Abstract
Regulation of the adhesion of mononuclear cells to endothelial cells is considered to be a critical step for the treatment of inflammatory diseases, including autoimmune diseases. K-13182 was identified as a novel inhibitor for these adhesions. K-13182 inhibited the expression of vascular cell adhesion molecule-1 (VCAM-1, CD106) on human umbilical vein endothelial cells (HUVECs) and on mouse vascular endothelial cell line (MAECs) induced by tumour necrosis factor (TNF)-α. K-13182 also inhibited the adhesion of mononuclear cells to these HUVECs and MAECs, indicating that K-13182 suppressed these adhesions mediated by cellular adhesion molecules including VCAM-1. To evaluate the therapeutic effect in autoimmune disease model mice, K-13182 was orally administered to non-obese diabetic (NOD) mice as Sjögren's syndrome (SS) model mice. Severe destructive inflammatory lesions were observed in the lacrimal glands of vehicle-treated control mice; however, 8-week administration of K-13182 inhibited the mononuclear cell infiltration into the inflammatory lesions of the lacrimal glands. In K-13182-treated mice, the decrease in tear secretion was also prevented compared to the control mice. In addition, the apoptosis and the expression of FasL (CD178), perforin, and granzyme A was suppressed in the lacrimal glands of K-13182-treated mice. Therefore, K-13182 demonstrated the possibility of therapeutic efficacy for the inflammatory region of autoimmune disease model mice. These data reveal that VCAM-1 is a promising target molecule for the treatment of autoimmune diseases as a therapeutic strategy and that K-13182 has the potential as a new anti-inflammatory drug for SS.
Keywords: autoimmune disease, endothelial cells, lacrimal gland, NOD mouse, VCAM-1
Introduction
Sjögren's syndrome (SS) is an organ-specific autoimmune disorder characterized by lymphocytic infiltration and progressive loss of exocrine glands resulting in symptoms of dry mouth and dry eye due to insufficient secretion, and systemic production of autoantibodies to the ribonucleoprotein [1]. Although a large number of studies have been conducted [2–5], the mechanism of the destruction of the exocrine glands is still unclear.
In the first stage of inflammatory diseases, leucocytes migrate from the circulation into the sites in which inflammation manifests. Leucocyte adherence to the blood vessel wall through cell adhesion molecules is the important step for leucocyte migration [6]. When activated by inflammatory cytokines, endothelial cells express adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1, CD54) and E-selectin (CD62E). VCAM-1 is a cell surface glycoprotein which belongs to the immunoglobulin superfamily, and is adhesive to certain blood leucocytes and tumour cells that bear α4 integrins. In the vascular system, VCAM-1 is expressed on activated endothelial cells, smooth muscle cells and fibroblasts in a variety of pathological conditions, including atherosclerosis and inflammation [7–9]. These former studies indicate that endothelial/lymphocyte adhesion involving VCAM-1/VLA-4 (CD49d/CD29) control the migration of lymphocytes into the inflamed lesion. These cell adhesion molecules offer potential therapeutic targets to block the development of inflammation and tissue destruction.
In SS, it has been reported that the expression of cell adhesion molecules on vascular endothelial cells, such as ICAM-1 and VCAM-1, increased in the salivary and lacrimal glands [10]. In addition, these adhesion molecules play predominant roles in controlling T cell recruitment into these tissues and in the regulation of inflammation [11].
In this study, we identified the newly synthesized, low molecular weight compound K-13182, which inhibited the VCAM-1 expression on human umbilical vein endothelial cells (HUVECs), mouse aortic vascular endothelial cell lines (MAECs) and inhibited cellular adhesion between HUVECs and U-937 human monocytic cell lines. The purpose of this study is to evaluate the therapeutic effect of K-13182 and to clarify the mechanism in detail in SS model mice.
Materials and methods
Cell cultures
HUVECs were purchased from Clontech (Palo Alto, CA, USA), and cultured in EGM-2 medium (Clontech). Three or four time-passaged cells were used for the experiments. U-937 was obtained from American Type Culture Collection (Manassa, VA, USA) and maintained in RPMI-1640 medium containing 10% fetal calf serum (FCS) (Invitrogen, Carlsbad, CA, USA). Murine myeloid leukaemia cell line, WEHI-3, was obtained from Riken Cell Bank (Ibaraki, Japan) and maintained in RPMI-1640 medium containing 10% FCS and 2-mercaptoethanol. MAECs were isolated and established from p53-deficient mice in our previous study [12].The cells were maintained in M199 (Sigma, St Louis, MO, USA) supplemented with 5% FCS, 10 U/ml heparin sodium (Shimizu Pharmaceutical, Shizuoka, Japan), 100 U/ml penicillin (Invitrogen) and 100 µg/ml streptomycin (Invitrogen).
Expression of VCAM-1 on HUVECs and MAECs
The expression of VCAM-1 on MAECs and HUVECs was analysed with cell enzyme-linked immunosorbent assay (ELISA). MAECs and HUVECs (1 × 104) were seeded onto 96-well culture plates (Becton Dickinson, Franklin Lakes, NJ, USA). Confluent cultures of cells were stimulated with 10 ng/ml tumour necrosis factor (TNF)-α in the presence of various doses of K-13182. Non-specific binding was blocked by the sequential addition of 3% non-fat dry milk/phosphate-buffered saline (PBS) and 5% goat serum/PBS for 1 h. Anti-human VCAM-1 (4B2, Genzyme Corporation, Cambridge, MA, USA) or anti-mouse VCAM-1 (MK2.7, American Type Culture Collection) and horseradish peroxidase (HRP)-conjugated goat anti-rat IgG antibody (R&D Systems, Minneapolis, MN, USA) were used as the first and second antibodies, respectively, followed by the addition of 3, 3′, 5, 5′-tetramethyl-benzidine (Moss Inc., Pasadena, MD, USA). The optical density (OD) of each well was determined by using a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA) at 450 nm. The relative VCAM-1 expression was calculated using the following formula: (expression in the presence of K-13182 [OD]/expression in the absence of K-13182 [OD]) × 100. Data are expressed as mean ± standard deviation (s.d.) of three individual experiments.
Cellular adhesion assay
Cellular adhesion assay using HUVECs or MAECs and U-937 or WEHI-3 was performed as described in previous reports [12–15], with some modifications. Endothelial cells (HUVECs or MAECs, 1 × 104/well) were seeded into each well of 96-well culture plates. Confluent cultures of endothelial cells were stimulated by 30 ng/ml TNF-α and varying concentrations of K-13182 for 16 h at 37°C. U-937 or WEHI-3 cells were labelled with 10 µmol/l 2′,7′-bis(carboxyethyl)-5(6′) carboxyfluorescein tetraacetoxymethyl ester (PKH-2; Dojindo Laboratories, Kumamoto, Japan) for 1 h at 37°C in each medium for HUVECs and MAECs and then washed three times with serum-free medium. PKH-2-labelled cells (2 × 104) were added to each well and incubated with TNF-α-stimulated endothelial cells for 1 h. Cells that were not bound to endothelial cells were removed by inverting the plates for 30 min. Wells were subsequently washed once with serum-free M199, and the remaining cells were lysed with 1% Nonidet P-40 (Calbiochem, La Jolla, CA, USA). Fluorescence intensity in cell lysate was measured by using an automated microplate fluorometer (Perkin Elmer, Boston, MA, USA) at an excitation wavelength of 485 nm and an emission wavelength of 535 nm. The relative adhesion cells were calculated using the following formula: (adhesion in the presence of K-13182 [OD]/adhesion in the absence of K-13182 [OD]) × 100.
Reverse transcription–polymerase chain reaction (RT–PCR) assay
MAECs and HUVECs were stimulated with 10 ng/ml TNF-α in the presence of K-13182 for 4 h. Total RNA was extracted with TRIzol reagent (Life Technologies, Rockville, MD, USA), and cDNA was prepared from RNA with 50 pmol of random hexamer and 200 U of reverse transcriptase (Invitrogen); 0.5 µl of a 20-µl cDNA mixture was used for PCR with 5 pmol each of forward and reverse primers and 2.5 U of Ex Taq DNA polymerase (Takara Shuzo, Kyoto, Japan). The sequences of the specific sense and anti-sense oligonucleotide primer pairs were as follows: VCAM-1 (human), GGATAATGTTTGCAGCTTCTC and TTCAGTAAGTCTATCTCCAGC; VCAM-1 (mouse), CCCAAGGATCCAGAGATTCA and TAAGGTGAGGGTGGCATTTC; β-actin, CTCTTTGATGTCACGCACGATTTC and GTGGGCCGCTCTAGGCACCAA.
Samples were amplified through 25 or 30 cycles in a PCR Thermal Cycler (Applied Biosystems, Foster City, CA, USA).
Mice
Male non-obese diabetic (NOD) mice were purchased from Clea Japan, Inc. (Tokyo, Japan) and maintained under specific-pathogen-free conditions in the animal facilities of Kowa Tokyo New Drug Research Laboratories. All experimental protocols were approved by the animal welfare committees of Tsurumi University and Kowa Tokyo New Drug Research Laboratories.
Administration of K-13182
K-13182, dissolved in 0.5% hydroxypropyl methylcellulose, was administered orally into the mice at a dose of 30 mg/kg/day from 4 to 12 weeks of age (8 week of administration, n = 12) or from 4 to 16 weeks of age (12 week of administration, n = 15). For control mice, 0.5% hydroxypropyl methylcellulose was administered as vehicle for a period of 8 (n = 12) or 12 weeks (n = 15).
Measurement of tear secretion
Tear secretion was compared before and after administration of K-13182. We measured the tear secretion of NOD mice before administration (control mice: n = 5; K-13182-treated mice: n = 5) and 12 weeks after administration (control mice: n = 10; K-13182-treated mice: n = 11). Mice were anaesthetized intraperitoneally with a mixture of 36 mg/kg ketamine (Sigma) and 16 mg/kg xylazine (Sigma). The amount of secreted tears was determined by the length of the Schirmer strip soaked by tears (1 mm in width; Showa Yakuhin Kako, Tokyo) after insertion into the inner aspect of an eyelid every 5 min in a 20-min period.
Histological analysis
NOD mice were anaesthetized with diethyl ether (Wako Pure Chemical Industries, Osaka, Japan) and were killed. The lacrimal glands were then removed from K-13182-treated NOD mice (n = 11, 8 weeks of administration, n = 15, 12 weeks of administration) and control mice (n = 11, 8 weeks of administration, n = 15, 12 weeks of administration). Removed lacrimal glands were fixed with 4% paraformaldehyde and embedded in paraffin. The sections (4 µm) were prepared and stained with haematoxylin and eosin (H&E) [16] using the standard method. Histological grading of the inflammatory lesions in the lacrimal glands was performed according to the method proposed by White and Casarett [17]. The number of mononuclear cells on H&E-stained sections (three areas/section/animal) obtained from each animal was counted under a light microscope (× 400), and the mean value was calculated for each animal.
TaqMan RT–PCR
Lacrimal glands were removed from NOD mice as mentioned above. Total RNA were obtained from the lacrimal glands of K-13182-treated mice or control mice. Reverse transcription was performed using a GeneAmp RNA PCR kit (Applied Biosystems). TaqMan–PCR was also performed according to the manufacturer's instructions (Applied Biosystems). Oligonucleotide primers and probes are described in Table 1. Sequence specific amplification was detected with an increased fluorescent signal of reporter dye 6-carboxy fluorescein (FAM) during the following amplification cycles: 1 cycle at 50°C for 2 min, 1 cycle at 95°C for 10 min and 40 cycles each at 95°C for 15 s and 60°C for 1 min. Gene-specific mRNA was normalized subsequently to rRNA. Primers and probes for rRNA were purchased from Applied Biosystems.
Table 1.
Primers and probes used for reverse transcription–polymerase chain reaction (RT–PCR) analysis.
| mRNA | Sequences |
|---|---|
| VCAM-1 | |
| Forward primer | ACAAGTCTACATCTCTCCCAGGAATAC |
| Reverse primer | CACAGCACCACCCTCTTGAA |
| Probe | CTGTACATCCCTCCACAAG |
| FasL | |
| Forward primer | TCAGCTCTTCCACCTGCAGAA |
| Reverse primer | TACTTTAAGGCTTTGGTTGGTGAA |
| Probe | AACTGGCAGAACTCCGT |
| Perforin | |
| Forward primer | GCAGGTCAGGCCAGCATAA |
| Reverse primer | ACCTTTGAATCCTGGCACTCA |
| Probe | AGTAGCCATGATTCATGCC |
| Granzyme A | |
| Forward primer | GGTGGAAAGGACTCCTGCAA |
| Reverse primer | GCCTCGCAAAATACCATCACA |
| Probe | ATTCTGGCAGCCCTC |
TUNEL assay
Lacrimal glands were removed from NOD mice, as mentioned above, and were embedded in optimal cutting temperature compound (OCT; Sakura Finetechnical, Tokyo, Japan) and frozen in liquid nitrogen. Cryostat sections (5 µm) were made, and apoptotic cells were detected in sections by terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) assay using the in situ apoptosis in situ detection kit (Wako Pure Chemical Industries), according to the manufacturer's instructions. The percentage of TUNEL-positive cells were counted under the light microscope (× 400) in three fields per one section (TUNEL index), and expressed as mean percentage ± s.d. in four (control mice) or three (K-13182-treated mice) sections.
Results
K-13182 inhibited cellular adhesion and the expression of VCAM-1 in HUVECs
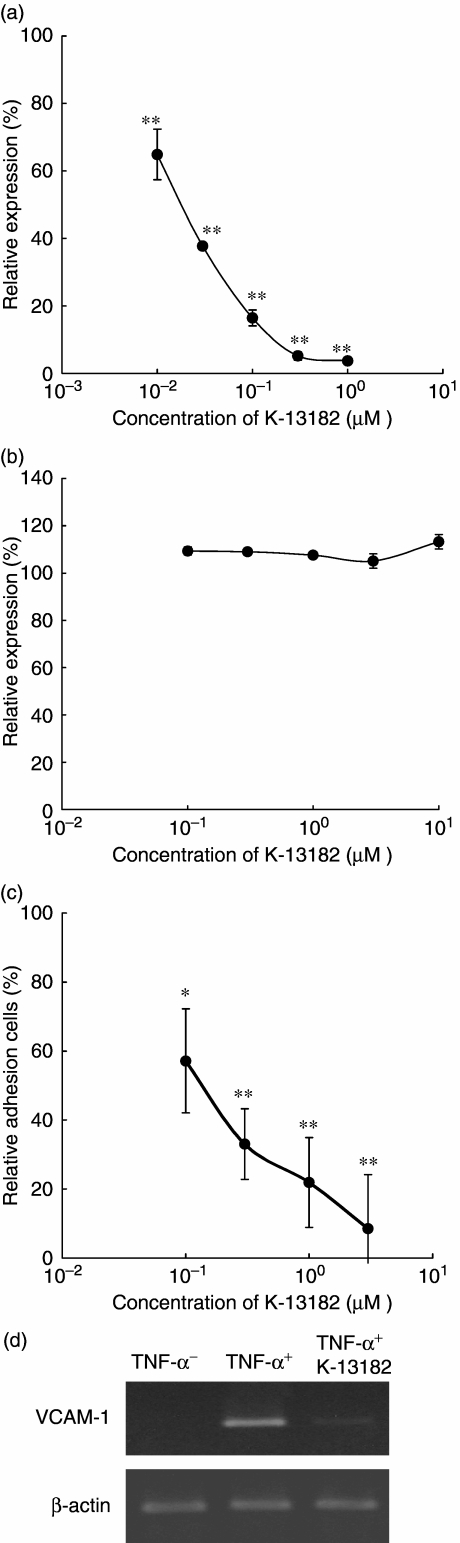
In our previous study, the expression of VCAM-1, ICAM-1 and E-selectin on the HUVECs in response to TNF-α was augmented markedly [15]. Among these cell adhesion molecules, K-13182 suppressed VCAM-1 expression in a dose-dependent manner (P < 0.0001 at 0.01, 0.03, 0.1, 0.3 and 1 µM compared to absence of K-13182, Dunnett's multiple comparisons, Fig. 1a). The observed half-maximal inhibitory concentration (IC50) for VCAM-1 expression was 0.019 µM. To examine the specificity of K-13182, ICAM-1 expression was also analysed by cell ELISA. K-13182 (0.1–10 µM) did not show a suppressive effect on ICAM-1 expression (Fig. 1b). Suppression of VCAM-1 expression was also confirmed at the transcriptional level using RT–PCR, and 1 µM of K-13182 clearly inhibited the VCAM-1 mRNA expression that was induced by 10 ng/ml TNF-α (Fig. 1d). These results demonstrated that K-13182 specifically inhibited VCAM-1 expression.
Fig. 1.
The cell adhesion molecule expression and human mononuclear cell/endothelial cell adhesion. (a) Effects of K-13182 on vascular cell adhesion molecule-1 (VCAM-1) expression in human umbilical vein endothelial cells (HUVECs). HUVECs were stimulated with tumour necrosis factor (TNF)-α in the presence of K-13182 for 4 h. The expression of VCAM-1 was then investigated by cell enzyme-linked immunosorbent assay (ELISA). Data are expressed as percentage of control expression (without K-13182) and represented as mean ± s.d. of triplicate samples. Inhibitory concentration (IC50) was 0.019 µM. Statistical analysis was made using Dunnett's multiple comparisons compared to absence of K-13182, **P < 0.001. The results are the average of four separate experiments. (b) Effects of K-13182 on intercellular adhesion molecule-1 (ICAM-1) expression in HUVECs. ICAM-1 expression was not down-regulated by K-13182 in doses of 0–10 µM. (c) U-937/HUVECs adhesion assay. HUVECs were stimulated with TNF-α in the presence of K-13182 (0–3 µM) for 4 h. Adhesion of fluorescent-labelled U-937 cells were analysed by fluorescent spectrophotometer. IC50 was 0.14 µM. Data are expressed as percentage adhesion of cells added and represented as mean ± s.d. of triplicate samples. Statistical analysis was made using Dunnett's multiple comparisons compared to absence of K-13182, *P < 0.05 and **P < 0.001. (d) Effects of K-13182 on VCAM-1 mRNA expression in HUVECs. HUVECs were stimulated with 10 ng/ml TNF-α in the presence of 1 µM of K-13182 for 4 h. The expression of VCAM-1 was then investigated by reverse transcription–polymerase chain reaction (RT–PCR).
We have established a mononuclear cell/endothelial cell adhesion assay system for the functional analysis of adhesion inhibitory compounds in a former report [15]. To assess whether K-13182 can function in lymphocytic cellular adhesion to activated HUVECs, this cellular adhesion assay was performed in the presence of K-13182. HUVECs were treated with TNF-α (10 ng/ml) for 4 h in the presence of K-13182, and the cellular adhesion of U-937 cells to HUVECs was measured thereafter. As shown in Fig. 1c, the adhesion of U-937 to TNF-α-stimulated HUVECs was inhibited significantly by the treatment of K-13182 in a dose-dependent manner (P < 0.01 at 0.1, 0.3, 1 and 3 µM compared to absence of K-13182, Dunnett's multiple comparisons). The observed IC50 for the cellular adhesion was 0.14 µM. Ten µM of K-13182 had no toxic effect on U-937 cells (data not shown).
K-13182 inhibited cellular adhesion and the expression of VCAM-1 in a murine endothelial cell line
We further confirmed the effects of K-13182 on a murine endothelial cell line. We used MAECs that had been established in our laboratory. Expression of VCAM-1 on MAECs was up-regulated in response to TNF-α stimulation and they retained cellular adhesion activity with WEHI-3 [12]. Stimulation with 10 ng/ml TNF-α showed a maximum VCAM-1 expression after 24 h (data not shown) and reached a plateau thereafter. We therefore decided to use a dose of TNF-α (10–30 ng/ml) for later experiments.
The expression of VCAM-1 on TNF-α-stimulated MAECs was significantly down-regulated by K-13182 in a dose-dependent manner (P < 0.001 at 0.01, 0.03 and 0.1 µM compared to absence of K-13182, Dunnett's multiple comparisons, Fig. 2a). The IC50 for VCAM-1 expression in MAECs was 0.05 µM. Cell viability was assessed by the trypan blue dye exclusion test, and no cytotoxicity of K-13182 to MAECs was observed. K-13182 (0.3 µM) also suppressed the VCAM-1 expression that was up-regulated by 10 ng/ml of TNF-α at the transcriptional level (Fig. 2c). There was more significant down-regulation of VCAM-1 mRNA in HUVECs and the concentration of K-13182 was 1 µM in HUVECs and 0.3 µM in MAECs. However, 1 µM of K-13182 did not show a significant effect compared with 0.3 µM of K-13182 in the RT–PCR in MAECs (data not shown).
Fig. 2.
Mouse vascular cell adhesion molecule-1 (VCAM-1) expression and mononuclear cell/endothelial cell adhesion. (a) Effects of K-13182 on the expression of VCAM-1 in mouse aortic vascular endothelial cell lines (MAECs). MAECs were stimulated with tumour necrosis factor (TNF)-α in the presence of K-13182 for 4 h. The expression of VCAM-1 was then investigated by cell enzyme-linked immunosorbent assay (ELISA). Inhibitory concentration (IC50) was 0.05 µM. Data are expressed as percentage of control expression (without K-13182) and represented as mean ± s.d. of triplicate samples. Statistical analysis was made using Dunnett's multiple comparisons compared to absence of K-13182, **P < 0.001. (b) WEHI-3/MAECs adhesion assay. MAECs were pretreated with K-13182 (0–1.0 µM) overnight and stimulated with TNF-α for 4 h. Adhesion of fluorescent-labelled WEHI-3 cells was analysed by fluorescent spectrophotometer. IC50 was 0.10 µM. Data are expressed as percent adhesionage of cells added and represented as mean ± s.d. of triplicate samples. Statistical analysis was made using Dunnett's multiple comparisons compared to absence of K-13182, *P < 0.05. (c) Effects of K-13182 on VCAM-1 mRNA expression in MAECs. MAECs were stimulated with 10 ng/ml TNF-α in the presence of 0.3 µM of K-13182 for 4 h. The expression of VCAM-1 was then investigated by reverse transcription–polymerase chain reaction (RT–PCR).
To assess whether K-13182 affects mononuclear cellular adhesion with activated mouse endothelium, a cellular adhesion assay was performed using WEHI-3 and TNF-α-stimulated MAECs in the presence of K-13182. The adhesion of WEHI-3 to TNF-α-stimulated MAECs was inhibited significantly by the treatment with K-13182 in a dose-dependent manner (P < 0.05 at 0.1 µM comparedto absence of K-13182, Dunnett's multiple comparisons, Fig. 2b). The IC50 for cellular adhesion assay was 0.10 µM.
Administration of K-13182 into NOD mice
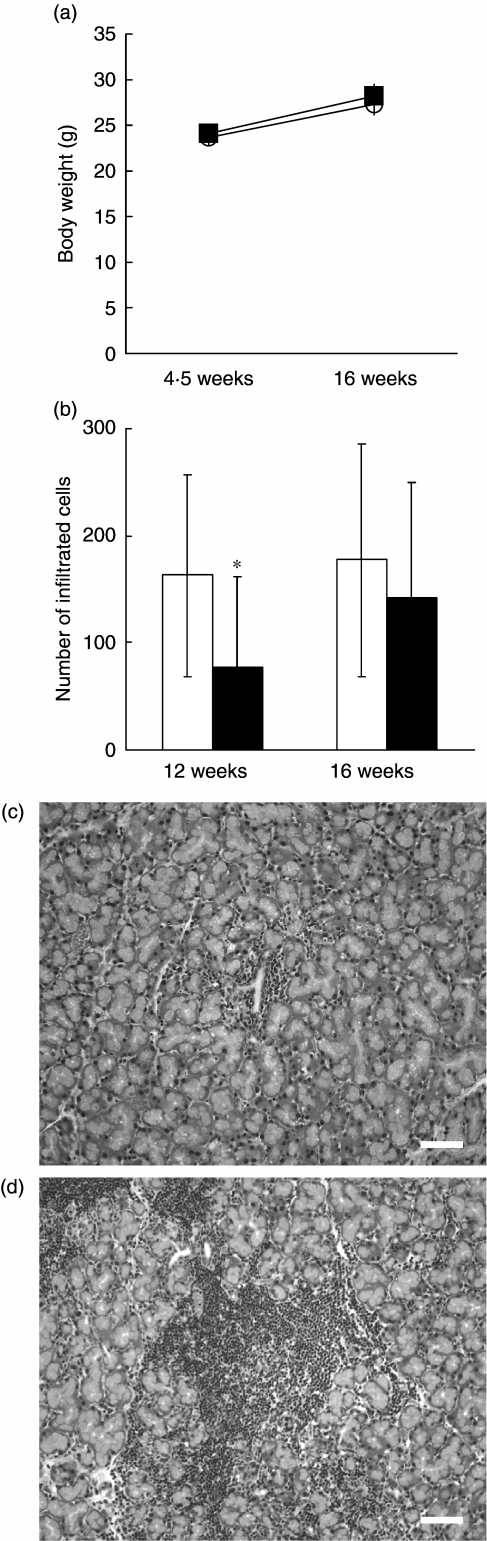
The NOD mouse has been proposed as a valuable animal model for SS in humans, because NOD mice develop spontaneously mononuclear cell infiltration into the lacrimal and submandibular glands. Although diabetes developed only in female NOD mice, lymphocyte infiltration into the lacrimal glands was detected in male mice [18]. On the other hand, the destruction of submandibular glands was seen in female NOD mice [19]. Thus, we examined the in vivo therapeutic effects of K-13182 in male NOD mice as a murine model of lacrimal gland destruction. Body weight was monitored before (4.5 weeks of age) and after (16 weeks of age) administration of K-13182. Body weight increased in a time-dependent manner in both groups, and no statistical difference was detected between control and K-13182-treated mice at both 4.5 and 16 weeks of age (Fig. 3a). Side effects were not observed during administration of K-13182. In order to examine the anti-inflammatory effect of K-13182 in the lacrimal glands, they were analysed histologically after administration of K-13182. We evaluated their inflammatory lesions by the number of infiltrated mononuclear cells or inflammation score at 12 or 16 weeks of age, as described in the Materials and methods section. The number of infiltrated mononuclear cells in K-13182-treated mice was decreased significantly compared to control mice at 12 weeks of age (P < 0.05, Student's t-test). At 16 weeks of age it was also slightly decreased; however, statistical difference was not detected (Fig. 3b). So far, we do not have an explanation for this discrepancy between 12 and 16 weeks. Further analysis, including the subset of infiltrated lymphocytes, may be useful to analyse this. The inflammation score was slightly decreased in K-13182-treated mice; however, no statistical difference was detected at 12 and 16 weeks of age (data not shown). Representative histological features in the lacrimal glands are shown in Fig. 3c,d. These results revealed that treatment with oral administration of K-13182 (30 mg/kg/day) was effective in preventing the infiltration of mononuclear cells in the lacrimal glands of the SS model mice.
Fig. 3.
Effects of in vivo administration of K-13182 in murine SS model. (a) The body weight of each mouse was measured at 4.5 weeks of age (control mice: open circle: n = 5; K-13182-treated mice: black square: n = 5) and at 16 weeks of age (control mice: n = 10; K-13182-treated mice: n = 11). Data are represented as mean ± s. d. of each time-point. (b) The number of infiltrated mononuclear cells in lacrimal glands at 12 weeks of age (control mice: n = 12; K-13182-treated mice: n = 11) was decreased significantly in K-13182-treated mice. Statistical difference was not detected at 16 weeks of age (control mice: n = 14; K-13182-treated mice: n = 15). The white and black bars indicate control mice and K-13182-treated mice, respectively. Data are represented as mean ± s.d. of each time-point. Statistical analysis was made using the Student's t-test, *P < 0.05. Representative histological features showing severe lesions in the lacrimal glands in (c) control mice but not in (d) K-13182-treated mice. Original magnification ×200, scale bar 20 µm.
Tear secretion was also measured to analyse whether an anti-inflammatory effect led to the recovery or prevention of lacrimal gland dysfunction. Tear secretion was decreased significantly in control mice (34.7% decrease, P < 0.05, Student's t-test) at 16 weeks of age compared to 4.5 weeks of age, while such a significant decrease was not shown in K-13182-treated mice at 16 weeks of age compared to 4.5 weeks of age (22.9% decrease, statistical difference was not detected). As tear secretion is decreased spontaneously in male NOD mice [20], this result demonstrated that K-13182 showed the preventive effect for tear decrease. As described previously, male NOD mice show mononuclear cell infiltration into only lacrimal glands; we therefore describe here the effect of K-13182 on the lacrimal glands. However, the effect of K-13182 on the pancreas and submandibular glands should be proved for further characterization of this compound.
Inhibition of apoptosis and apoptosis-related genes in lacrimal glands
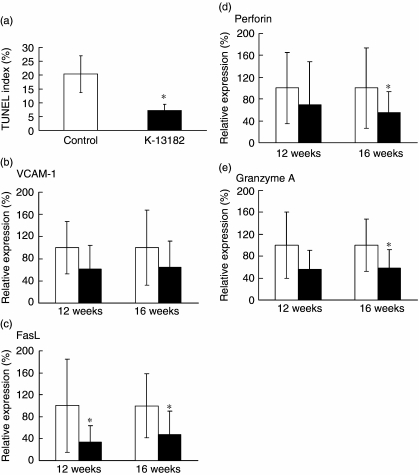
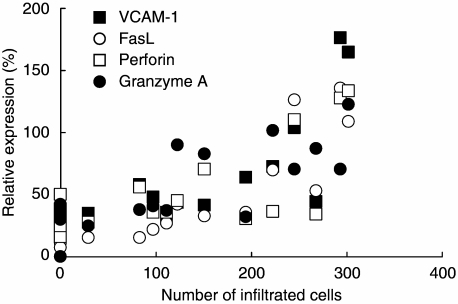
Apoptosis of the acinar and ductal epithelial cells of the lacrimal glands has been proposed as a possible mechanism responsible for the impairment of secretory function in NOD mice [20,21]. In our study, decreased infiltration of mononuclear cells was demonstrated in K-13182-treated mice, as described above. From our results, we investigated the apoptosis in the lacrimal glands of NOD mice by TUNEL assay. Interestingly, the percentage of apoptotic cells was decreased in the lacrimal glands of K-13182-treated mice compared to control mice at 12 weeks of age (Fig. 4a; P < 0.05, Student's t-test). In order to analyse the further anti-inflammatory mechanism of K-13182 in lacrimal glands, mRNA levels of FasL, perforin, granzyme A and VCAM-1 were measured by quantitative PCR. The mRNA of FasL (P < 0.05, Fig. 4c) at 12 weeks of age, and FasL (P < 0.05, Fig. 4c), perforin (P < 0.05, Fig. 4d) and granzyme A (P < 0.05, Fig. 4e) at 16 weeks of age were reduced significantly in K-13182-treated mice compared with control mice (Student's t-test). VCAM-1 was down-regulated slightly in K-13182-treated mice; however, statistical significance was not detected at 12 and 16 weeks of age (Fig. 4b). Correlation coefficients between the number of infiltrated mononuclear cells and the results of quantitative PCR were calculated to investigate the relation between the inflammation and expression of analysed genes in the lacrimal glands. As shown in Fig. 5, the expression of VCAM-1, FasL, perforin and granzyme A was proportional to the infiltrated mononuclear cell numbers at 16 weeks of age. Correlation coefficients (R2) to infiltrated mononuclear cell number was 0.59 for VCAM-1, 0.74 for FasL, 0.46 for perforin and 0.59 for granzyme A.
Fig. 4.
Down-regulation of inflammatory molecules and apoptosis in K-13182-treated mice. (a) Apoptosis in K-13182-treated mice lacrimal glands was analysed at 12 weeks of age by terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) assay and the percentage of TUNEL-positive cells (TUNEL index) was measured. Data were analysed in three fields per one section, and were expressed as mean ± s. d. in four (control mice) or three (K-13182-treated mice) examined in each group. Down-regulated (c) FasL; (d) perforin; (e) granzyme A mRNA in K-13182-treated mice was examined at 12 and 16 weeks of age with real-time polymerase chain reaction (PCR). (b) Vascular cell adhesion molecule-1 (VCAM-1) was slightly down-regulated but statistical significance was not detected. rRNA is the internal control for the reverse transcriptase–PCR. The white and black bars indicate control mice and K-13182-treated mice, respectively. Statistical analysis was performed using Student's t-test, *P < 0.05.
Fig. 5.
Correlation coefficients between the number of infiltrated mononuclear cells and the results of quantitative polymerase chain reaction (PCR). Correlation coefficients (R2) between the number of infiltrated mononuclear cells and the analysed gene mRNA levels were 0.59 [▪: vascular cell adhesion molecule-1 (VCAM-1)], 0.74 (○: FasL), 0.46 (□ perforin) and 0.59 (□: granzyme A).
Discussion
In the present study, our purpose was to assess the therapeutic effect of K-13182 as a therapeutic agent for SS in vitro and in vivo.
Regarding the inhibition of cellular adhesion of K-13182 between endothelial cells and mononuclear cells, as shown in in vitro experiments (Figs 1 and 2), K-13182 inhibited VCAM-1 expression in HUVECs and MAECs. In addition, cellular adhesion with endothelial cells and mononuclear cells was prevented in both human and murine co-culture assay systems. These results indicated that K-13182 retains the ability to inhibit mononuclear infiltration into the inflammatory region via endothelial cells, and the therapeutic effect shown in the in vivo analyses might be mediated by the prevention of cellular adhesion by K-13182.
Considering the effect of K-13182 on the VCAM-1 expression and cellular adhesion in vitro, IC50 for VCAM-1 expression was 0.019 µM (HUVECs) and 0.05 µM (MAECs), while IC50 for cellular adhesion was 0.14 µM (HUVECs) and 0.10 µM (MAECs). These results suggest that adhesion molecules other than VCAM-1 also contribute to cellular adhesion. Nakao et al. have shown that ICAM-1/β2 integrin also participated in the adhesion of U-937 cells on HUVECs [22]. In the VCAM-1 gene promoter region, functional transcription factor binding motifs have been reported, including nuclear factor (NF)-κB, interferon regulatory factor, AP-1 and GATA [23–27], while the ICAM-1 gene promoter also has SP-1, NF-κB and AP-1 binding sites; however, it does not contain the GATA binding motifs [28]. Taken together, K-13182 might have inhibited VCAM-1 but not ICAM-1 expression through the regulation of GATA in HUVECs and MAECs. Human VCAM-1 promoter region contains two conserved consensus binding sites for the GATA family, while the murine VCAM-1 promoter contains one site [29,30]. This number of GATA binding motifs may explain the smaller IC50 in HUVECs than in MAECs in VCAM-1 expression assays and cellular adhesion assays. K-13182 was developed from a compound (K-7174) that had shown an inhibitory effect for the GATA family [15]. However, details of the mechanisms regarding the inhibition of GATA and characteristics of K-13182 and K-7174 should be investigated in future study.
Following 12-week administration of K-13182, decreased infiltration of mononuclear cells to lacrimal glands was shown by histological analysis (Fig. 3b) and the preventive effect of K-13182 for tear decrease was also confirmed. These anti-inflammatory effects of K-13182 might be mediated by the down-regulation of VCAM-1 expression and mononuclear cell adhesion, considering the in vitro analyses (Figs 1 and 2). However, a statistically significant difference was not detected in the VCAM-1 expression in lacrimal glands of NOD mice (Fig. 4b). This discrepancy between the in vitro and in vivo experiments may be caused by the sample; we used endothelial cells in the in vitro experiment, while lacrimal glands used for quantitative PCR included various kind of cells. This sampling may lead to a slight suppression of VCAM-1 in K-13182-treated mice. Furthermore, the smaller IC50 of K-13182 for MAECs compared with the IC50 for HUVECs discussed above and the results of the RT–PCR (Figs 1d and 2c) may explain partly the low therapeutic efficacy including VCAM-1 mRNA expression in lacrimal glands in a mouse model. Although further investigation is needed to find in which cells K-13182 is effective in lacrimal glands, K-13182 is considered to contribute to the down-regulation of VCAM-1 expression and mononuclear cell infiltration in lacrimal glands because high correlation coefficients were detected between VCAM-1 expression and infiltrated cell number in K-13182-treated mice (Fig. 5).
Considering the decreased mononuclear infiltration in K-13182-treated mice lacrimal glands and proposed mechanism of apoptosis in NOD mice [20,21], we speculated that the apoptosis suppression that may be induced due to the reduced mononuclear cell infiltration via cell adhesion molecules including VCAM-1. TUNEL assay showed the decreased number of apoptotic cells in K-13182-treated mice lacrimal glands. Furthermore, quantitative PCR analysis in K-13182-treated mice lacrimal glands also demonstrated the down-regulation of FasL, perforin and granzyme A, which are involved in SS [31–34]. Fas/FasL-mediated tissue destruction in SS lacrimal glands were also reported [31,32]. Granzyme A is a serine protease that is contained in the granules of activated lymphocytes and plays a role in the destruction of the SS lacrimal glands [33], and perforin was also reported to be expressed in the lacrimal glands of SS [34]. Taken together with our results, the prevention of the tear decrease in K-13182-treated mice can be explained partly by the suppression of apoptosis and molecules such as FasL, perforin and granzyme A that may be decreased due to the reduced mononuclear cell infiltration via cell adhesion molecules including VCAM-1.
Saito et al. determined that several cell adhesion molecules are involved in the lymphoid cell infiltration of salivary and lacrimal glands in SS patients [10]. Furthermore, previous studies have demonstrated that neutralizing monoclonal antibodies (mAb) of cell adhesion molecules prevented the development of experimental autoimmune encephalomyelitis in the murine model [35,36]. Therefore, the inhibition of signal transduction pathways involved in lymphocyte adhesion is also valuable in treating these autoimmune diseases. VCAM-1 plays a critical role in inflammatory reactions, from the original recruitment of leucocytes through successive steps in the continuing process [11]. In fact, VCAM-1/VLA-4 has been studied as a target for a variety of autoimmune diseases. The humanized mAb to alpha4 integrin, natalizumab, was approved recently in the United States for the treatment of relapsing multiple sclerosis [37]. Neutralizing mAb to VCAM-1 reduced clinical severity in collagen-induced arthritis in mice [38], and peptide inhibitor for VCAM-1 also prevented airway hyperresponsiveness in sheep [39]. Therefore, specific inhibitors of VCAM-1 induction in endothelial cells have been sought actively to develop a new therapeutic approach that would not elicit severe side effects in the case of the treatment of atherosclerosis or various chronic inflammatory disorders, including SS [40].
We have demonstrated here the potent effect of K-13182 in SS model mice, which might be caused by the preventive effect of cellular adhesion mediated by VCAM-1. Our results are consistent with a former report that showed antibodies against VCAM-1 almost completely blocked lymphocyte migration from the blood into inflamed lacrimal glands in NOD mice [18]. K-13182 showed similar effects on VCAM-1 expression and mononuclear adhesion both in murine and human endothelial cells. In this report, K-13182 was administered prior to disease onset for the prevention of the disease, therefore the therapeutic effect of K-13182 should be analysed further. However, from our results, K-13182 is a promising candidate for a SS therapeutic reagent.
Acknowledgments
The authors gratefully thank Roger E. Morgan for helpful comments and critical reading of the manuscript, Wakako Suzuki, Koichi Yamada, Aki Sato and Noriko Hitosugi for technical assistance, Naohiro Saito for maintenance of mice and Judith Nishino for helpful discussions during the preparation of this manuscript. This work was supported partially by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The Sjögren's syndrome Project of Keio University was supported by Kowa Co., Ltd.
References
- 1.Fox RI, Saito I. Criteria for diagnosis of Sjogren's syndrome. Rheum Dis Clin North Am. 1994;20:391–407. [PubMed] [Google Scholar]
- 2.Kang HI, Fei HM, Saito I, et al. Comparison of HLA class II genes in Caucasoid, Chinese, and Japanese patients with primary Sjogren's syndrome. J Immunol. 1993;150:3615–23. [PubMed] [Google Scholar]
- 3.Fox RI, Chilton T, Scott S, Benton L, Howell FV, Vaughan JH. Potential role of Epstein–Barr virus in Sjogren's syndrome. Rheum Dis Clin North Am. 1987;13:275–92. [PubMed] [Google Scholar]
- 4.Fox RI, Pearson G, Vaughan JH. Detection of Epstein–Barr virus-associated antigens and DNA in salivary gland biopsies from patients with Sjogren's syndrome. J Immunol. 1986;137:3162–8. [PubMed] [Google Scholar]
- 5.Saito I, Servenius B, Compton T, Fox RI. Detection of Epstein–Barr virus DNA by polymerase chain reaction in blood and tissue biopsies from patients with Sjogren's syndrome. J Exp Med. 1989;169:2191–8. doi: 10.1084/jem.169.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol. 1998;18:842–51. doi: 10.1161/01.atv.18.5.842. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien KD, Allen MD, McDonald TO, et al. Vascular cell adhesion molecule-1 is expressed in human coronary atherosclerotic plaques. Implications for the mode of progression of advanced coronary atherosclerosis. J Clin Invest. 1993;92:945–51. doi: 10.1172/JCI116670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morales-Ducret J, Wayner E, Elices MJ, Alvaro-Gracia JM, Zvaifler NJ, Firestein GS. Alpha 4/beta 1 integrin (VLA-4) ligands in arthritis. Vascular cell adhesion molecule-1 expression in synovium and on fibroblast-like synoviocytes. J Immunol. 1992;149:1424–31. [PubMed] [Google Scholar]
- 10.Saito I, Terauchi K, Shimuta M, et al. Expression of cell adhesion molecules in the salivary and lacrimal glands of Sjogren's syndrome. J Clin Lab Anal. 1993;7:180–7. doi: 10.1002/jcla.1860070309. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function-associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J Exp Med. 1994;179:1145–54. doi: 10.1084/jem.179.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiyama T, Mishima K, Ide F, et al. Functional analysis of an established mouse vascular endothelial cell line. J Vasc Res. 2007;44:138–48. doi: 10.1159/000098520. [DOI] [PubMed] [Google Scholar]
- 13.Kaneko M, Hayashi J, Saito I, Miyasaka N. Probucol downregulates E-selectin expression on cultured human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 1996;16:1047–51. doi: 10.1161/01.atv.16.8.1047. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko M, Inoue H, Nakazawa R, et al. Pirfenidone induces intercellular adhesion molecule-1 (ICAM-1) down-regulation on cultured human synovial fibroblasts. Clin Exp Immunol. 1998;113:72–6. doi: 10.1046/j.1365-2249.1998.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umetani M, Nakao H, Doi T, et al. A novel cell adhesion inhibitor, K-7174, reduces the endothelial VCAM-1 induction by inflammatory cytokines, acting through the regulation of GATA. Biochem Biophys Res Commun. 2000;272:370–4. doi: 10.1006/bbrc.2000.2784. [DOI] [PubMed] [Google Scholar]
- 16.Delporte C, O'Connell BC, He X, et al. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci USA. 1997;94:3268–73. doi: 10.1073/pnas.94.7.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White SC, Casarett GW. Induction of experimental autoallergic sialadenitis. J Immunol. 1974;112:178–85. [PubMed] [Google Scholar]
- 18.Mikulowska-Mennis A, Xu B, Berberian JM, Michie SA. Lymphocyte migration to inflamed lacrimal glands is mediated by vascular cell adhesion molecule-1/alpha(4) beta(1) integrin, peripheral node addressin/1-selectin, and lymphocyte function-associated antigen-1 adhesion pathways. Am J Pathol. 2001;159:671–81. doi: 10.1016/s0002-9440(10)61738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toda I, Sullivan BD, Rocha EM, Da Silveira LA, Wickham LA. Sullivan DA. Impact of gender on exocrine gland inflammation in mouse models of Sjogren's syndrome. Exp Eye Res. 1999;69:355–66. doi: 10.1006/exer.1999.0715. [DOI] [PubMed] [Google Scholar]
- 20.Tsubota K, Fujita H, Tadano K, et al. Improvement of lacrimal function by topical application of CyA in murine models of Sjogren's syndrome. Invest Ophthalmol Vis Sci. 2001;42:101–10. [PubMed] [Google Scholar]
- 21.Kong L, Robinson CP, Peck AB, et al. Inappropriate apoptosis of salivary and lacrimal gland epithelium of immunodeficient NOD-scid mice. Clin Exp Rheumatol. 1998;16:675–81. [PubMed] [Google Scholar]
- 22.Nakano H, Doi T, Suda M, et al. An inhibitor of VCAM-1 expression and its implication as a novel treatment of inflammatory diseases. J Atheroscler Thromb. 1998;4:149–55. [Google Scholar]
- 23.Iademarco MF, McQuillan JJ, Rosen GD, Dean DC. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1) J Biol Chem. 1992;267:16323–9. [PubMed] [Google Scholar]
- 24.Neish AS, Read MA, Thanos D, Pine R, Maniatis T, Collins T. Endothelial interferon regulatory factor 1 cooperates with NF-kappa B as a transcriptional activator of vascular cell adhesion molecule 1. Mol Cell Biol. 1995;15:2558–69. doi: 10.1128/mcb.15.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neish AS, Khachigian LM, Park A, Baichwal VR, Collins T. Sp1 is a component of the cytokine-inducible enhancer in the promoter of vascular cell adhesion molecule-1. J Biol Chem. 1995;270:28903–9. doi: 10.1074/jbc.270.48.28903. [DOI] [PubMed] [Google Scholar]
- 26.Lechleitner S, Gille J, Johnson DR, Petzelbauer P. Interferon enhances tumor necrosis factor-induced vascular cell adhesion molecule 1 (CD106) expression in human endothelial cells by an interferon-related factor 1-dependent pathway. J Exp Med. 1998;187:2023–30. doi: 10.1084/jem.187.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papi A, Johnston SL. Respiratory epithelial cell expression of vascular cell adhesion molecule-1 and its up-regulation by rhinovirus infection via NF-kappaB and GATA transcription factors. J Biol Chem. 1999;274:30041–51. doi: 10.1074/jbc.274.42.30041. [DOI] [PubMed] [Google Scholar]
- 28.Voraberger G, Schafer R, Stratowa C. Cloning of the human gene for intercellular adhesion molecule 1 and analysis of its 5′-regulatory region. Induction by cytokines and phorbol ester. J Immunol. 1991;147:2777–86. [PubMed] [Google Scholar]
- 29.Cybulsky MI, Allan-Motamed M, Collins T. Structure of the murine VCAM1 gene. Genomics. 1993;18:387–91. doi: 10.1006/geno.1993.1480. [DOI] [PubMed] [Google Scholar]
- 30.Cybulsky MI, Fries JW, Williams AJ, et al. Gene structure, chromosomal location, and basis for alternative mRNA splicing of the human VCAM1 gene. Proc Natl Acad Sci USA. 1991;88:7859–63. doi: 10.1073/pnas.88.17.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito I, Haruta K, Shimuta M, et al. Fas ligand-mediated exocrinopathy resembling Sjogren's syndrome in mice transgenic for IL-10. J Immunol. 1999;162:2488–94. [PubMed] [Google Scholar]
- 32.Ishimaru N, Yoneda T, Saegusa K, et al. Severe destructive autoimmune lesions with aging in murine Sjogren's syndrome through Fas-mediated apoptosis. Am J Pathol. 2000;156:1557–64. doi: 10.1016/S0002-9440(10)65027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox RI. Sjogren's syndrome: immunobiology of exocrine gland dysfunction. Adv Dent Res. 1996;10:35–40. doi: 10.1177/08959374960100010601. [DOI] [PubMed] [Google Scholar]
- 34.Tsubota K, Saito I, Miyasaka N. Expression of granzyme A and perforin in lacrimal gland of Sjogren's syndrome. Adv Exp Med Biol. 1994;350:637–40. doi: 10.1007/978-1-4615-2417-5_106. [DOI] [PubMed] [Google Scholar]
- 35.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356:63–6. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 36.Merrill JE, Benveniste EN. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 1996;19:331–8. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- 37.Rice GP, Hartung HP, Calabresi PA. Anti-alpha4 integrin therapy for multiple sclerosis: mechanisms and rationale. Neurology. 2005;64:1336–42. doi: 10.1212/01.WNL.0000158329.30470.D0. [DOI] [PubMed] [Google Scholar]
- 38.Carter RA, Campbell IK, O'Donnel KL, Wicks IP. Vascular cell adhesion molecule-1 (VCAM-1) blockade in collagen-induced arthritis reduces joint involvement and alters B cell trafficking. Clin Exp Immunol. 2002;128:44–51. doi: 10.1046/j.1365-2249.2002.01794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh J, Van Vlijmen H, Liao Y, et al. Identification of potent and novel alpha4beta1 antagonists using in silico screening. J Med Chem. 2002;45:2988–93. doi: 10.1021/jm020054e. [DOI] [PubMed] [Google Scholar]
- 40.Foster CA. VCAM-1/alpha 4-integrin adhesion pathway: therapeutic target for allergic inflammatory disorders. J Allergy Clin Immunol. 1996;98:S270–7. doi: 10.1016/s0091-6749(96)70075-1. [DOI] [PubMed] [Google Scholar]