 8; sham/saline 1.00 ± 0.31, n = 11) (Fig. 5A). There was no difference in expression of PC-1 between groups (Neph/saline 0.98 ± 0.23, n = 18; Neph/ghrelin 1.08 ± 0.23, n = 19; Neph/BIM-28131 1.03 ± 0.061, n = 8; sham/saline 1.0 ± 0.24, n = 11) (Fig. 5C). Regarding PC-2, however, the Neph/saline group had an increased expression of PC-2 relative to the BIM-28131 and the sham/saline groups [Neph/saline 1.46 ± 0.53, n = 18; Neph/ghrelin 1.22 ± 0.26, n = 19; Neph/BIM-28131 0.97 ± 0.33, n = 8 (P < 0.01 vs. Neph/saline); sham/saline 1.00 ± 0.49, n = 11 (P < 0.01 vs. Neph/saline)] (Fig. 6D). There was no difference between any of the groups in expression of POMC in the hypothalamus (Neph/saline 1.06 ± 0.66, n = 15; Neph/ghrelin 0.95 ± 0.68, n = 15; Neph/BIM-28131 0.35 ± 0.095, n = 7; sham/saline 1.00 ± 0.59, n = 7) (Fig. 5E), but there was a decrease in POMC transcript in the brainstem among each of the Neph groups relative to sham [Neph/saline 0.46 ± 0.27, n = 13 (P < 0.01 vs. sham/saline); Neph/ghrelin 0.35 ± 0.23, n = 8 (P < 0.01 vs. sham/saline); BIM-28131 0.21 ± 0.10, n = 8 (P < 0.001 vs. sham/saline); sham/saline 1.00 ± 0.59, n = 7] (Fig. 5F).
Figure 5.
Appetite-regulating neuropeptide gene expression. Change in expression of neuropeptide transcript as determined by real-time PCR. Data are reported as fold change vs. sham/saline rats for Neph rats treated with saline, ghrelin, or BIM-28131. Expression levels are shown in the hypothalamus for NPY (A), AgRP (B), PC-1 (C), PC-2 (D), and POMC (E) and in the brainstem for POMC (F). Significant differences are indicated: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
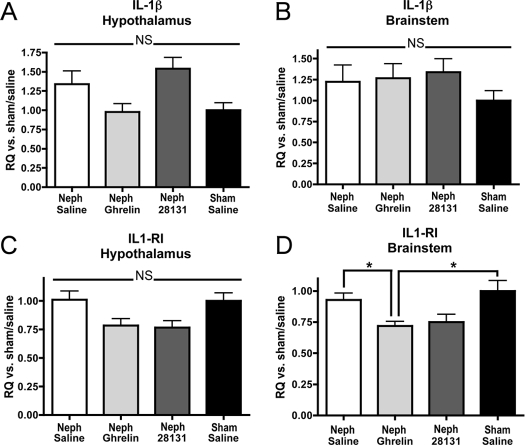
Figure 6.
IL-1β and IL-1RI gene expression. Change in expression of IL-1β and IL-1RI transcript was determined by real-time PCR. Data are reported as fold change vs. sham/saline rats for Neph rats treated with saline, ghrelin, or BIM-28131. Expression levels are shown for IL-1β and IL-1RI in the hypothalamus (A and B) and in the brainstem (C and D). Significant differences are indicated: *, P < 0.05.
Central inflammatory transcripts.
There was no difference among the groups for transcript levels of IL-1 β in the hypothalamus (Neph/saline 1.34 ± 0.74, n = 18; Neph/ghrelin 0.98 ± 0.46, n = 19; Neph/BIM-28131 1.54 ± 0.42, n = 8; sham/saline 1.00 ± 0.32, n = 11) (Fig. 6A), IL-1R in the hypothalamus (Neph/saline 1.01 ± 0.31, n = 17; Neph/ghrelin 0.78 ± 0.26, n = 19; Neph/BIM-28131 0.77 ± 0.17, n = 8; sham/saline 1.00 ± 0.22, n = 10) (Fig. 6B), or IL-1β in the brainstem (Neph/saline 1.22 ± 0.75, n = 14 n = 17; Neph/ghrelin 1.27 ± 0.60, n = 12; Neph/BIM-28131 1.34 ± 0.46, n = 8; sham/saline 1.00 ± 0.33, n = 8) (Fig. 6C). However, there was a significant decrease in expression of IL-1 receptor I (IL1-RI) in the brainstem among ghrelin-treated animals relative to both the Neph/saline group and the sham/saline group [Neph/saline 0.93 ± 0.21, n = 14; Neph/ghrelin 0.72 ± 0.12, n = 12 (P < 0.05 relative to both Neph/saline and sham/saline); Neph/BIM-28131 0.75 ± 0.18, n = 8; sham/saline 1.00 ± 0.24, n = 8] (Fig. 6D).
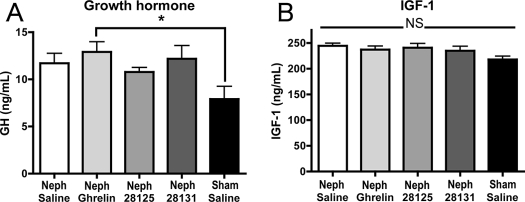
GH
There was an increase in random GH levels among animals treated with ghrelin relative to the sham group [Neph/saline 11.7 ± 1.05, n = 10; Neph/ghrelin, 12.9 ± 1.07 n = 11 (P < 0.05 vs. sham/saline); Neph/BIM-28125 10.78 ± 0.46, n = 9; Neph/BIM-28131 12.18 ± 1.39, n = 11; sham/saline 7.9 ± 1.34, n = 10] (Fig. 7A), but there was no difference in IGF-I levels among the groups (Neph/saline 244.4 ± 5.02, n = 10; Neph/ghrelin 237.1 ± 6.89, n = 11; Neph/BIM-28125 240.7 ± 8.01, n = 9; Neph/BIM-28131 234.7 ± 8.67, n = 11; sham/saline 217.9 ± 6.10, n = 10) (Fig. 7B).
Figure 7.
GH (A) and IGF-I (B) levels. GH and IGF-I levels at the time of euthanasia for Neph rats treated with saline, ghrelin, BIM-28125, or BIM-28131 as well as for sham-operated rats treated with saline. Significance is indicated: *, P < 0.05.
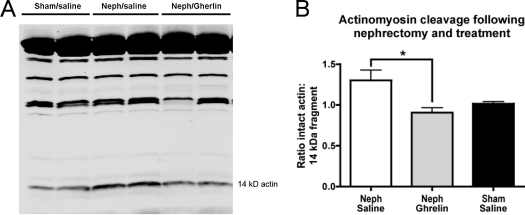
Muscle proteolysis
There was a decrease in the level of the 14-kDa actin fragment in muscle of CKD rats treated with ghrelin relative to levels in muscle of the Neph/saline animals. In these analyses, we corrected for differences in loading by calculating the ratio of intact actin to the level of the 14-kDa fragment (Neph/saline 1.299 ± 0.129, n = 22; Neph/ghrelin 0.906 ± 0.06, n = 13; sham/saline 1.016 ± 0.023, n = 13) (Fig. 8). We did not have a full set of muscles from CKD rats treated with BIM-28131. The level of the 14-kDa actin fragment in available muscles from a subset of BIM-28131 animals was intermediate between the Neph/saline and Neph/ghrelin animals (1.141 ± 0.107, n = 8). If we included this limited set results in our ANOVA (i.e. analyzed all four groups with any data), the significance between the Neph/saline and Neph/ghrelin animals was absent.
Figure 8.
Muscle actinomycin degradation. A, Representative Western blot of calf muscle lysate from Neph animals receiving saline or ghrelin and sham-operated rats receiving saline. Western blot was performed using an antibody to the carboxy terminus of actin, showing the 42-kDa intact actin band (top band) and the cleaved 14-kDa fragment (labeled). B, Ratio of intact actin to 14-kDa fragment for Neph/saline, Neph/ghrelin, or sham/saline animals. Significance is indicated: *, P < 0.05.
Discussion
Advanced, stage 4 CKD affects more than 500,000 Americans, and there are approximately 350,000 patients with end-stage renal disease being treated by maintenance hemodialysis (43,44). Among patients with advanced CKD, 10–70% suffer from symptoms of cachexia including anorexia and loss of lean body mass, affecting both quality of life and survival (15,45,46). There has been some success in treating patients with end-stage renal disease using anabolic steroids or GH to increase lean body mass, but no treatment has proven successful in improving other symptoms of cachexia (1,15,45,46,47,48).
We have studied the use of both ghrelin and ghrelin-receptor agonists to improve weight gain and lean body mass accrual in a rat model of CKD. These results mirror those seen when these compounds were administered in a rat model of cancer cachexia and raise the potential that ghrelin-receptor agonists may prove useful in treating cachexia in multiple settings. Ghrelin’s efficacy in the treatment of the complications of CKD is intriguing given that circulating ghrelin levels have been shown to be elevated when there is renal insufficiency (34). A limitation of our study is that we did not test higher doses of the compounds (having used 150 nmol/kg·d for CKD vs. 500 nmol/kg·d for cancer cachexia) or more extended periods of treatment to determine whether there would be more significant benefits. Such studies would be important for designing clinical trials of ghrelin in CKD patients and might suggest how ghrelin could be used prophylactically, administered over extended periods of time and in conjunction with dialysis or other treatments.
A potential mechanism for the improvement in food intake and lean body mass despite the presence of CKD could be via the central melanocortin system in the hypothalamus and brainstem. This system involves two classes of neurons; the first includes anorexigenic neurons that produce POMC, which is cleaved to form α-MSH to stimulate melanocortin-4 receptors, producing anorexia and a decrease in muscle mass (27,49). The other class of neurons in the melanocortin system are orexigenic, producing AgRP, a natural antagonist of the melanocortin-4 receptor (thereby blocking signals from POMC neurons). Another orexigenic hormone is NPY, which increases feeding behavior by acting on the Y1 receptor. In our previous experiments investigating ghrelin’s role in treating cancer cachexia, we showed that tumor-bearing animals treated with 500 nmol/kg·d ghrelin had an increase in expression of both AgRP and NPY relative to CKD rats treated with saline (35). In the present study, we treated the animals with 150 nmol/kg·d and did not find a significant difference in expression of these orexigenic transcripts in CKD rats treated with ghrelin vs. saline. In addition to dose differences, the lack of a difference in orexigenic transcripts may represent a difference in the severity of the cachexia because animals with cancer have a more severe loss of body weight.
We also did not find any significant difference in the expression of POMC between Neph animals treated with ghrelin and those treated with saline, although there was a generalized decrease in POMC transcripts among all Neph rats relative to sham-operated rats. This decrease in POMC transcript was likely related to the decrease in food intake among cachectic rats because pair-feeding experiments in rats with cancer cachexia revealed a similar decrease in POMC transcript in sham/pair-fed animals relative to sham animals fed ad libitum.
Regarding the lack of changes in gene expression between Neph animals treated with ghrelin and those given saline, it is important to note that ghrelin may exert effects on the melanocortin system via an increase in neuropeptide release without measurable differences in long-term gene expression. Among animals treated with the ghrelin-mimetic BIM-28131, we did find a decrease in the expression of PC-2, an enzyme involved in the cleavage of POMC to α-MSH. PC-2 has been shown to be regulated during manipulation of the melanocortin system (50,51,52). A decrease in PC-2 transcript levels suggests there could be a decrease in the processing of POMC and thus possibly a decrease in the secretion of anorexigenic hormones. Nevertheless, changes in gene expression of PC-2 itself may not be reflected in the overall level of translational and posttranslational processing of the peptides involved. In future studies, it will be important to measure the overall peptide content in these appetite-regulating nuclei.
However, it may also be that there is a change in ghrelin’s mechanism of action in short-term vs. long-term treatment of cachexia. The model of cancer cachexia involved 5 d of treatment with ghrelin, whereas the model of CKD involved 14 d of treatment. It is possible that there is a shift in ghrelin’s effects on melanocortin output over time, with a dampening of ghrelin’s stimulation of the orexigenic peptide expression during longer courses of treatment. Interestingly, however, the Neph groups treated with ghrelin and ghrelin receptor agonists did have a significant increase in food intake and lean body mass over Neph/saline animals, results that may be explained by a previous increase in orexigenic expression that decreased over time. Alternatively, it may be that the improvements in food intake and lean mass are not driven significantly by the melanocortin system.
Another means by which ghrelin may produce its effects is via changes in the animal’s inflammatory state. There is a strong link between inflammation and cachexia, including both an association of inflammatory markers and cytokines with evidence of cachexia and evidence for a direct stimulation of central neurological centers, including the melanocortin system, by specific cytokines (14,15,53). Circulating cytokines can permeate the relatively weak blood-brain barrier at the level of the hypothalamus, or cytokines expressed centrally could activate the system (54). Indeed, many of the interventions attempted thus far to improve cachexia have targeted the associated inflammation (1).
The potential for ghrelin to decrease inflammatory processes is raised because GHS-1a receptors are expressed on leukocytes; macrophages pretreated with ghrelin exhibited a decreased release of inflammatory cytokines after lipopolysaccharide stimulation (36). We have investigated inflammatory changes associated with cancer cachexia and found a decrease in expression of the IL1-RI in the hypothalamus and brainstem among tumor-bearing rats treated with ghrelin relative to their saline-treated controls. In our CKD model, we noted a similar decrease in IL1-RI expression in the brainstem among ghrelin-treated animals, suggesting that ghrelin reduces the response to inflammation. In the cancer cachexia model, there also was a decrease in central expression of cytokine receptors, but this did not appear to be mediated by peripheral inflammatory signals, because there were no differences in circulating cytokine levels (35).
In CKD rats, we found an overall decrease in circulating proinflammatory cytokines in CKD animals treated with ghrelin, yielding levels that approximate those of sham-treated animals. There also was an increase in circulating levels of the antiinflammatory cytokine IL-10 among CKD rats treated with BIM-28125. Besides the longer period of treatment, it is not clear why there was a decrease in inflammation in CKD rats but not in the model of cancer cachexia. It is also not clear why this increase in IL-10 levels occurred after treatment with BIM-28125 but not with ghrelin itself, although this may be due to increased serum half-life or tighter receptor-binding constants of the ghrelin-receptor antagonists. Given that inflammation is related to cachexia, an antiinflammatory response to ghrelin may represent a major mechanism for the beneficial responses to ghrelin as well as a desirable potential for future applications of ghrelin.
Ghrelin’s improvement in lean body mass accrual in the CKD rats was due at least partly to a decrease in muscle catabolism (Fig. 8). CKD is known to stimulate muscle protein degradation via activation of caspase-3 and the ubiquitin-proteasome pathway (55,56). In CKD and other catabolic conditions such as cancer and chronic heart failure, there appears to be a link to elevated cytokines causing activation of intracellular proteases including caspase-3 and apoptosis (programmed cell death) (57,58,59,60). Caspase 3 cleaves actinomyosin in muscle to produce a 14-kDa fragment that is quickly degraded by the ubiquitin-proteasome pathway (5). The 14-kDa actin fragment is found in muscle of animals with increased protein degradation and in patients with catabolic conditions (5,40,41,42,61). We observed a decrease in this marker of muscle proteolysis in CKD rats treated with ghrelin compared with CKD rats treated with saline, compatible with previously demonstrated muscle proteolysis (3,5,8,40,41,42,61,62). However, it should be pointed out that if the partial set of muscles from Neph/28131 animals was included in this ANOVA, the significance between Neph/ghrelin- and Neph/saline-treated animals was lost. Nevertheless, we did find it interesting that given that muscle protein degradation worsens in catabolic conditions and is related to increases in circulating inflammatory factors, the decrease in muscle degradation we found may be induced in part by ghrelin’s antiinflammatory effect.
Other potential mechanisms that could account for the influence of ghrelin on the improvement in lean body mass include a potential improvement in kidney function. A recent report concluded that ghrelin improved kidney function (63). We did not observe significant differences in BUN or creatinine in our experiments, suggesting that the improvement in lean body mass retention was related to factors other than a change in renal function.
In evaluating ghrelin’s effect on the GH-IGF-I axis, we found an increase in random levels of GH in the Neph/ghrelin rats relative to sham-treated rats (but not relative to the Neph/saline animals). However, there were no significant differences in IGF-I levels between any of the treatment groups, suggesting (as we also observed in the model of cancer cachexia) that ghrelin’s effects are not mediated by IGF-I in these models.
The goal of cachexia research is to improve the lives of humans suffering from cachexia. Small-molecule agonists of the ghrelin receptor GHS-1a had equal efficacy to ghrelin, using compounds that have a longer half-life and oral bioavailability with better potential for clinical use. Ghrelin itself has a short half-life and is not bioavailable, limiting its potential clinical application. The benefit of the present results suggest that these agonists could prevent or ameliorate the development of cachexia in CKD
Conclusion
In conclusion, we have used a rat model of surgically induced renal failure and found that ghrelin treatment improves food intake, total body mass, and lean body mass. Administration of human ghrelin or two synthetic ghrelin-receptor agonists exerted similar benefits. The improvement in lean body mass accrual was in part mediated by a decrease in muscle protein degradation. Ghrelin treatment also caused an overall decrease in circulating cytokines, which may be one mechanism of action of its effects and further highlights potential benefits for therapeutic use.
Footnotes
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) K08 NIDDK 1 K08 DK062207-01, National Institutes of Health NIDDK R01 DK 70333-01, F32 DK072820-01A1, and an unrestricted research grant from IPSEN, Inc.
Disclosure Statement: M.D.D., X.Z., P.R.L., A.I., Z.H., G.H., and W.E.M. have nothing to declare. J.E.T, H.A.H., J.Z.D., R.D., and M.D.C. are employees of IPSEN. D.L.M. consults for IPSEN.
First Published Online November 26, 2007
Abbreviations: AgRP, Agouti-related peptide; BUN, blood urea nitrogen; CKD, chronic kidney disease; DEXA, dual-energy x-ray absorptiometry; GHS, GH secretagogue; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL1-RI, IL-1 receptor I; Neph, nephrectomized; NPY, neuropeptide Y; PC-2, prohormone convertase-2; POMC, proopiomelanocortin.
References
- DeBoer MD, Marks DL 2006 Therapy insight: use of melanocortin antagonists in the treatment of cachexia in chronic disease. Nat Clin Pract Endocrinol Metab 2:459–466 [DOI] [PubMed] [Google Scholar]
- Mak RH, Cheung W 2006 Energy homeostasis and cachexia in chronic kidney disease. Pediatr Nephrol 21:1807–1814 [DOI] [PubMed] [Google Scholar]
- Bailey JL, Wang X, England BK, Price SR, Ding X, Mitch WE 1996 The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J Clin Invest 97:1447–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertow GM, Ackert K, Lew NL, Lazarus JM, Lowrie EG 2000 Prealbumin is as important as albumin in the nutritional assessment of hemodialysis patients. Kidney Int 58:2512–2517 [DOI] [PubMed] [Google Scholar]
- Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE 2004 Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113:115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen GA, Dubin JA, Muller HG, Rosales L, Levin NW, Mitch WE 2004 Inflammation and reduced albumin synthesis associated with stable decline in serum albumin in hemodialysis patients. Kidney Int 65:1408–1415 [DOI] [PubMed] [Google Scholar]
- Mitch WE 2002 Malnutrition: a frequent misdiagnosis for hemodialysis patients. J Clin Invest 110:437–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering WP, Price SR, Bircher G, Marinovic AC, Mitch WE, Walls J 2002 Nutrition in CAPD: serum bicarbonate and the ubiquitin-proteasome system in muscle. Kidney Int 61:1286–1292 [DOI] [PubMed] [Google Scholar]
- Benigni A, Remuzzi G 2001 How renal cytokines and growth factors contribute to renal disease progression. Am J Kidney Dis 37:S21–S24 [DOI] [PubMed] [Google Scholar]
- Bologa RM, Levine DM, Parker TS, Cheigh JS, Serur D, Stenzel KH, Rubin AL 1998 Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis 32:107–114 [DOI] [PubMed] [Google Scholar]
- Kaysen GA 2005 Effects of inflammation on plasma composition and endothelial structure and function. J Ren Nutr 15:94–98 [DOI] [PubMed] [Google Scholar]
- Maruyama Y, Stenvinkel P, Lindholm B 2005 Role of interleukin-1β in the development of malnutrition in chronic renal failure patients. Blood Purif 23:275–281 [DOI] [PubMed] [Google Scholar]
- Mitch WE, Du J, Bailey JL, Price SR 1999 Mechanisms causing muscle proteolysis in uremia: the influence of insulin and cytokines. Miner Electrolyte Metab 25:216–219 [DOI] [PubMed] [Google Scholar]
- Pereira BJ, Shapiro L, King AJ, Falagas ME, Strom JA, Dinarello CA 1994 Plasma levels of IL-1β, TNFα and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients. Kidney Int 45:890–896 [DOI] [PubMed] [Google Scholar]
- Qureshi AR, Alvestrand A, Divino-Filho JC, Gutierrez A, Heimburger O, Lindholm B, Bergstrom J 2002 Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol 13(Suppl 1):S28–S36 [PubMed] [Google Scholar]
- DeBoer MD, Marks DL 2006 Cachexia: lessons from melanocortin antagonism. Trends Endocrinol Metab 17:199–204 [DOI] [PubMed] [Google Scholar]
- Mak RH, Cheung W, Cone RD, Marks DL 2006 Mechanisms of disease: cytokine and adipokine signaling in uremic cachexia. Nat Clin Pract Nephrol 2:527–534 [DOI] [PubMed] [Google Scholar]
- Marks DL, Ling N, Cone RD 2001 Role of the central melanocortin system in cachexia. Cancer Res 61:1432–1438 [PubMed] [Google Scholar]
- Scarlett JM, Jobst EE, Enriori PJ, Bowe DD, Batra AK, Grant WF, Cowley MA, Marks DL 2007 Regulation of central melanocortin signaling by interleukin-1β. Endocrinology 148:4217–4225 [DOI] [PubMed] [Google Scholar]
- Bossola M, Muscaritoli M, Tazza L, Panocchia N, Liberatori M, Giungi S, Tortorelli A, Rossi Fanelli F, Luciani G 2005 Variables associated with reduced dietary intake in hemodialysis patients. J Ren Nutr 15:244–252 [DOI] [PubMed] [Google Scholar]
- Chertow GM, Johansen KL, Lew N, Lazarus JM, Lowrie EG 2000 Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int 57:1176–1181 [DOI] [PubMed] [Google Scholar]
- Dwyer JT, Larive B, Leung J, Rocco M, Burrowes JD, Chumlea WC, Frydrych A, Kusek JW, Uhlin L 2002 Nutritional status affects quality of life in Hemodialysis (HEMO) Study patients at baseline. J Ren Nutr 12:213–223 [DOI] [PubMed] [Google Scholar]
- Shinaberger CS, Kilpatrick RD, Regidor DL, McAllister CJ, Greenland S, Kopple JD, Kalantar-Zadeh K 2006 Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am J Kidney Dis 48:37–49 [DOI] [PubMed] [Google Scholar]
- Sreedhara R, Avram MM, Blanco M, Batish R, Avram MM, Mittman N 1996 Prealbumin is the best nutritional predictor of survival in hemodialysis and peritoneal dialysis. Am J Kidney Dis 28:937–942 [DOI] [PubMed] [Google Scholar]
- Vigano A, Donaldson N, Higginson IJ, Bruera E, Mahmud S, Suarez-Almazor M 2004 Quality of life and survival prediction in terminal cancer patients: a multicenter study. Cancer 101:1090–1098 [DOI] [PubMed] [Google Scholar]
- Wang AY, Sea MM, Tang N, Sanderson JE, Lui SF, Li PK, Woo J 2004 Resting energy expenditure and subsequent mortality risk in peritoneal dialysis patients. J Am Soc Nephrol 15:3134–3143 [DOI] [PubMed] [Google Scholar]
- DeBoer MD 2007 Melanocortin interventions in cachexia: how soon from bench to bedside? Curr Opin Clin Nutr Metab Care 10:457–462 [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S 2001 A role for ghrelin in the central regulation of feeding. Nature 409:194–198 [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang P, Zheng H, Smith RG 2004 Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA 101:4679–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JM, Garcia-Touza M, Hijazi RA, Taffet G, Epner D, Mann D, Smith RG, Cunningham GR, Marcelli M 2005 Active ghrelin levels and active to total ghrelin ratio in cancer-induced cachexia. J Clin Endocrinol Metab 90:2920–2926 [DOI] [PubMed] [Google Scholar]
- Hataya Y, Akamizu T, Hosoda H, Kanamoto N, Moriyama K, Kangawa K, Takaya K, Nakao K 2003 Alterations of plasma ghrelin levels in rats with lipopolysaccharide-induced wasting syndrome and effects of ghrelin treatment on the syndrome. Endocrinology 144:5365–5371 [DOI] [PubMed] [Google Scholar]
- Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, Ueno K, Kitakaze M, Miyatake K, Kangawa K 2004 Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation 110:3674–3679 [DOI] [PubMed] [Google Scholar]
- Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, Frost GS, Ghatei MA, Coombes RC, Bloom SR 2004 Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab 89:2832–2836 [DOI] [PubMed] [Google Scholar]
- Rodriguez Ayala E, Pecoits-Filho R, Heimburger O, Lindholm B, Nordfors L, Stenvinkel P 2004 Associations between plasma ghrelin levels and body composition in end-stage renal disease: a longitudinal study. Nephrol Dial Transplant 19:421–426 [DOI] [PubMed] [Google Scholar]
- DeBoer MD, Zhu XX, Levasseur P, Meguid MM, Suzuki S, Inui A, Taylor JE, Halem HA, Dong JZ, Datta R, Culler MD, Marks DL 2007 Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model of cancer cachexia. Endocrinology 148:3004–3012 [DOI] [PubMed] [Google Scholar]
- Granado M, Priego T, Martin AI, Villanua MA, Lopez-Calderon A 2005 Anti-inflammatory effect of the ghrelin agonist growth hormone-releasing peptide-2 (GHRP-2) in arthritic rats. Am J Physiol Endocrinol Metab 288:E486–E492 [DOI] [PubMed] [Google Scholar]
- Datta R DJ, Taylor JE, Halem HA, Zhang J, Strassburg, Tschop M, Springer J, Anker S, Marks D, Culler MD, Ghrelin and ghrelin analogs. Proc Third International Cachexia Conference, Rome, Italy, 2005 [Google Scholar]
- Halem HA TJ, Dong JZ, Eynon J, Zhang J, Datta R, Culler MD, A unique agonist of the GHS-1a receptor that induces an enhanced biphasic pattern of weight gain. Program of the 86th Annual Meeting of The Endocrine Society, New Orleans, LA, 2004, p 34 (Abstract P2-512) [Google Scholar]
- Halem HA TJ, Datta R, Mussulli L, Eynon J, Zhang J, Dong JZ, Culler MD, BIM-28131, a uniquely potent agonist of the GHS-1a receptor that is highly efficacious in stimulating body weight gain and food intake. Program of the 87th Annual Meeting of The Endocrine Society, San Diego, CA, 2005, p 407 (Abstract P2-210) [Google Scholar]
- Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE 2006 Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J Am Soc Nephrol 17:1388–1394 [DOI] [PubMed] [Google Scholar]
- Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P 2005 Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest 115:451–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hu Z, Hu J, Du J, Mitch WE 2006 Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 147:4160–4168 [DOI] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Balakrishnan VS 2006 The kidney disease wasting: inflammation, oxidative stress, and diet-gene interaction. Hemodial Int 10:315–325 [DOI] [PubMed] [Google Scholar]
- Xue JL, Ma JZ, Louis TA, Collins AJ 2001 Forecast of the number of patients with end-stage renal disease in the United States to the year 2010. J Am Soc Nephrol 12:2753–2758 [DOI] [PubMed] [Google Scholar]
- Bergstrom J 1995 Nutrition and mortality in hemodialysis. J Am Soc Nephrol 6:1329–1341 [DOI] [PubMed] [Google Scholar]
- Burrowes JD, Cockram DB, Dwyer JT, Larive B, Paranandi L, Bergen C, Poole D 2002 Cross-sectional relationship between dietary protein and energy intake, nutritional status, functional status, and comorbidity in older versus younger hemodialysis patients. J Ren Nutr 12:87–95 [DOI] [PubMed] [Google Scholar]
- Hansen TB, Gram J, Jensen PB, Kristiansen JH, Ekelund B, Christiansen JS, Pedersen FB 2000 Influence of growth hormone on whole body and regional soft tissue composition in adult patients on hemodialysis. A double-blind, randomized, placebo-controlled study. Clin Nephrol 53:99–107 [PubMed] [Google Scholar]
- Johansen KL, Mulligan K, Schambelan M 1999 Anabolic effects of nandrolone decanoate in patients receiving dialysis: a randomized controlled trial. JAMA 281:1275–1281 [DOI] [PubMed] [Google Scholar]
- Cone RD 2005 Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578 [DOI] [PubMed] [Google Scholar]
- Helwig M, Khorooshi RM, Tups A, Barrett P, Archer ZA, Exner C, Rozman J, Braulke LJ, Mercer JG, Klingenspor M 2006 PC1/3 and PC2 gene expression and post-translational endoproteolytic pro-opiomelanocortin processing is regulated by photoperiod in the seasonal Siberian hamster (Phodopus sungorus). J Neuroendocrinol 18:413–425 [DOI] [PubMed] [Google Scholar]
- Jing E, Nillni EA, Sanchez VC, Stuart RC, Good DJ 2004 Deletion of the Nhlh2 transcription factor decreases the levels of the anorexigenic peptides α-melanocyte-stimulating hormone and thyrotropin-releasing hormone and implicates prohormone convertases I and II in obesity. Endocrinology 145:1503–1513 [DOI] [PubMed] [Google Scholar]
- Laurent V, Jaubert-Miazza L, Desjardins R, Day R, Lindberg I 2004 Biosynthesis of proopiomelanocortin-derived peptides in prohormone convertase 2 and 7B2 null mice. Endocrinology 145:519–528 [DOI] [PubMed] [Google Scholar]
- Scarlett JM JE, Enriori PJ, Bowe DD, Batra AK, Grant WF, Cowley MA, Marks DM 2007 Regulation of central melanocortin signaling by interleukin-1β. Endocrinology 148:4217–4225 [DOI] [PubMed] [Google Scholar]
- Cheunsuang O, Morris R 2005 Astrocytes in the arcuate nucleus and median eminence that take up a fluorescent dye from the circulation express leptin receptors and neuropeptide Y Y1 receptors. Glia 52:228–233 [DOI] [PubMed] [Google Scholar]
- Lecker SH, Solomon V, Mitch WE, Goldberg AL 1999 Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr 129:227S–237S [DOI] [PubMed] [Google Scholar]
- Mitch WE, Goldberg AL 1996 Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med 335:1897–1905 [DOI] [PubMed] [Google Scholar]
- Belizario JE, Lorite MJ, Tisdale MJ 2001 Cleavage of caspases-1, -3, -6, -8 and -9 substrates by proteases in skeletal muscles from mice undergoing cancer cachexia. Br J Cancer 84:1135–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libera LD, Vescovo G 2004 Muscle wastage in chronic heart failure, between apoptosis, catabolism and altered anabolism: a chimaeric view of inflammation? Curr Opin Clin Nutr Metab Care 7:435–441 [DOI] [PubMed] [Google Scholar]
- Raj DS, Shah H, Shah VO, Ferrando A, Bankhurst A, Wolfe R, Zager PG 2003 Markers of inflammation, proteolysis, and apoptosis in ESRD. Am J Kidney Dis 42:1212–1220 [DOI] [PubMed] [Google Scholar]
- Vescovo G, Zennaro R, Sandri M, Carraro U, Leprotti C, Ceconi C, Ambrosio GB, Dalla Libera L 1998 Apoptosis of skeletal muscle myofibers and interstitial cells in experimental heart failure. J Mol Cell Cardiol 30:2449–2459 [DOI] [PubMed] [Google Scholar]
- Workeneh BT, Rondon-Berrios H, Zhang L, Hu Z, Ayehu G, Ferrando A, Kopple JD, Wang H, Storer T, Fournier M, Lee SW, Du J, Mitch WE 2006 Development of a diagnostic method for detecting increased muscle protein degradation in patients with catabolic conditions. J Am Soc Nephrol 17:3233–3239 [DOI] [PubMed] [Google Scholar]
- Reaich D, Channon SM, Scrimgeour CM, Daley SE, Wilkinson R, Goodship TH 1993 Correction of acidosis in humans with CRF decreases protein degradation and amino acid oxidation. Am J Physiol 265:E230–E235 [DOI] [PubMed] [Google Scholar]
- Takeda R, Nishimatsu H, Suzuki E, Satonaka H, Nagata D, Oba S, Sata M, Takahashi M, Yamamoto Y, Terauchi Y, Kadowaki T, Kangawa K, Kitamura T, Nagai R, Hirata Y 2006 Ghrelin improves renal function in mice with ischemic acute renal failure. J Am Soc Nephrol 17:113–121 [DOI] [PubMed] [Google Scholar]