Abstract
The aim of the present study was to elucidate the impact of liver transplantation (LTX) on myeloid dendritic cell (MDC) homeostasis. We observed a threefold reduction of circulating CD1c+ MDC immediately after LTX (n = 16; P < 0.01), and normalization between 3 and 12 months after LTX. This decline was not due to recruitment of MDC into the liver graft, as numbers of MDC in post-LTX liver graft biopsies were not increased compared to pre-LTX biopsies (n = 7). Moreover, no change in chemokine receptor expression on circulating MDC was observed, suggesting that their homing properties were not altered. Normalization of circulating MDC was associated with withdrawal of corticosteroid therapy, and not with changes in calcineurin inhibitor intake, indicating that corticosteroids are responsible for the observed changes in numbers of circulating MDC. During high-dose corticosteroid treatment early after LTX, circulating MDC showed a lowered maturation status with decreased expression of human leucocyte antigen D-related (HLA-DR) and CD86 compared to pre-LTX values (P < 0.01). However, when MDC from blood of LTX recipients were matured ex vivo, they up-regulated HLA-DR and co-stimulatory molecules to a comparable extent as MDC from healthy individuals. In addition, ex vivo matured MDC from both groups had equal allogeneic T cell stimulatory capacity. In conclusion, during the first months after LTX numbers and maturational status of circulating MDC are impaired significantly, probably due to a suppressive effect of corticosteroids on MDC. However, corticosteroid therapy does not imprint MDC with an intrinsic resistance to maturation stimuli.
Keywords: chemokine receptor, corticosteroids, dendritic cell, liver biopsy, liver transplantation
Introduction
The earliest stage of the T cell response against allogeneic grafts consists of priming the recipient's T cells against donor antigens by dendritic cells (DC). In humans there are two main types of DC: myeloid DC (MDC) and plasmacytoid DC (PDC). Both donor- and host-derived MDC can activate allogeneic T cells after organ transplantation. Donor-derived MDC migrate from the graft via the blood circulation into the recipient's spleen and lymph nodes, and activate T cells of the recipient by direct presentation of donor alloantigens [1–4]. Host-derived MDC are recruited into the graft in response to ischaemic injury [5], and migrate subsequently to the recipient's spleen and lymph nodes to prime recipient T cells by indirect presentation of donor antigens [3]. In contrast, PDC in the transplantation setting are probably involved in the development of allograft tolerance [6,7].
Whereas considerable knowledge is available on circulating donor-specific T cells after organ transplantation, only few studies have investigated changes in DC homeostasis. Longitudinal studies that have been performed show that numbers of circulating MDC and PDC decrease immediately after cardiac [8], kidney [9,10] and liver (LTX) [11] transplantation, but considerable differences in the duration of DC impairment have been reported. After renal [9] and cardiac [8] transplantation, numbers of circulating MDC and PDC remained consistently lowered during the follow-up of 3 and 8 months. We reported that after LTX circulating PDC numbers decrease immediately and are only restored partially during the first year after transplantation [12]. This was confirmed by cross-sectional studies, which showed consistently that numbers of circulating PDC are reduced in patients late after organ transplantation compared to those in healthy controls [10,13–15]. Study results regarding the duration of MDC impairment after organ transplantation are, however, not uniform. Some studies reported reduced [10,13], whereas others found normal [14,15] numbers of circulating MDC in organ transplant recipients late after transplantation. Moreover, one study found that circulating MDC numbers were already restored to pretransplant values 1 month after transplantation [11]. Besides, it is unclear whether the maturational status and functional capacity of MDC are altered after organ transplantation.
We found that the decrease in circulating PDC numbers after LTX was due to induction of apoptosis in PDC by corticosteroids [12]. Whether corticosteroid treatment is also responsible for the observed decline in circulating MDC numbers after organ transplantation is unknown. One cross-sectional study found that the decline in circulating MDC numbers after organ transplantation was associated with corticosteroid therapy [14], while two other studies did not [13,15].
The aim of the present study was to establish the longitudinal course of numbers and maturational status of circulating MDC during the first year after LTX. In addition, we compared the functional capacity of blood MDC of LTX recipients with healthy individuals. In an attempt to explain our observations, we analysed chemokine receptor expression on circulating MDC, quantified MDC in liver grafts, investigated associations between changes in circulating MDC and alterations in immunosuppressive therapy, and studied whether immunosuppressive drugs induce apoptosis of blood MDC.
Patients and methods
Patients and materials
Circulating MDC after LTX were analysed in peripheral blood mononuclear cells (PBMC) obtained pre-, and at different time-points post-transplantation from 16 LTX recipients transplanted between 1999 and 2001. Paraffin-embedded and frozen pre- and post-LTX liver graft biopsies were available from seven patients. Pre-LTX biopsies had been collected at the end of the cold preservation of the graft. Post-LTX biopsies had been obtained as protocol biopsies or suspicion of graft dysfunction. The clinical characteristics of the included patients are summarized in Table 1. Immunosuppressive therapy consisted of a calcineurin inhibitor [either cyclosporin A (CsA) or tacrolimus (Tac)] and corticosteroids. Before reperfusion the patients were given a single intravenous dose of 500 mg methylprednisolone (Solu-Medrol®, Pfizer, Capelle aan den IJssel, the Netherlands). During the first week after LTX intravenous prednisolone was given according to the following scheme: 100 mg on days 1 and 2, 75 mg on days 3 and 4, 50 mg on days 5 and 6 post-LTX. Thereafter, treatment was changed to oral prednisone (15 mg per day) for the next 3 months. Subsequently, corticosteroid medication was tapered slowly over time, and was discontinued completely in 10 of 16 patients between 6 and 12 months (median: 10 months) after LTX. Ten patients were treated with CD25 antibody during the early post-transplant period.
Table 1.
Characteristics of liver transplantation (LTX) recipients in which the longitudinal course of numbers and maturation status of circulating myeloid dendritic cells (MDC) were studied.
| Patients (n = 16) | |
|---|---|
| Age, years, mean (range) | 48 (16–66) |
| Gender, male/female | 7/9 |
| Indication for liver transplantation | |
| Hepatitis B | 3 |
| Hepatitis C | 3 |
| PBC | 3 |
| PSC | 3 |
| Alcoholic liver cirrhosis | 1 |
| Insulinoma with liver metastasis | 1 |
| Autoimmune hepatitis | 2 |
| Immunosuppression | |
| CsA + prednisone | 3 |
| CsA + prednisone +αCD25 | 4 |
| TAC + prednisone | 3 |
| TAC + prednisone +αCD25 | 6 |
CsA: cyclosporin; PBC: primary biliary cirrhosis; PSC: primary sclerosing cholangitis; Tac: tacrolimus.
For functional studies with purified blood MDC, fresh blood was collected from eight LTX recipients at 2 weeks after transplantation, and from 11 healthy volunteers. These LTX recipients were transplanted for hepatitis B virus (HBV) cirrhosis (n = 3), acute fulminant HBV infection (n = 1) or hepatitis C virus (HCV) cirrhosis (n = 4). Mean age was 47 (range: 23–61) years, and immunosuppressive therapy consisted of Tac or CsA supplemented with corticosteroids and CD25 antibody. The Medical Ethical Committee of the Erasmus Medical Center, Rotterdam approved the study protocol, and informed written consent was obtained from all patients.
Analysis of numbers and maturation status of circulating DC and monocytes
PBMC were isolated by Ficoll density centrifugation from heparinized venous blood and stored in liquid nitrogen until analysis. After thawing, PBMC were incubated with combinations of the following monoclonal antibodies (mAb): CD1c-phycoerythrin (PE), CD14-peridinin chlorophyll (PerCP), CD20-PerCP, CD80-fluorescein isothiocyanate (FITC), CD86-allophycocyanin (APC), CCR7-FITC, CXCR4-FITC, anti-human leucocyte antigen D-related (HLA-DR)-FITC or anti-HLA-DR-PerCP. MDC were defined as CD1c+CD14–CD20– cells. For determination of the relative numbers of monocytes and MDC, and for analysis of expression of HLA-DR and co-stimulatory molecules on monocytes, 50 000 events were collected on a fluorescence activated cell sorter (FACS)Calibur flow cytometer. To analyse the expression of cell surface molecules on MDC, at least 200 CD1c+CD14–CD20– events were live-gated. Appropriate isotype control mAb were used to set gates for analysis. All mAb were obtained from BD Biosciences, Erembodegem, Belgium, except CD1c PE (Miltenyi Biotech, Bergisch Gladbach, Germany), CD80-FITC (Beckman Coulter Immunotech, Marseille, France), CCR7-FITC and CXCR4-FITC (R&D Systems, Abingdon, Oxon, UK). Analysis was performed using CellQuest Pro software.
Effect of immunosuppressive drugs on MDC survival in vitro
PBMC (2 × 106/ml) from healthy individuals were cultured for 18 h in RPMI-1640 supplemented with penicillin (100 U/ml), streptomycin (100 µg/ml) (all from Gibco brl Life Technologies, Breda, the Netherlands) and 10% fetal bovine serum (Hyclone, Logan, UT, USA), with or without CsA, Tac, prednisolone or methylprednisolone. After 18 h, expression of active caspase 3 was determined by flow cytometry after intracellular labelling with anti-active caspase 3-FITC mAb (BD Biosciences) using Intraprep permeabilization reagent (Beckman Coulter Immunotech). Before intracellular staining, surface antigens were labelled with CD1c-PE and CD20-APC mAb to determine MDC, CD14-PE mAb to determine monocytes or anti-blood dendritic cell antigens (BDCA)-4-PE (Miltenyi Biotech) and CD11c-APC (BD Biosciences) to determine BDCA-4+CD11c– PDC.
Quantification of intragraft MDC and macrophages
Consecutive cryosections were stained with CD1c mAb (Miltenyi Biotec) and secondary peroxidase-conjugated goat anti-mouse Envision complex (Dako, Glostrup, Denmark) as described previously [4], or with DC-Lamp mAb (CD208; clone 104.G4) followed by rabbit anti-mouse antibodies and alkaline phosphatase–anti-alkaline phosphatase complex (APAAP; Serotec, Oxford, UK), as described previously [16]. Sections of paraffin-embedded biopsies were stained with CD68-Envision-peroxdiase (Dako, Glostrup, Denmark) after blocking for endogenous peroxidase by incubation with 0.2% sodium azide and 0.05% H2O2 in citric acid/phosphate buffer (pH: 5.8), and antigen retrieval by Pronase (Sigma, Zwijndrecht, the Netherlands) incubation for 15 min and 37°C. CD1c and CD68 mAb were visualized using 3-amino-9-ethylcarbazole, and DC-Lamp mAb using Fast Blue/ASBI-phosphate substrate. Sections were examined only if stains with appropriate isotype-matched control mAb were negative. Positive cells were counted simultaneously by two observers. Numbers of positive cells in the parenchyma were counted in six randomly chosen microscopic fields of 400 × magnification. In addition, positive cells in portal fields were counted. If the difference in counts between the two observers exceeded 15%, recounting was carried out. For each biopsy the mean number of positive cells per portal field and per microscopic field of 400 × magnification of the parenchyma were calculated, and these numbers were used in the analysis.
Purification of blood MDC and determination of their functional capacity
MDC were isolated from blood of LTX recipients or healthy volunteers by immunomagnetic selection of CD1c+CD20– cells, as described previously [17]. The purity of MDC (defined as CD1c+ CD20– cells) isolated from LTX recipients was 94 ± 4% and from healthy individuals 96 ± 2%, and viability (determined by trypan blue exclusion) was 88 ± 4 and 89 ± 3%, respectively. For determination of their maturational capacity, MDC (1 × 104/200 μl) were stimulated for 24 h at 37°C with tumour necrosis factor (TNF)-α (25 ng/ml) and interleukin (IL)-1β (50 ng/ml) (both from Strathmann Biotech, Hannover, Germany) in RPMI-1640 supplemented with penicillin, streptomycin, 10% fetal bovine serum and granulocyte–macrophage colony-stimulating factor (GM-CSF) (500 U/ml; Leucomax, Novartis Pharma, Arnhem, the Netherlands). Before and after culture, MDC were labelled with CD1c-PE, CD20-PerCP, anti-HLA-DR-FITC, CD80-FITC and CD86-APC or appropriate isotype control mAb, and analysed by flow cytometry.
To determine the capacity of MDC to acquire allogeneic T cell stimulatory capacity, graded numbers (5, 2.5 and 1.25 × 103 cells per well of a 96-well flat-bottomed plate) were stimulated to mature with TNF-α, IL-1β and GM-CSF for 24 h. Thereafter, the culture medium was aspirated, MDC were washed twice to remove additives, and 1.5 × 105 allogeneic T cells were added. In all experiments the same batch of T cells was used, which were enriched by nylon wool filtration of PBMC from a buffy coat of a healthy blood donor, and contained 83% CD3+ cells. After 5 days, T cell proliferation was assessed by determination of the incorporation of 0.5 µCi [3H]-thymidine (Radiochemical Centre, Amersham, Little Chalfont, UK) during 18 h. Each condition was tested in triplicate from which means were calculated. These means were used in the analyses.
Statistical analysis
All data are expressed as mean ± standard error of the mean (s.e.m.). Numbers of DC and macrophages in pre- and post-LTX biopsies were compared using the Wilcoxon test for paired data. All other differences were analysed afterlog-transformation of data to obtain normal distribution, using either the independent-samples t-test or the paired-samples t-test. Statistical analysis was performed using spss version 11.0 software. A two-sided P-value of less than 0.05 was considered significant.
Results
The longitudinal course of numbers of circulating MDC after LTX in relation to immunosuppressive medication
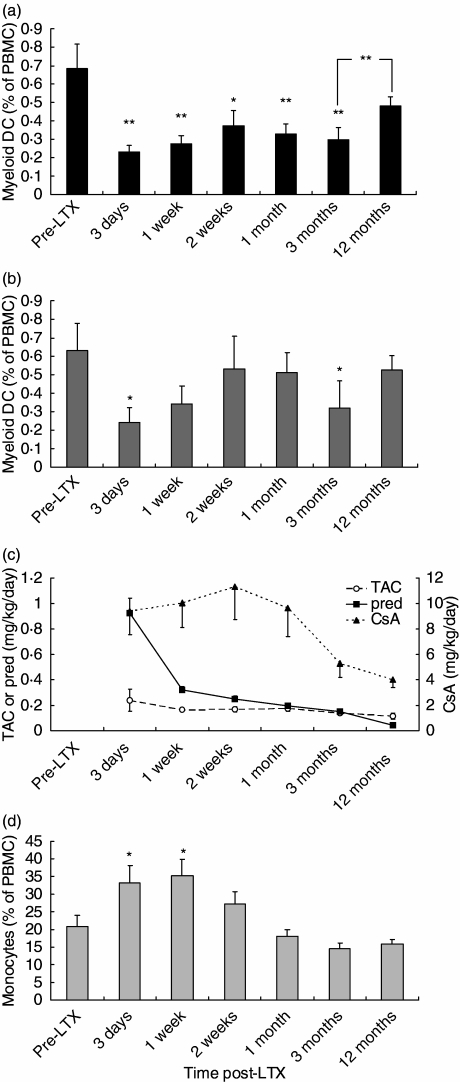
The relative numbers of circulating MDC and monocytes were determined in PBMC from 16 LTX recipients. To preclude the effects of rejection treatment, only patients who had never been treated for acute rejection were included. The frequencies of CD1c+ MDC in liver transplant recipients before transplantation were comparable to those in healthy individuals which we have reported previously [17]. Figure 1a shows that as early as 3 days after LTX the relative numbers of circulating MDC decreased threefold compared to pre-LTX values. This decline persisted up to 3 months post-LTX, and between 3 and 12 months after LTX the numbers of circulating MDC were restored to pretransplant values.
Fig. 1.
Longitudinal course of the numbers of circulating myeloid dendritic cells (MDC) and monocytes in liver transplantation (LTX) recipients. (a) Relative numbers of CD1c+CD14–CD20– MDC were determined in 16 LTX recipients at baseline, 3 days, 1 and 2 weeks, 1, 3 and 12 months after transplantation. (b) Idem for the LTX recipients that were treated with CsA (n = 7). (c) Treatment doses of tacrolimus (Tac), cyclosporin (CsA) and prednisolone/prednisone in the LTX recipients at the indicated time-points. The left y-axis indicates daily Tac or prednisone doses, and the right y-axis daily CsA doses. (d) Relative numbers of CD14+ monocytes in the 16 LTX recipients. **P < 0.01; *P < 0.05 in comparison with pre-LTX values, or between indicated time-points.
To investigate the cause of the changes in numbers of circulating MDC, we compared longitudinal alterations in immunosuppressive therapy with MDC numbers (Fig. 1c).
All LTX patients received prednisone in combination with either CsA or Tac. In the CsA-treated group the intake of CsA was reduced between 1 and 3 months after LTX. This reduction did not coincide with recovery of circulating MDC. MDC numbers even tended to decrease in the period of CsA reduction (Fig. 1b; P = 0.43). During the period that MDC numbers were restored to pre-LTX values, i.e. between 3 and 12 months, CsA dosage was only slightly reduced. These data were confirmed in the Tac-treated group, as between months 3 and 12 MDC numbers also increased (from 0.27 ± 0.5 to 0.45 ± 0.07; P = 0.04) in this group, even though there were no alterations in the Tac dosage. Together, these observations support that the changes in circulating MDC numbers after LTX were not related to treatment with calcineurin inhibitors.
However, the recovery of MDC numbers between 3 and 12 months after LTX did coincide with tapering of prednisone treatment. In the subgroup of patients in which prednisone treatment was completely stopped in this time-period (n = 10), relative numbers of circulating MDC increased from 0.28 ± 0.10% of PBMC at 3 months to 0.50 ± 0.05% at 12 months after LTX (P = 0.008). In the remaining six patients, prednisone intake was tapered (from 0.17 ± 0.03 mg/kg/day at 3 months to 0.11 ± 0.04 mg/kg/day at 12 months), which coincided with an increase in numbers of circulating MDC from 0.32 ± 0.08% to 0.45 ± 0.12% of PBMC (P = 0.04). This suggests that the alterations in numbers of circulating MDC after LTX may be related to corticosteroid treatment. The strong decline in MDC numbers immediately after LTX may be caused by high-dose prednisolone treatment during and shortly after transplantation.
In contrast to MDC, the relative numbers of circulating monocytes increased immediately after LTX (Fig. 1d) and normalized again after 2 weeks, showing that immunosuppressive therapy did not reduce all types of circulating APC.
Numbers of intragraft MDC and macrophages before and after LTX
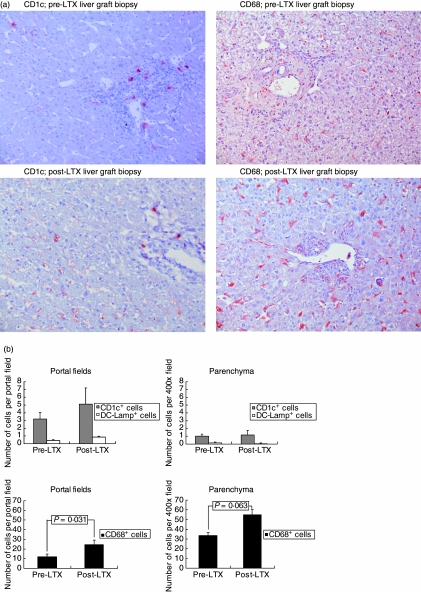
To investigate whether the decline in circulating MDC after LTX resulted from MDC recruitment into the liver graft, MDC and, for comparison, CD68+ monocytes/macrophages were quantified immunohistochemically in liver graft biopsies taken before and after LTX. Paired pre- and post-LTX biopsies were available from seven patients. The post-LTX biopsies had been taken, on average, 12 (range: 6–24) days after transplantation, and did not show significant histological rejection activity [mean rejection activity index (RAI) score 2; range: [0–5]. CD1c mAb was used as a marker for both immature and mature liver MDC [4,16,18], and DC-Lamp as a marker for mature MDC [19]. Figure 2a shows that CD1cbright MDC were located predominantly in portal fields, while CD68+ macrophages/monocytes were observed both in portal fields and parenchyma. In the parenchyma these represent Kupffer cells. In post-LTX biopsies additional faint staining with CD1c mAb was observed in the parenchyma, in a pattern reminiscent of Kupffer cell staining. Therefore, only CD1cbright cells were considered as MDC. DC-Lamp+ mature MDC were only observed sporadically both before and after LTX (not shown). Quantification of CD1cbright and DC-Lamp+ MDC showed that their numbers were not increased significantly in post-LTX compared to pre-LTX liver graft biopsies (Fig. 2b). In contrast, numbers of intragraft CD68+ monocytes/macrophages increased after transplantation, the increase being significant in portal fields and borderline significant in the parenchyma. These data show that it is unlikely that the decline in circulating MDC after LTX is due to accumulation in the liver graft.
Fig. 2.
Myeloid dendritic cells (MDC) and macrophages/monocytes in pre- and post-liver transplantation (LTX) liver graft biopsies. (a) Photomicrographs showing CD1c stains of cryosections and of CD68 stains of paraffin sections from pre- and post-LTX liver graft biopsies (200 × magnification). (b) Numbers of CD1cbright and DC-Lamp+ MDC and CD68+ monocytes/macrophages in paired pre- and post-LTX liver graft biopsies from seven LTX patients. Positive cells were quantified per portal field and per microscopic field of 400 × magnification in the parenchyma, as described in the Materials and methods section.
Expression of chemokine receptors on circulating MDC before and after LTX
To elucidate whether the migratory potential of circulating MDC changed after LTX, expression of CCR-7, the chemokine receptor responsible for homing to secondary lymphoid tissues [20], and of CXCR-4, the receptor for CXCL-12 which is expressed constitutively in lymphoid and non-lymphoid tissues [21,22] including liver grafts [23], were analysed. Before LTX, 56 ± 9% of circulating MDC expressed CXCR-4 and 12 ± 4% expressed CCR-7. Expression of these chemokine receptors measured at 3 days and 1, 2 and 4 weeks after LTX, was not altered, suggesting that the decrease in relative numbers of circulating MDC is not due to enhanced homing capacity to secondary lymphoid organs (data not shown).
Effect of immunosuppressive drugs on MDC survival
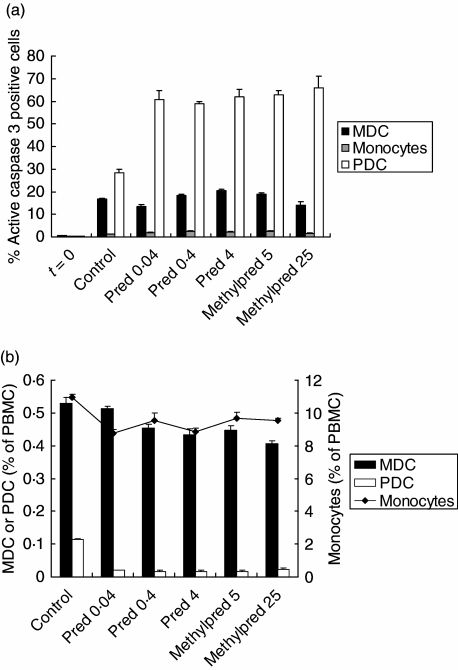
Previously, we reported that PDC are very sensitive to apoptosis induction by corticosteroids [12]. To study the pro-apoptotic effects of the immunosuppressive drugs on blood MDC, PBMC were cultured in the presence or absence of immunosuppressive drugs and after 18 h, expression of the active form of caspase 3, the most downstream effector caspase, was determined in MDC, and for comparison also in monocytes and PDC. The effects of both corticosteroids used in the treatment of the LTX recipients (prednisolone and methylprednisolone) were studied. Different concentrations, corresponding with expected plasma concentrations in the patients were tested. Figure 3a shows that prednisolone or methylprednisolone did not induce expression of active caspase-3 in MDC or monocytes, but only in PDC (Fig. 3a). In addition, relative numbers of MDC, monocytes and PDC were quantified after the cultures. Corticosteroids had almost no effects on the survival of MDC or monocytes in vitro (Fig. 3b). In contrast, in accordance with our previous data, survival of PDC was clearly impaired [12]. No effects of CsA or Tac on expression of active caspase 3 in MDC, monocytes or PDC, or on the survival of these cells, were observed (data not shown). Together, these data show that the reduction in circulating MDC after LTX, in contrast to the decline in PDC, is not due to impairment of MDC survival by immunosuppressive drugs.
Fig. 3.
Effects of corticosteroids on survival of and active caspase 3 expression in myeloid dendritic cells (MDC), monocytes and plasmacytoid dendritic cell (PDC). (a) After 18 h incubation of peripheral blood mononuclear cells (PBMC) from a healthy individual with different concentrations of corticosteroids, percentages of MDC, monocytes and PDC expressing active caspase 3 were determined by flow cytometry. Prednisolone was tested in a concentration of 0.4 μg/ml, which corresponds to the peak plasma level reached during daily treatment with 10 mg prednisone [37], at a 10-fold higher concentration because immediately after liver transplantation (LTX) the patients had been treated with daily doses of 100 mg, and at a 10-fold lower concentration. Methylprednisolone was tested at high concentrations, which corresponded with the range of peak plasma concentrations reached after treatment with 500 mg, according to the manufacturer. Data are means with s.e.m. of triplicate incubations from one of two independent experiments; t = 0: expression before culture; control = after incubation without corticosteroid; pred = after incubation with prednisolone; methylpred = after incubation with methylprednisolone. Concentrations (μg/ml) used are depicted in the numbers beneath the x-axis. (b) Relative numbers of CD1c+CD20– MDC, CD14+ monocytes and blood dendritic cell antigens (BDCA)-4+CD11c– PDC were determined by flow cytometry after culture with different concentrations of corticosteroids. Data are means ± s.e.m. of triplicate incubations from one of three independent experiments. The left y-axis indicates percentages of MDC or PDC, and the right y-axis percentages of monocytes within PBMC.
Maturation status and functional capacity of circulating MDC after LTX in relation to immunosuppressive therapy
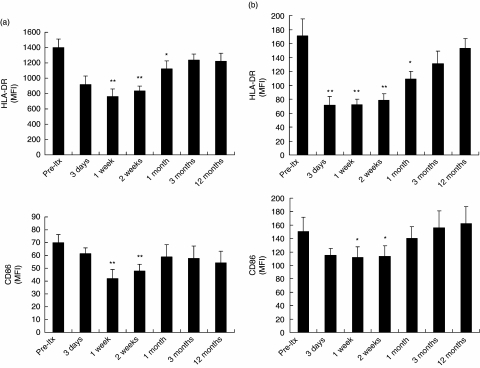
The expression of CD80 on circulating MDC was very low at baseline (0.6 ± 0.4% and positive MDC), and did not change after transplantation. In contrast, all circulating MDC expressed HLA-DR, and 71 ± 4% of circulating MDC expressed CD86 before LTX. The expression of both molecules on circulating MDC was reduced significantly during the first 2 weeks after LTX (Fig. 4a). A similar pattern was observed for the expression of HLA-DR and CD86 on circulating monocytes (Fig. 4b). These data show that the maturation status of circulating MDC and monocytes was reduced significantly during the early post-transplant period. Comparison with the immunosuppressive drug regimen (Fig. 1b) suggests that the depressed maturation status of MDC and monocytes may be related to high-dose corticosteroid intake during the first few weeks after transplantation.
Fig. 4.
Maturation status of circulating myeloid dendritic cells (MDC) and monocytes after liver transplantation (LTX). (a) Expression of HLA-DR and CD86 on circulating CD1c+CD14–CD20– MDC in 16 LTX recipients, shown as mean fluorescence intensity (MFI). (b) Expression of HLA-DR and CD86 on circulating CD14+ monocytes in 16 LTX recipients. **P < 0.01; *P < 0.05 compared to pre-LTX values.
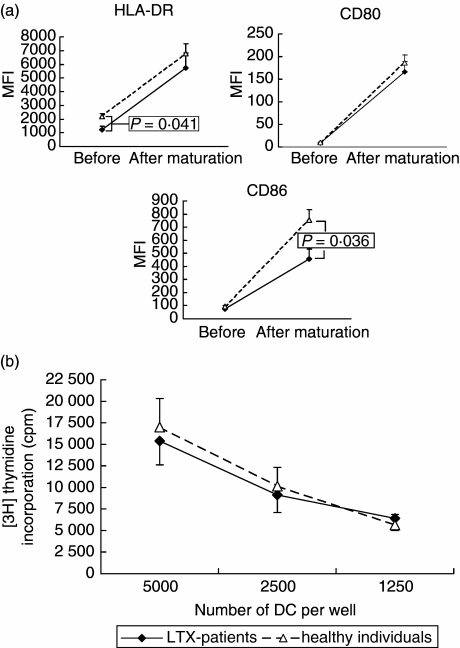
We next examined whether circulating MDC early after LTX were intrinsically impaired in their maturational capacity, or could still respond to maturation stimuli ex vivo in the absence of immunosuppressive drugs. For this purpose, MDC were purified from blood collected prospectively from eight LTX recipients at 2 weeks after transplantation, and from blood of 11 healthy individuals. MDC from both sources were stimulated ex vivo for 24 h with proinflammatory cytokines (TNF-α and IL-1β). Figure 5a shows that MDC from LTX recipients and healthy individuals responded equally to the proinflammatory cytokines with up-regulation of HLA-DR and CD80. Only up-regulation of CD86 was slightly impaired in MDC from the LTX recipients. The capacity of the ex vivo matured MDC from LTX recipients and from healthy individuals to stimulate proliferation of allogeneic T cells was similar (Fig. 5b). Together, these data show that circulating MDC of LTX recipients are not refractory to maturational stimuli, but functionally competent after they have been removed from the environment with immunosuppressive drugs.
Fig. 5.
Comparison of maturational capacity and allogeneic T cell stimulatory capacity of purified blood myeloid dendritic cells (MDC) from liver transplantation (LTX) recipients and healthy individuals. MDC were purified immunomagnetically from blood collected 2 weeks after transplantation from eight LTX recipients, and from blood collected from 11 healthy individuals. MDC were stimulated ex vivo with tumour necrosis factor (TNF)-α and interleukin (IL)-1β for 24 h. (a) Expression of HLA-DR, CD80 and CD86 before and after stimulation. Closed diamonds connected by solid lines show expression on MDC from LTX recipients; open triangles with dotted lines show expression on MDC from healthy individuals. (b) Allogeneic T cell stimulatory capacity after ex vivo maturation. Allogeneic T cells (15 × 104) were added to graded numbers of ex vivo matured DC. After 5 days [3H]-thymidine incorporation was determined.
Discussion
The first key observation in this study is the reduction of the numbers and attenuation of the maturation status of circulating MDC after LTX. The maturation status was restored after 1 month, but the lower numbers of circulating MDC persisted until at least 3 months after LTX. The second key observation is that the circulating MDC in LTX recipients were nevertheless functionally competent and responded well to maturation stimuli ex vivo.
Several factors might contribute to the decline in circulating MDC after LTX
First, the reduction could be due to recruitment of these cells into the liver graft. This possibility is contradicted, however, by our observation that MDC did not accumulate in the liver graft during the early post-transplant period, when circulating MDC numbers were most profoundly decreased.
Secondly, the reduction of circulating MDC might be caused by increased potential of these cells to home into lymphoid tissues. Immunosuppressive drugs can influence chemokine receptor expression [24] and migration of MDC [25]. However, no change in the expression of CCR-7, the chemokine receptor responsible for homing to secondary lymphoid tissues [20], and of CXCR-4, the receptor for the homeostatic chemokine CXCL-12 which is expressed constitutively in many lymphoid and non-lymphoid tissues [21,22], was observed upon transplantation. Although migration of circulating MDC into tissues might also be dependent upon other factors, these results do not support the possibility that the decrease of circulating MDC after LTX is due to altered homing properties.
A third explanation for the reduced numbers of circulating MDC may be that immunosuppressive drug treatment influence their survival negatively, although neither corticosteroids nor CsA or Tac affected survival of blood MDC in vitro, nor induced expression of the active form of effector caspase 3. These observations are in agreement with data showing that these drugs do not induce apoptosis in MDC derived from monocytes [26] or CD34+ haematopoietic progenitors in vitro[24,27].
Fourthly, the decrease in circulating MDC numbers might be due to the surgical intervention. Indeed, numbers of circulating MDC are lowered immediately after abdominal surgery [9,28], but this reduction is only temporary and MDC counts normalize within 4 days [28].
Finally, immunosuppressive drugs may impair the generation of MDC from their precursors. The longitudinal association with corticosteroid intake strongly suggests that the impairment in circulating MDC is caused by corticosteroid treatment. In vitro, it has been established clearly that corticosteroids inhibit the differentiation of MDC from monocytes [29,30] and from CD34+ haematopoietic progenitors [27] with concomitant increased generation of macrophages [31]. Moreover, in vivo, corticosteroid treatment resulted in reduced numbers of MDC and increased numbers of macrophages in the spleen in mice [32]. In addition, corticosteroids inhibit maturation of MDC in vitro, suppressing the up-regulation of HLA and co-stimulatory molecules [33,34]. Similarly, corticosteroids suppress maturation of monocytes in vitro[35]. Thus, the observed decrease in HLA-DR and CD86 expression on circulating MDC and monocytes may also be caused by corticosteroid treatment. Therefore, we hypothesize that the lowered numbers and maturation status of circulating MDC after LTX is due largely to inhibition of their generation and maturation by corticosteroid treatment. This hypothesis in congruent with the observed increase in numbers of monocytes in the circulation and monocytes/macrophages in the graft after LTX which may be due, at least partially, to suppression of their differentiation into MDC. Because all LTX recipients in our centre are treated with corticosteroids, we were unable to test this hypothesis formally.
Previous cross-sectional studies in LTX and kidney transplant recipients [13–15] were discordant as to whether corticosteroid therapy influences the numbers of circulating MDC. These studies investigated the association between corticosteroids and circulating MDC several years after transplantation, when only low doses of corticosteroids are given. In contrast, by investigating the longitudinal course of circulating MDC early after transplantation, the present study provided the opportunity to observe a strongly suggestive association between corticosteroid therapy and circulating MDC.
Although circulating MDC during the first few weeks after LTX showed an impaired maturational status, these cells were functionally fully competent. Upon isolation they matured in a comparable way in response to proinflammatory cytokines to T cell stimulatory APC, as did MDC from non-treated individuals. Apparently, corticosteroid therapy does not result in MDC that are refractory to maturation stimuli. The effect of corticosteroid treatment on MDC is thus quite different from the effect of chronic infection with, e.g. HBV, which results in an intrinsic impairment, making MDC resistant to maturation stimuli ex vivo[18]. This observation shows that circulating MDC in organ transplant recipients treated with corticosteroids differ from the ‘corticosteroid DC’ that have been generated in vitro from monocytes in the presence of corticosteroids. The latter are refractory to maturation stimuli, and unable to acquire potent allogeneic T cell stimulatory capacity [30,36]. Indeed, ‘corticosteroid DC’ are macrophage-like cells, with a high expression of CD14 and no expression of CD1a [29–31].
In conclusion, during the first few weeks after transplantation LTX recipients have reduced numbers of circulating MDC with an impaired maturational status. The paucity of circulating MDC persists up to at least 3 months after transplantation. Our results suggest strongly that the MDC impairment is caused by corticosteroid treatment. However, corticosteroid therapy does not result in the generation of maturation-resistant tolerogenic MDC. The unique role of corticosteroids in immunosuppressive protocols may be related at least partly to their inhibitory effects on MDC, thereby suppressing allogeneic T cell activation by the graft in its earliest phase, namely by inhibition of donor antigen presentation. Therefore, in the design of corticosteroid-free immunosuppressive protocols it should be realized that omission of a drug that suppresses MDC requires compensational immunosuppressive drug treatment to avoid rejection.
Acknowledgments
The authors would like to thank Dr Andrea Woltman, Department of Gastroenterology and Hepatology, Erasmus MC, Rotterdam, for critically reading the manuscript.
References
- 1.Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171:307–14. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saiki T, Ezaki T, Ogawa M, Maeda K, Yagita H, Matsuno K. In vivo roles of donor and host dendritic cells in allogeneic immune response: cluster formation with host proliferating T cells. J Leukoc Biol. 2001;69:705–12. [PubMed] [Google Scholar]
- 3.Saiki T, Ezaki T, Ogawa M, Matsuno K. Trafficking of host- and donor-derived dendritic cells in rat cardiac transplantation: allosensitization in the spleen and hepatic nodes. Transplantation. 2001;71:1806–15. doi: 10.1097/00007890-200106270-00017. [DOI] [PubMed] [Google Scholar]
- 4.Bosma BM, Metselaar HJ, Mancham S, et al. Characterization of human liver dendritic cells in liver grafts and perfusates. Liver Transplant. 2006;12:384–93. doi: 10.1002/lt.20659. [DOI] [PubMed] [Google Scholar]
- 5.Penfield JG, Wang Y, Li S, et al. Transplant surgery injury recruits recipient MHC class II-positive leukocytes into the kidney. Kidney Int. 1999;56:1759–69. doi: 10.1046/j.1523-1755.1999.00741.x. [DOI] [PubMed] [Google Scholar]
- 6.Abe M, Wang Z, de Creus A, Thomson AW. Plasmacytoid dendritic cell precursors induce allogeneic T-cell hyporesponsiveness and prolong heart graft survival. Am J Transplant. 2005;5:1808–19. doi: 10.1111/j.1600-6143.2005.00954.x. [DOI] [PubMed] [Google Scholar]
- 7.Ochando JC, Homma C, Yang Y, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–62. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 8.Athanassopoulos P, Vaessen LM, Maat AP, et al. Preferential depletion of blood myeloid dendritic cells during acute cardiac allograft rejection under controlled immunosuppression. Am J Transplant. 2005;5(4)(Part 1):810–20. doi: 10.1111/j.1600-6143.2005.00777.x. [DOI] [PubMed] [Google Scholar]
- 9.Hesselink DA, Vaessen LM, Hop WC, et al. The effects of renal transplantation on circulating dendritic cells. Clin Exp Immunol. 2005;140:384–93. doi: 10.1111/j.1365-2249.2005.02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Womer KL, Peng R, Patton PR, et al. The effects of renal transplantation on peripheral blood dendritic cells. Clin Transplant. 2005;19:659–67. doi: 10.1111/j.1399-0012.2005.00405.x. [DOI] [PubMed] [Google Scholar]
- 11.Schvoerer E, Thumann C, Spohrer S, et al. Early decrease in circulating dendritic cells number after liver transplantation could favor hepatitis C virus recurrence. J Med Virol. 2006;78:1070–5. doi: 10.1002/jmv.20664. [DOI] [PubMed] [Google Scholar]
- 12.Boor PP, Metselaar HJ, Mancham S, Tilanus HW, Kusters JG, Kwekkeboom J. Prednisolone suppresses the function and promotes apoptosis of plasmacytoid dendritic cells. Am J Transplant. 2006;6:2332–41. doi: 10.1111/j.1600-6143.2006.01476.x. [DOI] [PubMed] [Google Scholar]
- 13.Hackstein H, Renner FC, Bohnert A, et al. Dendritic cell deficiency in the blood of kidney transplant patients on long-term immunosuppression: results of a prospective matched-cohort study. Am J Transplant. 2005;5:2945–53. doi: 10.1111/j.1600-6143.2005.01101.x. [DOI] [PubMed] [Google Scholar]
- 14.Lim WH, Kireta S, Thomson AW, Russ GR, Coates PT. Renal transplantation reverses functional deficiencies in circulating dendritic cell subsets in chronic renal failure patients. Transplantation. 2006;81:160–8. doi: 10.1097/01.tp.0000188620.72969.56. [DOI] [PubMed] [Google Scholar]
- 15.Mazariegos GV, Zahorchak AF, Reyes J, Chapman H, Zeevi A, Thomson AW. Dendritic cell subset ratio in tolerant, weaning and non-tolerant liver recipients is not affected by extent of immunosuppression. Am J Transplant. 2005;5:314–22. doi: 10.1111/j.1600-6143.2004.00672.x. [DOI] [PubMed] [Google Scholar]
- 16.Tanis W, Mancham S, Binda R, et al. Human hepatic lymph nodes contain normal numbers of mature myeloid dendritic cells but few plasmacytoid dendritic cells. Clin Immunol. 2004;110:81–8. doi: 10.1016/j.clim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 17.van der Molen RG, Sprengers D, Binda RS, et al. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40:738–46. doi: 10.1002/hep.20366. [DOI] [PubMed] [Google Scholar]
- 18.Kwekkeboom J, Boor PP, Sen E, et al. Human liver myeloid dendritic cells maturate in vivo into effector DC with a poor allogeneic T-cell stimulatory capacity. Transplant Proc. 2005;37:15–16. doi: 10.1016/j.transproceed.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 19.de Saint-Vis B, Vincent J, Vandenabeele S, et al. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–36. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 20.Gunn MD. Chemokine mediated control of dendritic cell migration and function. Semin Immunol. 2003;15:271–6. doi: 10.1016/j.smim.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Sozzani S. Dendritic cell trafficking: more than just chemokines. Cytokine Growth Factor Rev. 2005;16:581–92. doi: 10.1016/j.cytogfr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Penna G, Vulcano M, Sozzani S, Adorini L. Differential migration behavior and chemokine production by myeloid and plasmacytoid dendritic cells. Hum Immunol. 2002;63:1164–71. doi: 10.1016/s0198-8859(02)00755-3. [DOI] [PubMed] [Google Scholar]
- 23.Goddard S, Williams A, Morland C, et al. Differential expression of chemokines and chemokine receptors shapes the inflammatory response in rejecting human liver transplants. Transplantation. 2001;72:1957–67. doi: 10.1097/00007890-200112270-00016. [DOI] [PubMed] [Google Scholar]
- 24.Chen T, Guo J, Yang M, et al. Cyclosporin A impairs dendritic cell migration by regulating chemokine receptor expression and inhibiting cyclooxygenase-2 expression. Blood. 2004;103:413–21. doi: 10.1182/blood-2003-07-2412. [DOI] [PubMed] [Google Scholar]
- 25.Cumberbatch M, Dearman RJ, Kimber I. Inhibition by dexamethasone of Langerhans cell migration: influence of epidermal cytokine signals. Immunopharmacology. 1999;41:235–43. doi: 10.1016/s0162-3109(99)00037-5. [DOI] [PubMed] [Google Scholar]
- 26.Vanderheyde N, Verhasselt V, Goldman M, Willems F. Inhibition of human dendritic cell functions by methylprednisolone. Transplantation. 1999;67:1342–7. doi: 10.1097/00007890-199905270-00009. [DOI] [PubMed] [Google Scholar]
- 27.Woltman AM, Massacrier C, de Fijter JW, Caux C, van Kooten C. Corticosteroids prevent generation of CD34+-derived dermal dendritic cells but do not inhibit Langerhans cell development. J Immunol. 2002;168:6181–8. doi: 10.4049/jimmunol.168.12.6181. [DOI] [PubMed] [Google Scholar]
- 28.Ho CS, Lopez JA, Vuckovic S, Pyke CM, Hockey RL, Hart DN. Surgical and physical stress increases circulating blood dendritic cell counts independently of monocyte counts. Blood. 2001;98:140–5. doi: 10.1182/blood.v98.1.140. [DOI] [PubMed] [Google Scholar]
- 29.Piemonti L, Monti P, Allavena P, et al. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol. 1999;162:6473–81. [PubMed] [Google Scholar]
- 30.Woltman AM, de Fijter JW, Kamerling SW, Paul LC, Daha MR, van Kooten C. The effect of calcineurin inhibitors and corticosteroids on the differentiation of human dendritic cells. Eur J Immunol. 2000;30:1807–12. doi: 10.1002/1521-4141(200007)30:7<1807::AID-IMMU1807>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 31.Canning MO, Grotenhuis K, de Wit HJ, Drexhage HA. Opposing effects of dehydroepiandrosterone and dexamethasone on the generation of monocyte-derived dendritic cells. Eur J Endocrinol. 2000;143:687–95. doi: 10.1530/eje.0.1430687. [DOI] [PubMed] [Google Scholar]
- 32.Moser M, De Smedt T, Sornasse T, et al. Glucocorticoids down-regulate dendritic cell function in vitro and in vivo. Eur J Immunol. 1995;25:2818–24. doi: 10.1002/eji.1830251016. [DOI] [PubMed] [Google Scholar]
- 33.Woltman AM, van Kooten C. Functional modulation of dendritic cells to suppress adaptive immune responses. J Leukoc Biol. 2003;73:428–41. doi: 10.1189/jlb.0902431. [DOI] [PubMed] [Google Scholar]
- 34.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 35.Blotta MH, DeKruyff RH, Umetsu DT. Corticosteroids inhibit IL-12 production in human monocytes and enhance their capacity to induce IL-4 synthesis in CD4+ lymphocytes. J Immunol. 1997;158:5589–95. [PubMed] [Google Scholar]
- 36.Woltman AM, van der Kooij SW, de Fijter JW, van Kooten C. Maturation-resistant dendritic cells induce hyporesponsiveness in alloreactive CD45RA and CD45RO T-cell populations. Am J Transplant. 2006;6:2580–91. doi: 10.1111/j.1600-6143.2006.01520.x. [DOI] [PubMed] [Google Scholar]
- 37.Hollander AA, van Rooij J, Lentjes GW, et al. The effect of grapefruit juice on cyclosporine and prednisone metabolism in transplant patients. Clin Pharmacol Ther. 1995;57:318–24. doi: 10.1016/0009-9236(95)90157-4. [DOI] [PubMed] [Google Scholar]