Abstract
Interleukin (IL)-32 is a recently described proinflammatory cytokine, characterized by induction of nuclear factor (NF)-κB activation. We studied IL-32α expression in the inflamed mucosa of inflammatory bowel disease (IBD). We also investigated mechanisms regulating IL-32α expression. Tissue samples were obtained endoscopically or surgically from patients with ulcerative colitis (UC) (n = 10), Crohn's disease (CD) (n = 10), ischaemic colitis (n = 4) and normal colorectal tissues (n = 10). IL-32α expression was evaluated by standard immunohistochemical procedure. IL-32 mRNA expression was analysed by Northern blot. IL-32α was expressed weakly by colonic epithelial cells from normal individuals and subjects with ischaemic colitis. In the inflamed mucosa of IBD patients, epithelial IL-32α expression was increased markedly. In UC and CD patients, IL-32α expression was enhanced in affected mucosa compared to non-affected mucosa. In intestinal epithelial cell lines, expression of IL-32α mRNA and protein was enhanced by IL-1β, interferon (IFN)-γ and tumour necrosis factor (TNF)-α. A combination of TNF-α plus IFN-γ exerted synergistic effects. IL-32α induction by IL-1β and/or TNF-α was mediated by NF-κB activation. Epithelial IL-32α expression was increased in IBD patients, and in CD patients in particular. IL-32α might be involved in the pathophysiology of IBD as a proinflammatory cytokine and a mediator of innate immune response.
Keywords: cytokine, IBD, NOD2
Introduction
Ulcerative colitis (UC) and Crohn's disease (CD), two common forms of idiopathic inflammatory bowel disease (IBD), are chronic, relapsing inflammatory disorders of the gastrointestinal tracts. Although the precise aetiology of IBD remains unclear, a widely accepted hypothesis is that ubiquitous, commensal intestinal bacteria trigger an inappropriate, overactive and ongoing mucosal immune response that mediates intestinal tissue damage in genetically susceptible individuals [1–3].
Interleukin (IL)-32 is a recently described cytokine produced by T lymphocytes, natural killer (NK) cells, monocytes and epithelial cells [4,5]. Although IL-32 was first reported as transcript in IL-2 activated NK and T cells, it appears that epithelial cells are a dominant and widespread source [6]. The gene encoding IL-32 is located on human chromosome 16p13.3 and organized into eight exons [7]. There are four splice variants (IL-32α, IL-32β, IL-32δ and IL-32γ), and IL-32α is the most abundant transcript. Of particular importance, IL-32 is prominently induced by interferon (IFN)-γ in lung epithelial cells and monocytes [4]. IL-32 exhibits several properties typical of proinflammatory cytokines [4,5]. For example, it stimulates the secretion of proinflammatory cytokines and chemokines such as IL-1α, TNF-α, IL-6 and IL-8 by means of the activation of nuclear factor-κB (NF-κB) and p38 mitogen-activated protein kinases (MAPKs) [4,5].
Netea et al. demonstrated recently that IL-32 augments the production of IL-1α and IL-6 induced by muramyl dipeptide (MDP), a peptidoglycan fraction of bacteria, by means of the nucleotide-binding oligomerization domain proteins (NOD1 and NOD2) through a caspase-1-dependent mechanism [5]. NODs are a family of intracytoplasmic bacterial sensors, and recognition of bacterial peptidoglycans subsequently induces NF-κB activation [8]. Mutations in NOD2 have been implicated in the pathogenesis of CD [9,10], and CD patients homozygous for the frameshift 3020insC mutated allele have defective responses to MDP in cytokine production [11,12]. Recently, it has been shown that the NOD2 mutation in CD patients potentiates NF-κB activity and IL-1α processing [13]. Thus, these suggest a pivotal role of IL-32 in the pathophysiology of IBD, and CD in particular.
IL-32 has been implicated in inflammatory disorders such as rheumatoid arthritis [6,14–16] and Mycobacterium tuberculosis infection [17,18], but the pathophysiological role of IL-32 in IBD remains unclear. In this study, we investigated IL-32α expression in the inflamed mucosa of IBD patients. Furthermore, we investigated in vitro mechanisms regulating IL-32α expression in intestinal epithelial cells (IECs). Our results provide evidence that IL-32α is overexpressed in the inflamed mucosa of IBD, and may therefore play a role in the pathophysiology of IBD.
Materials and methods
Reagents
Recombinant human IL-1β, IL-17 and IFN-γ were purchased from R&D Systems (Minneapolis, MN, USA), and other cytokines were obtained from PeproTech (Rocky Hill, NJ, USA). Anti-human IL-32α antibodies were purchased from R&D Systems. All other reagents were purchased from Sigma Chemical Co. (St Louis, MO, USA).
Cells
Human colon cancer cell lines HT-29 [19], Caco-2 [20] and T84 [21,22] were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were grown as described previously [19–21].
Tissue samples
Diagnoses for UC and CD were based on conventional clinical, endoscopic and histopathological criteria. Surgically obtained or biopsy specimens from 10 patients with UC (five male and five female, mean 34 years) and 10 patients with CD (five male and five female, mean 28 years) were used for this analysis, with informed consent. The Ethics Committee of the Shiga University of Medical Science approved this study. During sample collection, all patients were clinically and endoscopically active with either a colitis activity index for UC [23] or the Crohn's disease activity index [24]. Three UC and three CD patients underwent surgical procedures due to resistance to medication or to other complications (e.g. massive bleeding, fistula formation or perforation). All patients were treated with salicylates, and four of 10 UC and five of 10 CD patients received treatment with corticosteroids. Five UC patients were treated with azathioprine. Biopsy samples derived from ischaemic colitis (n = 4) were obtained by colonoscopies. Normal colorectal tissues were obtained by the surgical resection of colon cancer at distal tumour sites (n = 10).
Immunohistochemistry
Fresh-frozen sections were air-dried, fixed in 1.0% paraformaldehyde (pH 7.4) for 10 min and acetone for 5 min, and washed in phosphate-buffered saline (PBS). Goat polyclonal anti-human IL-32 antibodies (R&D) were used as the primary antibodies. After incubation with the primary antibodies, the sections were treated with biotin-conjugated goat anti-rabbit IgG (Vector, Burlingame, CA, USA) and avidin–biotin–peroxidase complexes (ABC; Vector).
Northern blot analyses
Total cellular RNA was isolated by the acid guanidinium thiocyanate–phenol–chloroform method [25]. Northern blots were performed according to a previously described method [26]. Hybridizations were performed with [32]P-labelled human probes, generated by a random primed DNA labelling kit (Amersham, Arlington Heights, IL, USA), and evaluated by autoradiography. Human IL-8 cDNA probes have been described in our previous reports [27].
Western blot analyses
Cells were exposed to cytokines for predetermined periods of time. Cells were then washed with PBS and lysed in sodium dodecyl sulphate (SDS) sample buffer containing 100 µM orthovanadate. Western blots were performed according to a method described previously [28]. Biotinylated anti-human IL-32α antibodies were purchased from Cell Signalling Technology (Beverly, MA, USA), and peroxidase-conjugated streptavidin was purchased from Dako Japan (Kyoto, Japan). Subsequently, detection was performed using the enhanced chemiluminescence Western blotting system (Amersham).
Adenovirus-mediated gene transfers
We used a recombinant adenovirus expressing a stable mutant form of IκBα (Ad-IκBΔN) [29], a recombinant adenovirus expressing a dominant negative mutant of c-Jun (Ad-DN-c-Jun) [30] and a recombinant adenovirus containing bacterial β-galactosidase cDNA (Ad-LacZ). The stable mutant form of IκBα (IκBΔN) lacks 54 NH2-terminal amino acids of wild-type IκBα, and is neither phosphorylated nor proteolysed in response to signal induction, but fully inhibits NF-κB activation. The dominant negative mutant c-Jun (TAM67) lacks the transactivational domain of amino acids 3–122 of wild-type c-Jun, but retains the DNA-binding domain. In preliminary experiments, Ad-LacZ infections of colonic myofibroblasts with a multiplicity of infection (MOI) of 10 showed a maximal expression (85% positive) of β-galactosidase. The recombinant adenovirus was transferred into the cells, and cells were made quiescent for 48 h before being assessed for the effects of the transferred gene.
Statistical analyses
The statistical significance of differences was determined by the Mann–Whitney U-test (Statview version 4.5). Differences resulting in P-values less than 0.05 were considered to be statistically significant.
Results
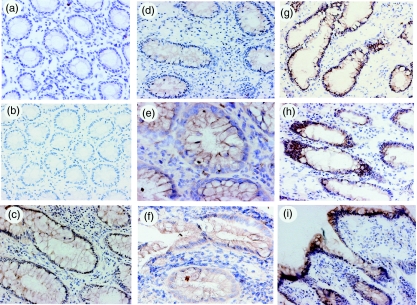
To evaluate the expression of IL-32α protein in the mucosa, biopsy specimens and/or surgically resected samples were stained with anti-IL-32α antibodies. As shown in Fig. 1a, IL-32α was expressed weakly by epithelial cells in normal colonic mucosa. The specificity of this staining was confirmed by staining with normal goat IgG (Fig. 1b). Similar staining to normal mucosa was observed in samples of ischaemic colitis (data not shown). In contrast, epithelial expression of IL-32α was enhanced markedly in the inflamed region of active UC (Fig. 1c–f) and CD patients (Fig. 1g–i). In particular, IL-32α expression tends to increase in samples from active CD patients. Enhanced IL-32α expression in epithelial cells was detected in all samples from active lesions of UC and CD patients. In contrast, IL-32α expression was scarcely detectable in leucocytes. Thus, these indicate that epithelial cells are major expression sites for IL-32α in intestinal mucosa, and that IL-32α expression is enhanced in IBD mucosa.
Fig. 1.
Immunohistochemical analyses of interleukin (IL)-32α expression in the human colon. (a) Normal mucosa; (b) normal mucosa stained with normal goat IgGs; (c–f) ulcerative colitis; and (g–i) Crohn's disease. Original magnification: (a,b,d,g) × 100; (c,f,h,i) × 200; and (e) × 400.
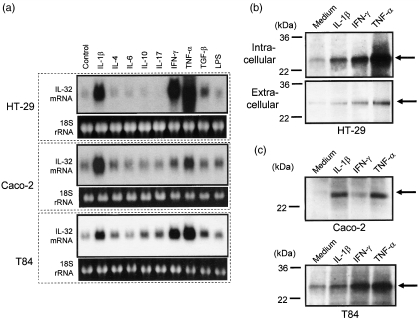
To investigate regulatory mechanisms underlying IL-32α induction, we used colon cancer cell lines, HT-29, Caco-2 and T84 cells [19–21]. These cells are well characterized and used frequently as models of intestinal epithelial cells. As shown in Fig. 2a, cells were stimulated with various cytokines for 12 h, and IL-32α mRNA expression was detected by Northern blot. In all cell lines, IL-32α mRNA was expressed weakly without any stimulus. Stimulation with IL-1β, IFN-γ and TNF-α enhanced IL-32α mRNA expression in all cell lines. In these factors, TNF-α was stronger than IL-1α and/or IFN-γ in both HT-29 and T84 cells, respectively. However, IL-1β was strongest in Caco-2 cells.
Fig. 2.
Interleukin (IL)-32α mRNA and protein expression in human intestinal epithelial cell lines. (a) Intestinal epithelial cell lines (HT-29, Cacco-2 and T84 cells) were stimulated with cytokines [IL-1β (10 ng/ml) and other cytokines (100 ng/ml)] for 12 h, and IL-32α mRNA expression was determined by Northern blot. (b) HT-29 cells were stimulated with cytokines [IL-1β (10 ng/ml) and other cytokines (100 ng/ml)] for 48 h, and intracellular (upper part) and secreted (lower part) IL-32α were detected by Western blot. (c) Caco-2 and T84 cells were stimulated with cytokines [IL-1β (10 ng/ml) and other cytokines (100 ng/ml)], and intracellular IL-32α was detected by Western blots.
Similar results were observed at the protein levels (Fig. 2b,c). Cells were stimulated for 48 h with IL-1β, IFN-γ and TNF-α, and IL-32α protein expression was analysed by Western blot. IL-32α was detected as a molecular weight protein of 25 kDa, which is comparable with a previous report [5]. In HT-29 cells, stimulation with IL-1β, IFN-γ and TNF-α enhanced intracellular accumulation (Fig. 2b, upper part) and extracellular secretion (Fig. 2b, lower part) of IL-32α protein. Intracellular IL-32α was detected relatively stronger than secreted IL-32α. In Caco-2 and T84 cells, intracellular IL-32 accumulation was also stimulated by IL-1β, IFN-γ and TNF-α (Fig. 2c), and IL-32α secretion was also stimulated by these cytokines (data not shown).
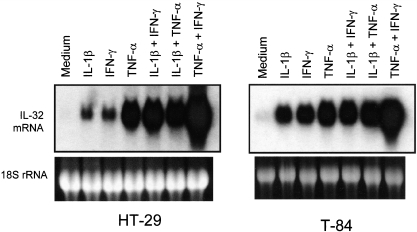
Next, we tested the effects of combinations of IL-1β, IFN-γ and TNF-α (Fig. 3). In HT-29 and T84 cells, a combination of IL-1α plus TNF-α showed additive effects. IL-1β plus IFN-γ showed synergistically enhanced IL-32α mRNA expression in HT-29 cells, but it was additive in T84 cells. In HT-29 and T84 cells, TNF-α plus IFN-γ enhanced synergistically IL-32α mRNA expression, and effects of this combination were strongest.
Fig. 3.
The combined effects of interleukin (IL)-1α, interferon (IFN)-γ and tumour necrosis factor (TNF)-α. Cells were stimulated with IL-1β (10 ng/ml), IFN-γ (100 ng/ml) and TNF-α (100 ng/ml), and combinations for 12 h and IL-32α mRNA expression were analysed by Northern blot.
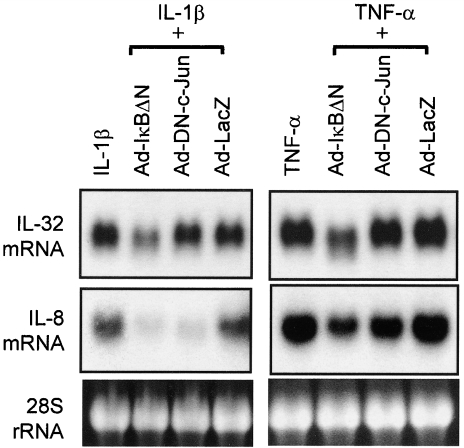
To assess the role of transcription factors NF-κB and AP-1, we evaluated the effects of a recombinant adenovirus containing a stable mutant form of IκBα (Ad-IκBΔN) and a dominant negative mutant of c-Jun (Ad-DN-c-Jun). As shown in Fig. 4, cells were infected with recombinant adenovirus, and were cultured for 48 h. Cells were then stimulated for 12 h with IL-1β (10 ng/ml) and TNF-α (100 ng/ml), and the expression of IL-32α mRNAs was determined by Northern blot. Ad-IκBΔN inhibited the effects of both IL-1β and TNF-α on IL-32α mRNA expression, but Ad-DN-c-Jun had no effect. In contrast, IL-8 mRNA expression was blocked by Ad-IκBΔN and Ad-DN-c-Jun, suggesting successful inhibitory effects of both mutant genes. Inhibitory effects were not induced by the Ad-LacZ gene, which was used as a negative control. These suggest that NF-κB plays a role in IL-1β- and TNF-α-induced IL-32α mRNA expression.
Fig. 4.
Molecular mechanisms underlying interleukin (IL)-32α mRNA expression. Effects of nuclear factor κB (NF-κB) and/or AP-1 inhibition on IL-32α and IL-8 mRNA expression. HT-29 cells were infected with an adenovirus expressing the IκBΔN or DN-c-Jun, and 48 h after infection cells were stimulated with IL-1β (10 ng/ml) and tumour necrosis factor (TNF)-α (100 ng/ml) for 12 h. IL-32α mRNA expression was determined by Northern blot.
Discussion
In the present study we demonstrate that the epithelial expression of IL-32α is enhanced in the inflamed mucosa of IBD patients. In vitro studies using human intestinal epithelial cell (IEC) lines suggest that IL-1β, IFN-γ and TNF-α are major inducers of IL-32α expression. TNF-α has not been reported previously as an inducer of IL-32α expression in any cell types, and to our knowledge this is the first description concerning this phenomenon. Because IL-32α is a proinflammatory cytokine characterized by NF-κB and p38 MAPK activating activities [4,15], and because IL-32 acts synergistically with NOD ligands to induce proinflammatory cytokines [5], overexpression of IL-32α in IBD mucosa suggests strongly that it plays an important role in inflammatory and anti-bacterial responses that are involved in the pathogenesis of IBD.
In this study, we observed the constitutive expression of IL-32α in IECs in vivo and in vitro, suggesting a role for this factor in intestinal homeostasis. Our finding is compatible with a recent report by Netea et al. [5], in which the authors show that IL-32 is expressed in the epithelium of normal human colonic mucosa. Furthermore, we found that epithelial IL-32α expression is enhanced markedly in IBD mucosa compared to normal mucosa, and prominently in CD patients. IL-32 acts as a proinflammatory cytokine, which is characterized by inducing the release of proinflammatory cytokines (TNF-α, IL-1β, IL-6 and chemokines) through NF-κB and p38 MAPK activation pathways [4,6]. The aforementioned suggests that enhanced secretion of IL-32α by IECs stimulates infiltrating immune cells to secrete proinflammatory cytokines and contributes to the deterioration of mucosal inflammation.
Recent studies focused on a role of innate immunity in the pathogenesis of IBD [31]. Initial sensing of innate immunity is mediated by the recognition of pathogen-associated molecular patters (PAMPs) through Toll-like receptors (TLRs) and NOD proteins (NODs) [32]. TLRs are located mainly on cell-surface membranes, but NODs function as intracellular recognition systems [8,9]. In human monocytes, IL-32 acts synergistically with NOD-specific peptidoglycans for the release of IL-1β and IL-6 [5]. The synergistic effect of IL-32 and NOD ligands on cytokine production is abolished in cells from CD patients bearing the NOD2 frameshift mutation 3020insC, indicating that the synergism of IL-32 plus MDP depends on NOD2 [5]. Interactions between NOD-1 and IL-32 also potentiate proinflammatory cytokine production [5]. Furthermore, Berrebi et al. previously reported overexpression of NOD2 in infiltrated monocytes and epithelial cells in IBD mucosa [33]. These findings suggest that overexpressed IL-32 causes specific and excessive stimulation of NOD pathways, which leads to a marked amplification in IL-1β and IL-6 production in IBD mucosa. IL-1β and IL-6 are representative proinflammatory cytokines, and these responses may contribute to an aggravation in IBD mucosal inflammation.
Previously, IL-1β, IL-12, IL-18 and IFN-γ were reported as inducers of IL-32 induction [4]. However, the regulatory mechanism of IL-32 expression in IECs remains unclear. In this study, we found that TNF-α, as well as IL-1β and IFN-γ, are potent stimulators of IL-32α induction in IECs. In addition, a synergistic effect of TNF-α and IFN-γ was observed. IL-32α mRNA expression by both IL-1β and TNF-α was mediated by NF-κB, but not AP-1. Thus, it became clear that TNF-α plays an important role in IL-32α induction in IECs. Because IL-32 was characterized initially as an inducer of TNF-α in circulating monocytes [4], inflammatory responses in the affected mucosa of IBD patients may be amplified by a consecutive loop of IL-32-induced TNF-α secretion from monocytes and TNF-α-stimulated IL-32 secretion from epithelial cells. This loop may be amplified further by Th1 cytokine IFN-γ. Previously, it was reported that TNF-α and IFN-γ induce synergistically NOD2 [34], which indicates a coupled regulation of IL-32α and NOD2. As described above, IL-32 and NOD proteins act synergistically in inflammatory response [5], so coupled regulation of IL-32α and NOD2 may account for the rapid and efficient induction of innate immune responses at the intestinal mucosa. Furthermore, these data suggest that an amelioration of IBD symptoms by TNF-α-targeting therapies may be dependent partially on an interference of the TNF-α-IL-32 loop.
Immunohistochemical studies showed cytoplasmic localization of IL-32α in IECs. This finding was supported by Western blot analyses in this study. In IEC lines IL-32α was secreted into supernatant, but abundant IL-32α was also detectable in lysates. Similar observations were reported in Cos7 cells transfected with IL-32α cDNA [4]. In these cells, intracellular IL-32α was approximately sixfold abundant compared to secreted IL-32α. In contrast, in Cos7 cells transfected with IL-32α cDNA, the abundance of IL-32α was comparable in supernatants and lysates. The IL-32 protein does not possess a typical hydrophobic signal peptide in its N terminus which is a typical feature of secreted cytokine, but contains predicted tyrosine sulphation sites which are common post-translational modification found in secretory proteins [7]. Although it remains unclear which of the IL-32 isoform is secreted effectively from particular cell types, IL-32 may play a role as a cytoplasmic protein. Recently, Goda et al. demonstrated that overexpression of intracellular IL-32β induced apoptosis in HeLa cells, which was blocked by interference of IL-32β transcription [35]. These results support the concept that high levels of intracellular IL-32 is associated with an induction of apoptosis. This may also be relevant to the in vivo finding of enhanced expression of IL-32α in IBD mucosa. Apoptosis of IECs is considered as a function to delete damaged epithelial cells and restore epithelial cell growth regulation and epithelial integrity [36]. Overexpression of cytoplasmic IL-32α might account for induction of apoptosis in damaged epithelial cells at the inflamed mucosa of IBD patients, leading to an efficient deletion and a rapid induction of mucosal repair. Apoptosis by accumulated IL-32 can be considered as a host defence mechanism against invading microorganisms, by which damaged epithelial cells are eliminated efficiently along with invading microorganisms and further invasions of microorganisms can be blocked.
In conclusion, we have demonstrated that IL-32α expression is enhanced in the inflamed mucosa of IBD patients. In human IECs, IL-32α was induced by IL-1β, IFN-γ and TNF-α. Furthermore, a combination of TNF-α and IFN-γ showed synergistic effects on IL-32α induction. IL-32 is a proinflammatory cytokine characterized by stimulatory activity on NF-κB and p38 MAP kinase [4], and IL-32 stimulation is amplified further by synergism with NOD pathways. Thus, IL-32-mediated inflammatory responses may play an important role in the pathophysiology of IBD. As enhanced IL-32 and NOD2 [33] in IBD mucosa co-localized, we speculate direct interactions between intracellular IL-32α and NOD2 in IECs. Our hypothesis is also supported by the synergism between extracellular stimulation of IL-32α and intracellular signalling by NODs [5]. To clarify further the role of the cytoplasmic accumulation of IL-32α in IECs, intracellular interactions between IL-32α and NOD proteins should be investigated.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Hibi T, Ogata H. Novel pathophysiological concepts of inflammatory bowel disease. J Gastroenterol. 2006;41:10–6. doi: 10.1007/s00535-005-1744-3. [DOI] [PubMed] [Google Scholar]
- 3.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 4.Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005;22:131–42. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Netea MG, Azam T, Ferwerda G, et al. IL-32 synergizes with nucleotide oligomerization domain (NOD) 1 and NOD2 ligands for IL-1beta and IL-6 production through a caspase 1-dependent mechanism. Proc Natl Acad Sci USA. 2005;102:16309–14. doi: 10.1073/pnas.0508237102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello CA, Kim SH. IL-32, a novel cytokine with a possible role in disease. Ann Rheum Dis. 2006;65(Suppl. 3):iii61–4. doi: 10.1136/ard.2006.058511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Q, Carroll HP, Gadina M. The newest interleukins: recent additions to the ever-growing cytokine family. Vitam Horm. 2006;74:207–28. doi: 10.1016/S0083-6729(06)74008-0. [DOI] [PubMed] [Google Scholar]
- 8.Peyrin-Biroulet L, Vignal C, Dessein R, Simonet M, Desreumaux P, Chamaillard M. NODs in defence: from vulnerable antimicrobial peptides to chronic inflammation. Trends Microbiol. 2006;14:432–8. doi: 10.1016/j.tim.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 10.Hugot JP. CARD15/NOD2 mutations in Crohn's disease. Ann NY Acad Sci. 2006;1072:9–18. doi: 10.1196/annals.1326.011. [DOI] [PubMed] [Google Scholar]
- 11.Netea MG, Ferwerda G, de Jong DJ, et al. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J Immunol. 2005;174:6518–23. doi: 10.4049/jimmunol.174.10.6518. [DOI] [PubMed] [Google Scholar]
- 12.Netea MG, Kullberg BJ, de Jong DJ, et al. NOD2 mediates anti-inflammatory signals induced by TLR2 ligands: implications for Crohn's disease. Eur J Immunol. 2004;34:2052–9. doi: 10.1002/eji.200425229. [DOI] [PubMed] [Google Scholar]
- 13.Maeda S, Hsu LC, Liu H, et al. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–8. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 14.Cagnard N, Letourneur F, Essabbani A, et al. Interleukin-32, CCL2, PF4F1 and GFD10 are the only cytokine/chemokine genes differentially expressed by in vitro cultured rheumatoid and osteoarthritis fibroblast-like synoviocytes. Eur Cytokine Netw. 2005;16:289–92. [PubMed] [Google Scholar]
- 15.Joosten LA, Netea MG, Kim SH, et al. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci USA. 2006;103:3298–303. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shoda H, Fujio K, Yamaguchi Y, et al. Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res Ther. 2006;8:R166. doi: 10.1186/ar2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kundu M, Basu J. IL-32: an emerging player in the immune response network against tuberculosis? PLoS Med. 2006;3:e274. doi: 10.1371/journal.pmed.0030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Netea MG, Azam T, Lewis EC, et al. Mycobacterium tuberculosis induces interleukin-32 production through a caspase-1/IL-18/interferon-gamma-dependent mechanism. PLoS Med. 2006;3:e277. doi: 10.1371/journal.pmed.0030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zweibaum A, Pinto M, Chevalier G, et al. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J Cell Physiol. 1985;122:21–9. doi: 10.1002/jcp.1041220105. [DOI] [PubMed] [Google Scholar]
- 20.Rousset M, Laburthe M, Pinto M, et al. Enterocytic differentiation and glucose utilization in the human colon tumor cell line Caco-2: modulation by forskolin. J Cell Physiol. 1985;123:377–85. doi: 10.1002/jcp.1041230313. [DOI] [PubMed] [Google Scholar]
- 21.Eckmann L, Kagnoff MF, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–74. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckmann L, Jung HC, Schurer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff MF. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology. 1993;105:1689–97. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 23.Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82–6. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn's disease activity index. Gastroenterol. 1976;70:439–44. National Cooperative Crohn's Disease Study. [PubMed] [Google Scholar]
- 25.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 26.Andoh A, Fujiyama Y, Sumiyoshi K, Sakumoto H, Bamba T. Interleukin 4 acts as an inducer of decay-accelerating factor gene expression in human intestinal epithelial cells. Gastroenterology. 1996;111:911–8. doi: 10.1016/s0016-5085(96)70058-6. [DOI] [PubMed] [Google Scholar]
- 27.Andoh A, Takaya H, Saotome T, et al. Cytokine regulation of chemokine (IL-8, MCP-1, and RANTES) gene expression in human pancreatic periacinar myofibroblasts. Gastroenterology. 2000;119:211–19. doi: 10.1053/gast.2000.8538. [DOI] [PubMed] [Google Scholar]
- 28.Shimada M, Andoh A, Hata K, et al. IL-6 secretion by human pancreatic periacinar myofibroblasts in response to inflammatory mediators. J Immunol. 2002;168:861–8. doi: 10.4049/jimmunol.168.2.861. [DOI] [PubMed] [Google Scholar]
- 29.Obara H, Takayanagi A, Hirahashi J, et al. Overexpression of truncated IkappaBalpha induces TNF-alpha-dependent apoptosis in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:2198–204. doi: 10.1161/01.atv.20.10.2198. [DOI] [PubMed] [Google Scholar]
- 30.Yasumoto H, Kim S, Zhan Y, et al. Dominant negative c-jun gene transfer inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia in rats. Gene Ther. 2001;8:1682–9. doi: 10.1038/sj.gt.3301590. [DOI] [PubMed] [Google Scholar]
- 31.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 32.Michelsen KS, Arditi M. Toll-like receptors and innate immunity in gut homeostasis and pathology. Curr Opin Hematol. 2007;14:48–54. doi: 10.1097/00062752-200701000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Berrebi D, Maudinas R, Hugot JP, et al. Card15 gene overexpression in mononuclear and epithelial cells of the inflamed Crohn's disease colon. Gut. 2003;52:840–6. doi: 10.1136/gut.52.6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenstiel P, Fantini M, Brautigam K, et al. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124:1001–9. doi: 10.1053/gast.2003.50157. [DOI] [PubMed] [Google Scholar]
- 35.Goda C, Kanaji T, Kanaji S, et al. Involvement of IL-32 in activation-induced cell death in T cells. Int Immunol. 2006;18:233–40. doi: 10.1093/intimm/dxh339. [DOI] [PubMed] [Google Scholar]
- 36.Kim JM, Eckmann L, Savidge TC, Lowe DC, Witthoft T, Kagnoff MF. Apoptosis of human intestinal epithelial cells after bacterial invasion. J Clin Invest. 1998;102:1815–23. doi: 10.1172/JCI2466. [DOI] [PMC free article] [PubMed] [Google Scholar]