Abstract
We have described previously the prophylactic and therapeutic effect of a DNA vaccine encoding the Mycobacterium leprae 65 kDa heat shock protein (DNA-HSP65) in experimental murine tuberculosis. However, the high homology of this protein to the corresponding mammalian 60 kDa heat shock protein (Hsp60), together with the CpG motifs in the plasmid vector, could trigger or exacerbate the development of autoimmune diseases. The non-obese diabetic (NOD) mouse develops insulin-dependent diabetes mellitus (IDDM) spontaneously as a consequence of an autoimmune process that leads to destruction of the insulin-producing β cells of the pancreas. IDDM is characterized by increased T helper 1 (Th1) cell responses toward several autoantigens, including Hsp60, glutamic acid decarboxylase and insulin. In the present study, we evaluated the potential of DNA-HSP65 injection to modulate diabetes in NOD mice. Our results show that DNA-HSP65 or DNA empty vector had no diabetogenic effect and actually protected NOD mice against the development of severe diabetes. However, this effect was more pronounced in DNA-HSP65-injected mice. The protective effect of DNA-HSP65 injection was associated with a clear shift in the cellular infiltration pattern in the pancreas. This change included reduction of CD4+ and CD8+ T cells infiltration, appearance of CD25+ cells influx and an increased staining for interleukin (IL)-10 in the islets. These results show that DNA-HSP65 can protect NOD mice against diabetes and can therefore be considered in the development of new immunotherapeutic strategies.
Keywords: cytokines, diabetes, DNA vaccine, heat shock protein (Hsp65)
Introduction
Genetic immunization has been used to generate protective humoral and cell-mediated immune responses in a wide variety of animal models of infectious diseases, allergy, cancer and autoimmunity [1–5]. After single or multiple injections of DNA, cellular and humoral immune responses to the expressed protein are elicited, and long-lived memory lymphocytes are induced [6]. We have reported previously that a DNA plasmid (DNAv), encoding the Mycobacterium leprae 65 kDa heat shock protein (DNA-HSP65), displayed prophylactic [7,8] as well as therapeutic effects in a murine model of tuberculosis [9,10]. The protection was attributed to the induction of a cellular immune response dominated by M. leprae Hsp65-specific T lymphocytes that produced interferon (IFN)-γ and were cytotoxic [11,12]. However, the principal argument against immune intervention with DNA-HSP65 in a clinical trial is that it could trigger an autoimmune response, because M. leprae Hsp65 has been shown to be 55% homologous to the equivalent mammalian protein [13]. Supporting this argument, other studies have shown humoral and cellular immune responses against mycobacterial Hsp65 in atherosclerosis [14,15], arthritis [16–19] and diabetes [20–23].
The non-obese diabetic (NOD) mouse develops insulin-dependent diabetes mellitus (IDDM) spontaneously as a consequence of an autoimmune process that leads to destruction of the insulin-producing β cells of the pancreas [24]. Several antigens have been identified as targets for diabetogenic T cells, including β cell-specific proteins such as insulin, non-β cell-restricted antigens such as glutamic acid decarboxylase (GAD), and even ubiquitous antigens such as 60 kDa heat shock protein (Hsp60) [24,25]. It has been shown that the onset of diabetes is preceded by an increase in T cell reactivity towards mycobacterial Hsp60 and human Hsp60 peptide, designated p277, which is located between amino acids 437 and 460 [21]. In contrast to the early T cell reactivity, antibodies to Hsp60 and p277 can be detected only late in the course of the disease, months after the onset of clinical diabetes, when the destructive process has terminated [26].
Despite the findings described above, the heat shock protein antigen has also been shown to participate in the protection against experimentally induced autoimmune diseases. Elias and Cohen showed that treatment of NOD mice with peptides derived from human Hsp60 (p277) emulsified in incomplete Freund's adjuvant inhibited the diabetogenic process [27]. Treated mice presented down-regulation of spontaneous T cell reactivity to p277 and induction of antibodies specific for Th2-associated isotypes [28,29]. In a more recent report, the same authors showed that NOD diabetes could be inhibited by vaccination with a DNA construct encoding human Hsp60. Diabetes prevention was attributed to decreased insulitis and down-regulation of spontaneous proliferative T cell responses to Hsp60 [30].
We have shown recently that the DNA-HSP65 construct, in addition to prophylactic and therapeutic effect in experimental tuberculosis, is also protective against pristane-induced experimental arthritis. This protective effect was attributed to significant down-modulation in the production of the proinflammatory interleukin (IL)-12, together with up-modulation of the anti-inflammatory cytokine IL-10 [31].
Within this context, the present study was designed to determine the ability of DNA-HSP65 to induce up- or down-modulation of the inflammatory immune response in the spontaneous diabetes seen in NOD mice. In addition, we investigated the possible effector mechanisms associated with the immunomodulation elicited by DNA-HSP65 injection in pancreatic islets. Our results show that neither DNAv nor DNA-HSP65 started or accelerated the development of spontaneous diabetes in NOD mice. Injection of both DNA-HSP65 and DNAv protected NOD mice against destruction insulitis, even though the vector effect was less striking. In comparison with controls and with mice injected with DNAv only, the DNA-HSP65-injected mice presented a marked decrease in CD4+ and CD8+ cell infiltrates, as well as more extensive staining for IL-10. In addition, a weaker staining for tumour necrosis factor (TNF)-α in the pancreatic islets associated with a significantly higher level of anti-Hsp65 IgG1 antibodies were observed in DNA-HSP65-injected animals. This protective effect of DNA-HSP65 injection in NOD mice seems to be related mainly to elicitation of a specific immune response, as injection of DNAv did not evoke a similar level of protection.
Materials and methods
Animals
NOD/Uni mice used in this study derived from the INSERM U-25 colony at the Hospital Necker (Paris, France) and had been maintained under germ-free conditions at the animal breeding centre of the State University of Campinas, São Paulo, Brazil [32]. In these animals, insulitis begins around 4 weeks of age and overt hyperglycaemia is observed clearly at around 16 weeks. The cumulative IDDM incidence reaches 60% or even more at 18 weeks [32]. The animals were maintained on a 12-h light/dark cycle and given free access to food and autoclaved water. Thirty female NOD mice were arranged into three groups: control, injected with phosphate-buffered saline (PBS); DNAv, injected with the vector (pVAX); and DNA-HSP65, injected with the vaccine (pVAX-HSP65). All experiments were performed twice.
Plasmid purification and immunization
The DNA-HSP65 construct was derived from its corresponding vector, designated DNAv (pVAX; Invitrogen, Carlsbad, CA, USA), which was digested with BamHI (Gibco-BRL, Gaithersburg, MD, USA) before insertion of a 3.3-kb fragment corresponding to the M. leprae Hsp65 gene and the cytomegalovirus intron A. DNAv without the Hsp65 gene was used as a control. DH5-αEscherichia coli transformed with pVAX alone or with pVAX carrying the Hsp65 gene (pVAX-HSP65) was grown in Luria–Bertani liquid medium (Gibco-BRL) containing kanamycin (50 µg/ml). The plasmids were purified with an Endofree plasmid Giga kit (Qiagen, Valencia, CA, USA). Plasmid concentration was determined by spectrophotometry at λ = 260 nm and at λ = 280 nm, using a GeneQuant II apparatus (Amersham Biosciences/GE Healthcare, Amersham, Bucks, UK). Four-week-old mice were immunized intramuscularly with three doses of 100 µg of DNA-HSP65 at 2-week intervals. Control animals received the corresponding vector concentration or PBS.
Glucose determination
Mice were monitored for the onset of diabetes by measurements of urine glucose using test-strips (Elililly do Brazil Ltda, São Paulo, SP, Brazil) twice a week. The glucose concentration in blood obtained from a tail vein was measured using Prestige LX Smart System test-strips (Home Diagnostic, Inc., Fort Lauderdale, FL, USA). Consecutive readings of blood glucose levels = 200 mg/dL (12 mmol/l) accompanied by glycosuria on 2 consecutive days were considered to be diagnostic of diabetes onset [33]. The incidence is expressed as percentages.
Recombinant Hsp65 protein
E. coli BL21 transformed with plasmid containing the mycobacterial Hsp65 gene was cultured in Luria–Bertani medium containing ampicillin (100 µg/µl). Bacterial growth was monitored by spectrophotometry at λ 600 nm in a Shimadzu UV 1650 spectrophotometer. When the OD reached 0.6, the culture was induced with 0.1 M of isopropylthio-β-D-galactoside (IPTG) (Gibco-BRL) and incubated at 30°C under agitation for 4 h. Protein purification was performed according to Portaro et al.[34].
Anti-Hsp65 antibodies
Individual sera were collected from 28-week-old NOD mice by retro-orbital puncture. In order to assess antigen-specific antibody levels, 96-well Nunc-Immuno plates (Maxisorp; Nalge Nunc International, Roskilde, Denmark) were coated with 0.1 ml of purified protein (recombinant Hsp65, 5 µg/ml) in coating solution (Na2CO3 14.3 mM, NaHCO3 10.3 mM NaN3; 0.02%, pH 9.6), incubated at 4°C overnight and then blocked with 1% bovine serum albumin (BSA) in PBS for 60 min at 37°C. Individual serum samples from vaccinated and control mice were applied at different dilutions. After incubation for 2 h at 37°C, anti-mouse IgG1 and IgG2a biotin conjugates (clones A85-1 and R19-15, respectively; Pharmingen, San Diego, CA, USA) were added. After washing, the plates were incubated at room temperature for 30 min using the Strept-AB kit (Dako, Glostrup, Denmark), and H2O2 plus o-phenylenediamine dihydrochloride (Sigma, St Louis, MO, USA) were added. The reaction was stopped by addition of 50 µl of 16% sulphuric acid. The optical density was measured at 490 nm. Antibody titres are shown as the highest dilution of serum that gave an absorbance value of 0.5 and are expressed as log10.
Histology and immunohistochemistry
Pancreata from 28-week-old mice were removed, and sections (4–5 µm thick) were cut from distinct portions of the gland. The sections were stained with haematoxylin–eosin (H&E; Merck, Whitehouse Station, NJ, USA) for evaluation of the insulitis score using the following scale: 0, intact islet; 1, peri-insulitis; 2, moderate insulitis (< 50% of the islet infiltrated); 3, severe insulitis ( = 50% of the islet infiltrated); and 4, destructive insulitis. At least 20 islets per pancreas were analysed. Staining for CD4, CD8, IL-10 and TNF-α was performed on acetone-fixed cryostat sections by incubation for 1 h with rat anti-mouse primary monoclonal antibody (PharMingen, San Jose, CA, USA) diluted to 1:200 in 3% BSA–PBS. This was followed by 1 h of incubation with biotin-conjugated rabbit anti-rat antibody (Pharmingen). Staining for CD25 was performed directly with rat anti-mouse CD25 (the IL-2 receptor α chain) biotinylated monoclonal antibody (PharMingen) diluted to 1:200 in 3% BSA–PBS. The colour was revealed using the 3,3′-diaminobenzidine (DAB) system (Vector kit; Vector Laboratories, Burlingame, CA, USA). As a control for non-specific staining, pancreas sections were treated without the anti-mouse primary monoclonal antibody.
Statistics
Statistical significance was determined by Fisher's test for diabetes incidence and insulitis degree. For enzyme-linked immunosorbent assay (ELISA) the statistical significance was determined by one-way analysis of variance (ANOVA) followed by Tukey's test, and values of P < 0.05 were considered significant.
Results
DNA injection protected NOD mice against severe diabetes
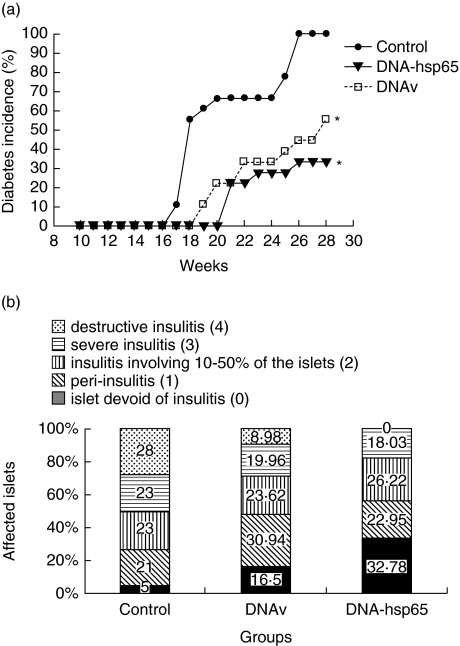
In NOD mice that received DNA-HSP65 or DNAv, the glycaemia levels were significantly lower than those levels observed in the control PBS group (Fig. 1a). By 28 weeks, only 33.3% of the mice injected with DNA-HSP65 and 50% from the DNAv group became diabetic, in contrast to a 100% incidence of the disease in animals not injected with DNA. Moreover, diabetes onset was delayed in DNA-HSP65 and DNAv-injected mice compared to the PBS control group (Fig. 1a). Figure 1b depicts the results obtained through histological examination of the pancreas. Despite the fact that the profile of diabetes incidence was similar in the two groups, DNA-HSP65 proved more potent in inducing a protective effect than did DNAv. In both groups (DNA-HSP65 and DNAv), animals presented strikingly higher proportions of insulitis-free islets (approximately 33% and 17% greater, respectively, than that seen in the control PBS group). In the DNA-HSP65-injected mice, however, there were no heavily infiltrated areas or areas of destructive insulitis, although such areas were identified in 28% and 8.98% of the non-DNA-injected (control) and DNAv-injected mice, respectively.
Fig. 1.
Diabetes incidence and insulitis severity in non-obese diabetic (NOD) mice injected with DNA. Female mice (4 weeks old) were inoculated intramuscularly (i.m.) with three 100-μg doses of DNA-HSP65 (pVAX-HSP65) or DNAv (pVAX) at 15-day intervals. Controls received identical injections of phosphate-buffered saline (PBS). Each group consisted of 10 mice. (a) Serum glucose levels were assayed weekly until mice reached the age of 28 weeks. (b) Pancreata from 28-week-old NOD mice were removed for histological analysis. To quantify islet infiltration, at least 20 islets from three sections per pancreas were examined in a blinded fashion. The degree of insulitis was evaluated using a semiquantitative scale: 0 = islet devoid of insulitis; 1 = peri-insulitis or insulitis occupying up to 10% of the islets; 2 = insulitis involving 10–50% of the islets; 3 = insulitis involving more than 80% of the islets; and 4 = destructive insulitis.
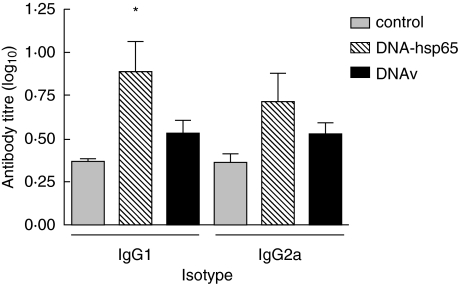
DNA-HSP65 injection induces significant increase in anti-Hsp65 IgG1 levels
To analyse the possible contribution of a T helper 2 (Th2) type of immune response in mice protected by DNA-HSP65, anti-Hsp65 IgG1 and IgG2a isotypes were measured in the sera at 28 weeks, when there was a clear difference in diabetes incidence among the groups (Fig. 2). Basal levels of both isotypes were found in sera of DNAv-injected mice, similar to the levels found in the control group not injected with DNA. As expected, DNA-HSP65-injected mice produced both IgG1- and IgG2a-specific antibodies. However, only IgG1 were statistically higher than control groups. Despite the increased levels of anti-Hsp65 IgG2a detected in the serum of DNA-HSP65-injected mice, these were not statistically different of either DNAv or control groups.
Fig. 2.
Anti-Hsp65 IgG1 and IgG2a antibody levels. Female mice (4 weeks old) were inoculated intramuscularly (i.m.) with three 100-μg doses of DNA-HSP65 (pVAX-HSP65) or DNAv (pVAX) at 15-day intervals. Controls received identical injections of phosphate-buffered saline (PBS). Serum samples were collected when the mice reached the age of 28 weeks. The anti-Hsp65 antibody levels were assayed individually using enzyme-linked immunosorbent assay (ELISA). Results are expressed as mean ± standard deviation from two independent experiments. *P < 0.05 for DNA-HSP65 versus control group.
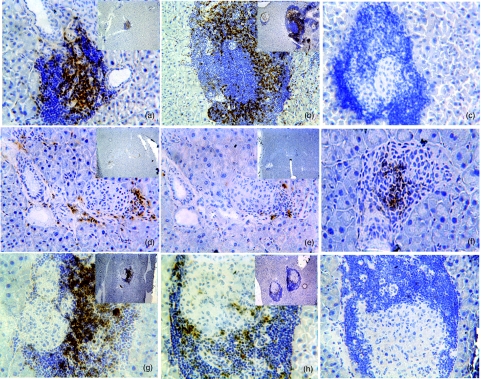
DNA-HSP65-injected mice present decreased CD4+, CD8+ but not CD25+ cellular influx
As can be observed in Fig. 3, a pronounced infiltration by CD4+ and CD8+ cells was observed in the islets of 28-week-old control group mice (Fig. 3a,b). A visible decrease in the infiltration of both cell subpopulations was observed in mice injected previously with DNA-HSP65 (Fig. 3d,e). Despite the marked infiltration of pancreatic islets, staining for CD8+ (Fig. 3h) but not for CD4+ (Fig. 3g) cells was also decreased in DNAv-injected mice. No staining for CD25+ was observed in the islets of the control group mice (Fig. 3c), while CD25+ cells were detected in the islets of DNA-HSP65-injected mice or DNAv-injected mice. Nevertheless, the CD25+ cells were clearly more evident in the pancreatic islets of DNA-HSP65 group mice than in those of DNAv group mice (Fig. 3f,i).
Fig. 3.
CD4+, CD8+ and CD25+ cell influx into pancreatic islets. Female mice (4 weeks old) were inoculated intramuscularly (i.m.) with three 100-μg doses of DNA-HSP65 (pVAX-HSP65) or DNAv (pVAX) at 15-day intervals. Controls received identical injections of phosphate-buffered saline (PBS). Pancreatic islets of 28-week-old mice were analysed. At least 20 islets from three sections per pancreas were examined. Samples from control group mice (a, b and c), DNA-HSP65 group mice (d, e and f) and DNAv group mice (g, h and i) were immunostained for CD4 (a, d and g), CD8 (b, e and h) and CD25 receptors (c, f and i). Original magnification, ×200; insets show the panoramic views (×40).
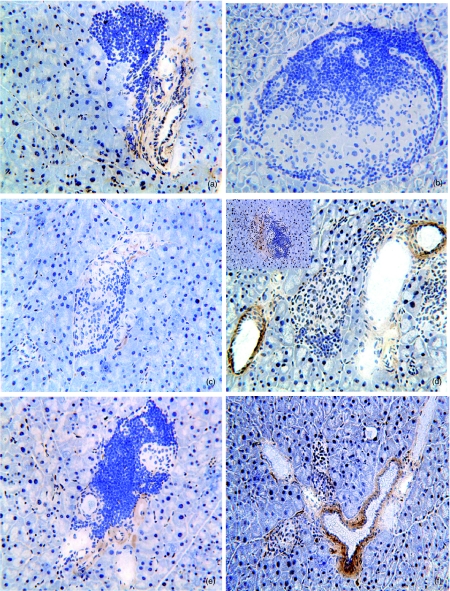
DNA-HSP65 alters cytokine pattern in the pancreas
We next investigated the presence of TNF-α and IL-10 in the pancreatic tissue. As can be seen in Fig. 4, there was an abundance of TNF-α in the islets of 28-week-old control group mice (Fig. 4a). A clearly different scenario was observed in the DNA-HSP65 group mice, in which the islets presented minimal TNF-α (Fig. 4c). In DNAv-injected mice, more extensive staining for TNF-α was observed when compared with the DNA-HSP65 group mice (Fig. 4e). In parallel with the decrease in local TNF-α, we observed that DNA injection clearly affected IL-10 production in islets. DNA-HSP65 and DNAv group mice presented an intense perivascular IL-10 staining (Fig. 4d,f). However, only DNA-HSP65-injected mice presented intra-islet IL-10 stain (Fig. 4d, in high left square). On the other hand, no IL-10 was detected in the islets of the control group (Fig. 4b).
Fig. 4.
Tumour necrosis factor (TNF)-α and interleukin (IL)-10 cytokine profiles in the pancreatic islets. Female mice (4 weeks old) were inoculated intramuscularly (i.m.) with three 100-μg doses of DNA-HSP65 (pVAX-HSP65) or DNAv (pVAX) at 15-day intervals. Controls received identical injections of phosphate-buffered saline (PBS). Pancreatic islets of 28-week-old mice were removed, after which the staining for TNF-α (a, c and e) and IL-10 (b, d and f) was evaluated. Control group (a and b), DNA-HSP65 group (c and d) and DNAv group mice (e and f). At least 20 islets from three sections per pancreas were examined. Original magnification, ×200.
Discussion
Vaccination with DNA is a recent and innovative means of protecting against experimental pathogenic infections and tumours [35–37]. Recently, it has also been used to prevent experimentally induced autoimmune diseases [5,38,39].
In the present study, we evaluated the potential of the DNA-HSP65 vaccine to induce (or not) mechanisms involved in the development of spontaneous diabetes. Interestingly, DNA-HSP65 was not diabetogenic. Our data show that prophylactic injection of DNA, either DNA-HSP65 or DNAv, did not accelerate the development of diabetes and indeed decreased the incidence of the disease. This conclusion was based on both glycaemic levels and analysis of pancreas histology. These results are considered very promising, and are in accordance with a previous study where DNA-HSP65, or its empty vector, did not induce arthritis in AIRmax mice, which are very prone to develop this autoimmune pathology after pristine inoculation [31].
Moreover, our results are in agreement with those described by Quintana et al., who showed that injection of empty plasmid DNA or CpG oligonucleotides inhibited diabetes in NOD mice [30]. Those authors showed that a DNA construct, encoding or not encoding human Hsp60, protected against the development of diabetes in NOD mice. They concluded that this protection was attributable to increased levels of circulating IgG2b. Moreover, these authors showed elegantly that DNA vaccination with human Hsp60, but not mycobacterial Hsp65 or an empty vector, inhibited cyclophosphamide-accelerated diabetes in NOD mice [40], suggesting that the efficacy of the Hsp60 construct might be based on regulatory Hsp60 epitopes not shared with its mycobacterial counterpart, Hsp65.
Here, we found that 66.7% of the mice injected with DNA-HSP65 were protected from diabetes development, as evidenced by the fact that they presented no destructive insulitis in the pancreatic islets, whereas 100% of the control group mice developed diabetes and presented destructive insulitis. Despite the fact that the DNAv group mice also presented a lower incidence of diabetes than that seen in control group mice, they presented destructive insulitis in the islets.
To unravel the mechanism by which this vaccine was preventing full-blown diabetes, we evaluated initially the amounts of specific IgG1 and IgG2a isotypes that could suggest a Th1/Th2 polarization. A clear polarization towards Th2, indicated by the higher IgG1 anti-Hsp65 antibody levels, was observed. This Th2 scenario could modulate or block immune response to other autoantigens from the pancreas and therefore delayed diabetes development [40].
It is well known that IDDM is a multi-step process that leads to destruction of the spontaneously insulin-producing β cells of the pancreas in NOD mice. Participation of both T cell subsets [41–43] and proinflammatory cytokines [44] seem to be essential to the development of diabetes in this model. On the other hand, immunization with microbial or mammalian stress proteins or heat-shock proteins in models of experimental autoimmunity has been observed to lead to increased disease resistance. Comparisons of microbial heat-shock proteins with other conserved immunogenic proteins of bacterial origin have indicated a unique capacity for heat-shock proteins to induce a regulatory phenotype in T cells, as reflected by the production of IL-10 [45].
To understand the possible mechanisms by which DNA-HSP65 triggers the partial arrest of diabetes development in NOD mice, we determined CD4+ and CD8+ cell influx into the pancreatic islets. In DNA-HSP65-injected mice, the higher numbers of conserved islets were accompanied by less influx of CD4+ and CD8+ cells in comparison with the control and DNAv group mice. In parallel with the diminished inflammatory response, islets of DNA-HSP65 group mice presented a focal distribution of CD25+ cells which was absent in the control and DNAv group mice.
In recent years, much evidence has been accumulated demonstrating that a unique population of CD4+ T cells can suppress autoaggressive T cell responses to host antigen, both in vitro and in vivo[46–48]. Although the phenotype of these T regulatory cells has yet to be defined fully, it is generally accepted that they express constitutively CD25 [47]. The mechanisms by which T regulatory cells suppress autoaggressive responses are not completely known, although cell–cell contact and the production of anti-inflammatory cytokines, such as IL-10, have been implicated [46,48–51].
Our results are in line with this hypothesis. First of all, we observed that DNA-HSP65-injected mice presented a clear reduction in insulitis. This reduction coincided not only with a reduction in both CD4+ and CD8+ but with the appearance of CD25+ cells in the islets. In addition, these findings also coincided with a reduction of TNF-α and an increase of IL-10 expression in the pancreas, the pathogenic and protective roles of which, respectively, in diabetes have been well accepted [49].
Two strong indications of the protection afforded by DNA vaccination in various models of experimentally induced autoimmune diseases include the generation of antigen-specific regulatory cells [5,38] and the induction of anergy in autoreactive T cells [39]. With this in mind, we propose that the presence of CD25+ cells, together with the increased staining for IL-10, indicate that regulatory T cells can be involved in DNA-HSP65-mediated protection. This finding suggests the hypothesis that DNA-HSP65 injection promotes the induction and infiltration of regulatory T cells into the islets, blocking further influx of inflammatory cells. In addition, less extensive staining for TNF-α in the islets of DNA-HSP65 group mice in relation to control and DNAv group mice also supports the hypothesis that regulatory T cells mediate down-modulation of the inflammatory immune response.
It should be borne in mind that the regulatory T cell effect might be dependent on the activation of a specific cellular immune response, as the protective effect mediated by DNA-HSP65 was more pronounced than that mediated by DNAv. In this context, T cell receptor (TCR) engagement would be essential for the activation of regulatory T cells. However, they perform their effector function in a non-specific way. Our data suggest that this non-specific effector role depends on the secretion of soluble mediators, such as IL-10, and seems to be independent of cell contact. Despite these original data we are conscious that whether the immune modulation played by DNA-HSP65 in NOD mice was attributed by skewing to a Th2 pattern, or by induction of T regulatory cells, remains to be investigated. With this in mind, the role of DNA-HSP65 immunization in the increase of natural T regulatory cells frequency is under investigation.
In conclusion, the data presented in this study encourage us to invest in exploring the regulatory potential of the DNA-HSP65 construct. Our findings have significant implications for the development of new immunotherapy strategies for combating autoimmune diseases.
Acknowledgments
The authors are grateful to Mrs Izaíra T Brandão and Mrs Ana Paula Masson for technical assistance. This study was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Foundation for the Support of Research in the State of São Paulo), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, National Council of Scientific and Technological Development) and the Rede Brazileira de Pesquisa em TB (REDE-TB, Brazilian Tuberculosis Research Network).
References
- 1.Gurunathan S, Wu CY, Freidag BL, Seder RA. DNA vaccines: a key for inducing long-term cellular immunity. Curr Opin Immunol. 2000;12:442–7. doi: 10.1016/s0952-7915(00)00118-7. [DOI] [PubMed] [Google Scholar]
- 2.Silva CL, Bonato VLD, Coelho Castelo AAM, Lima KM, Rodrigues JM., Jr . In: Mir L, editor. Genômica: Ciência da Vida; 2004. pp. 463–93. [Google Scholar]
- 3.Stevenson FK, Link CJ, Traynor A, Jr, Yu H, Corr M. DNA vaccination against multiple myeloma. Semin Hematol. 1999;36(Suppl. 3):38–42. [PubMed] [Google Scholar]
- 4.Ulmer JB, Fu TM, Deck RR, et al. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J Virol. 1998;72:5648–53. doi: 10.1128/jvi.72.7.5648-5653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waisman A, Ruiz PJ, Hirschberg DL, et al. Suppressive vaccination with DNA encoding a variable region gene of the T-cell receptor prevents autoimmune encephalomyelitis and activates Th2 immunity. Nat Med. 1996;2:899–905. doi: 10.1038/nm0896-899. [DOI] [PubMed] [Google Scholar]
- 6.Hassett DE, Zhang J, Slifka M, Whitton JL. Immune responses following neonatal DNA vaccination are long-lived, abundant, and qualitatively similar to those induced by conventional immunization. J Virol. 2000;74:2620–7. doi: 10.1128/jvi.74.6.2620-2627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonato VL, Lima VM, Tascon RE, Lowrie DB, Silva CL. Identification and characterization of protective T cells in hsp65 DNA-vaccinated and Mycobacterium tuberculosis-infected mice. Infect Immun. 1998;66:169–75. doi: 10.1128/iai.66.1.169-175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva CL, Bonato VL, Lima VM, Faccioli LH, Leao SC. Characterization of the memory/activated T cells that mediate the long-lived host response against tuberculosis after bacillus Calmette–Guerin or DNA vaccination. Immunology. 1999;97:573–81. doi: 10.1046/j.1365-2567.1999.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonato VL, Goncalves ED, Soares EG, et al. Immune regulatory effect of pHSP65 DNA therapy in pulmonary tuberculosis. activation of CD8+ cells, interferon-gamma recovery and reduction of lung injury. Immunology. 2004;113:130–8. doi: 10.1111/j.1365-2567.2004.01931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowrie DB, Tascon RE, Bonato VL, et al. Therapy of tuberculosis in mice by DNA vaccination. Nature. 1999;400:269–71. doi: 10.1038/22326. [DOI] [PubMed] [Google Scholar]
- 11.Silva CL, Lowrie DB. Identification and characterization of murine cytotoxic T cells that kill Mycobacterium tuberculosis. Infect Immun. 2000;68:3269–74. doi: 10.1128/iai.68.6.3269-3274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva CL, Silva MF, Pietro RC, Lowrie DB. Characterization of T cells that confer a high degree of protective immunity against tuberculosis in mice after vaccination with tumor cells expressing mycobacterial hsp65. Infect Immun. 1996;64:2400–7. doi: 10.1128/iai.64.7.2400-2407.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feige U, van Eden W. Infection, autoimmunity and autoimmune disease. Experientia. 1996;77:359–73. doi: 10.1007/978-3-0348-9088-5_24. [DOI] [PubMed] [Google Scholar]
- 14.Afek A, George J, Gilburd B, et al. Immunization of low-density lipoprotein receptor deficient (LDL-RD) mice with heat shock protein 65 (HSP-65) promotes early atherosclerosis. J Autoimmun. 2000;14:115–21. doi: 10.1006/jaut.1999.0351. [DOI] [PubMed] [Google Scholar]
- 15.Keren P, George J, Shaish A, et al. Effect of hyperglycemia and hyperlipidemia on atherosclerosis in LDL receptor-deficient mice: establishment of a combined model and association with heat shock protein 65 immunity. Diabetes. 2000;49:1064–9. doi: 10.2337/diabetes.49.6.1064. [DOI] [PubMed] [Google Scholar]
- 16.Danieli MG, Markovits D, Gabrielli A, et al. Juvenile rheumatoid arthritis patients manifest immune reactivity to the mycobacterial 65-kDa heat shock protein, to its 180–188 peptide, and to a partially homologous peptide of the proteoglycan link protein. Clin Immunol Immunopathol. 1992;64:121–8. doi: 10.1016/0090-1229(92)90189-u. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka S, Kimura Y, Mitani A, et al. Activation of T cells recognizing an epitope of heat-shock protein 70 can protect against rat adjuvant arthritis. J Immunol. 1999;163:5560–5. [PubMed] [Google Scholar]
- 18.Thompson SJ, Francis JN, Siew LK, et al. An immunodominant epitope from mycobacterial 65-kDa heat shock protein protects against pristane-induced arthritis. J Immunol. 1998;160:4628–34. [PubMed] [Google Scholar]
- 19.van Eden W. Heat-shock proteins as immunogenic bacterial antigens with the potential to induce and regulate autoimmune arthritis. Immunol Rev. 1991;121:5–28. doi: 10.1111/j.1600-065x.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 20.Bras A, Aguas AP. Diabetes-prone NOD mice are resistant to Mycobacterium avium and the infection prevents autoimmune disease. Immunology. 1996;89:20–5. doi: 10.1046/j.1365-2567.1996.d01-717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elias D, Reshef T, Birk OS, van der Zee R, Walker MD, Cohen IR. Vaccination against autoimmune mouse diabetes with a T-cell epitope of the human 65-kDa heat shock protein. Proc Natl Acad Sci USA. 1991;88:3088–91. doi: 10.1073/pnas.88.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta MM, Raghunath D, Kher SK, Radhakrishnan AP. Human leucocyte antigen and insulin dependent diabetes mellitus. J Assoc Phys India. 1991;39:540–3. [PubMed] [Google Scholar]
- 23.Kallmann BA, Lampeter EF, Hanifi-Moghaddam P, Hawa M, Leslie RD, Kolb H. Cytokine secretion patterns in twins discordant for Type I diabetes. Diabetologia. 1999;42:1080–5. doi: 10.1007/s001250051274. [DOI] [PubMed] [Google Scholar]
- 24.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–7. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 25.Birk OS, Elias D, Weiss AS, et al. NOD mouse diabetes: the ubiquitous mouse hsp60 is a beta-cell target antigen of autoimmune T cells. J Autoimmun. 1996;9:159–66. doi: 10.1006/jaut.1996.0019. [DOI] [PubMed] [Google Scholar]
- 26.Krause I, Tomer Y, Elias D, et al. Inhibition of diabetes in NOD mice by idiotypic induction of SLE. J Autoimmun. 1999;13:49–55. doi: 10.1006/jaut.1999.0292. [DOI] [PubMed] [Google Scholar]
- 27.Elias D, Cohen IR. Treatment of autoimmune diabetes and insulitis in NOD mice with heat shock protein 60 peptide p277. Diabetes. 1995;44:1132–8. doi: 10.2337/diab.44.9.1132. [DOI] [PubMed] [Google Scholar]
- 28.Ablamunits V, Elias D, Reshef T, Cohen IR. Islet T cells secreting IFN-gamma in NOD mouse diabetes: arrest by p277 peptide treatment. J Autoimmun. 1998;11:73–81. doi: 10.1006/jaut.1997.0177. [DOI] [PubMed] [Google Scholar]
- 29.Elias D, Meilin A, Ablamunits V, et al. Hsp60 peptide therapy of NOD mouse diabetes induces a Th2 cytokine burst and downregulates autoimmunity to various beta-cell antigens. Diabetes. 1997;46:758–64. doi: 10.2337/diab.46.5.758. [DOI] [PubMed] [Google Scholar]
- 30.Quintana FJ, Rotem A, Carmi P, Cohen IR. Vaccination with empty plasmid DNA or CpG oligonucleotide inhibits diabetes in nonobese diabetic mice: modulation of spontaneous 60-kDa heat shock protein autoimmunity. J Immunol. 2000;165:6148–55. doi: 10.4049/jimmunol.165.11.6148. [DOI] [PubMed] [Google Scholar]
- 31.Santos RR, Jr, Sartori A, De Franco M, et al. Immunomodulation and protection induced by DNA-hsp65 vaccination in an animal model of arthritis. Hum Gene Ther. 2005;16:1338–45. doi: 10.1089/hum.2005.16.1338. [DOI] [PubMed] [Google Scholar]
- 32.Pavin EA, Zollner RL. Implantation of NOD mice in Brazil: contribution of this animal model to diabetes and autoimmune diseases studies. Brazilian Archives of Endocrinology and Metabolism. 1994;38:105–8. [Google Scholar]
- 33.Ventura-Oliveira VCD, Zanin ME, Castro GM, Moreira Filho DC, Zollner RL. Kinetics of TNF-alpha and IFN-gamma mRNA expression in islets and spleen of NOD mice. Braz J Med Biol Res. 2002;35:1347–55. doi: 10.1590/s0100-879x2002001100013. [DOI] [PubMed] [Google Scholar]
- 34.Portaro FC, Hayashi MA, de Arauz LJ, et al. The Mycobacterium leprae hsp65 displays proteolytic activity. Mutagenesis studies indicate that the M. leprae hsp65 proteolytic activity is catalytically related to the HsIVU protease. Biochemistry. 2002;41:7400–6. doi: 10.1021/bi011999l. [DOI] [PubMed] [Google Scholar]
- 35.Lowrie DB, Silva CL, Colston MJ, Ragno S, Tascon RE. Protectionagainst tuberculosis by a plasmid DNA vaccine. Vaccine. 1997;15:834–8. doi: 10.1016/s0264-410x(97)00073-x. [DOI] [PubMed] [Google Scholar]
- 36.Lowrie DB, Tascon RE, Colston MJ, Silva CL. Towards a DNA vaccine against tuberculosis. Vaccine. 1994;12:1537–40. doi: 10.1016/0264-410x(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 37.Syrengelas AD, Chen TT, Levy R. DNA immunization induces protective immunity against B-cell lymphoma. Nat Med. 1996;2:1038–41. doi: 10.1038/nm0996-1038. [DOI] [PubMed] [Google Scholar]
- 38.Coon B, An LL, Whitton JL, von Herrath MG. DNA immunization to prevent autoimmune diabetes. J Clin Invest. 1999;104:189–94. doi: 10.1172/JCI7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruiz PJ, Garren H, Ruiz IU, et al. Suppressive immunization with DNA encoding a self-peptide prevents autoimmune disease: modulation of T cell costimulation. J Immunol. 1999;162:3336–41. [PubMed] [Google Scholar]
- 40.Quintana FJ, Carmi P, Cohen IR. DNA vaccination with heat shock protein 60 inhibits cyclophosphamide-accelerated diabetes. J Immunol. 2002;169:6030–5. doi: 10.4049/jimmunol.169.10.6030. [DOI] [PubMed] [Google Scholar]
- 41.DiLorenzo TP, Serreze DV. The good turned ugly: immunopathogenic basis for diabetogenic CD8+ T cells in NOD mice. Immunol Rev. 2005;204:250–63. doi: 10.1111/j.0105-2896.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 42.Goudy KS, Tisch R. Immunotherapy for the prevention and treatment of type 1 diabetes. Int Rev Immunol. 2005;24:307–26. doi: 10.1080/08830180500379721. [DOI] [PubMed] [Google Scholar]
- 43.Walter U, Toepfer T, Dittmar KE, et al. Pancreatic NOD beta cells express MHC class II protein and the frequency of I-A (g7) mRNA-expressing beta cells strongly increases during progression to autoimmune diabetes. Diabetologia. 2003;46:1106–14. doi: 10.1007/s00125-003-1164-y. [DOI] [PubMed] [Google Scholar]
- 44.Jaeschke A, Rincon M, Doran B, et al. Disruption of the Jnk2 (Mapk9) gene reduces destructive insulitis and diabetes in a mouse model of type I diabetes. Proc Natl Acad Sci USA. 2005;102:6931–5. doi: 10.1073/pnas.0502143102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–30. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- 46.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–18. [PubMed] [Google Scholar]
- 47.Takahashi T, Sakaguchi S. Naturally arising CD25+CD4+ regulatory T cells in maintaining immunologic self-tolerance and preventing autoimmune disease. Curr Mol Med. 2003;3:693–706. doi: 10.2174/1566524033479429. [DOI] [PubMed] [Google Scholar]
- 48.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 49.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Homann D, Holz A, Bot A, et al. Autoreactive CD4+ T cells protect from autoimmune diabetes via bystander suppression using the IL-4/Stat6 pathway. Immunity. 1999;11:463–72. doi: 10.1016/s1074-7613(00)80121-1. [DOI] [PubMed] [Google Scholar]
- 51.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]