Abstract
The intracellular pathogens Propionibacterium acnes and Mycobacterium tuberculosis have been leading suspects as the cause of sarcoidosis, a systemic disorder characterized by the formation of non-caseating granulomas. Toll-like receptor (TLR) 2 is important in the innate immune response against both pathogens, and is therefore of interest in sarcoidosis research. In the present study, three single nucleotide polymorphisms and one dinucleotide repeat polymorphism in the TLR-2 gene were genotyped in 419 sarcoidosis patients, divided into a study cohort and a validation cohort, and 196 healthy controls. In the study cohort we found a significant increase in prevalence of the AA-genotype at promotor location −16934 in patients with chronic disease compared to patients with acute/self-remitting sarcoidosis (34.5% versus 15.9%, respectively, P = 0.006, Pc = 0.019). These results could not be confirmed in our validation cohort, implicating a possible role for TLR-2 genetics in only a small percentage of sarcoidosis patients. Furthermore, linkage was found between the promotor polymorphism −16934 A/T and the number of GT repeats in intron 1 (P < 0.0001). After in vitro stimulation of peripheral blood mononuclear cells (PMBCs) with different TLR-2 agonists, a correlation between induction of TNF-α (P = 0.008), interleukin (IL)-12 (P = 0.008) as well as IL-6 (P = 0.02), and the number of GT repeats was observed. In conclusion, the data show that polymorphisms in TLR-2 might be important in a small group of sarcoidosis patients and that their functional consequences explain partly some of the variance in cytokine pattern observed in different clinical phenotypes of this disease.
Keywords: linkage, polymorphisms, sarcoidosis, Toll-like receptor 2
Introduction
Sarcoidosis is a systemic disorder characterized by the formation of non-caseating granulomas, most often affecting the lungs, lymph nodes and skin. The aetiology remains unknown but it is attractive to speculate, given the variation in the clinical spectrum of sarcoidosis, that this disease reflects a collection of different granulomatous diseases, each with its own antigen. Sarcoidosis is characterized by a strong cell-mediated immune reaction, making pathogens such as viruses or intracellular bacteria the leading suspects as causative agents. In particular, Propionibacterium acnes and Mycobacterium tuberculosis have been studied extensively and may well play a role in disease pathogenesis [1–5].
Recent years have seen a dramatic improvement in our understanding of the role of innate immunity in human host defence. Innate immunity is able to discriminate between self and a variety of pathogens and can initiate inflammatory and anti-microbial responses, thereby dictating the ensuing adaptive immune response. To date, relatively little attention has been paid to the role of innate immune receptors in the pathogenesis of sarcoidosis. Single nucleotide polymorphisms (SNPs) in genes encoding for mannose binding lectin (MBL2) and the nucleotide-binding oligomerization domains (NOD) 1 and 2 have been studied in sarcoidosis patients, showing that only NOD1 is likely to play a role in disease pathogenesis [6–9]. Regarding CD14, the classical lipopolysaccharide (LPS) receptor, it has been shown that the concentration of its soluble form (sCD14) in bronchoalveolar lavage (BAL) fluid is higher in patients with active sarcoidosis compared with inactive sarcoidosis [10]. Furthermore, Gazouli et al. demonstrated that the CD14 promotor polymorphism −159C/T is associated with sarcoidosis patients in Greece [11]. These studies suggest that altered regulation of the innate immune response can play a role in sarcoidosis pathogenesis, which has already been demonstrated for other granulomatous disorders such as Crohn's disease and tuberculosis [12–15].
An important group of innate immunity receptors is the family of Toll-like receptors (TLRs), consisting of 10 receptors, each recognizing a distinct, but limited, repertoire of microbial products. At present, three genetic studies have been published addressing the role of this receptor family in sarcoidosis. The influence of TLR-4 polymorphisms on the disease course of sarcoidosis patients has been studied by several research groups, including ours, but with conflicting results [11,16,17].
Taken together, when considering a causative role for intracellular bacteria in the pathogenesis of sarcoidosis and the possible influence of altered innate immunity, TLRs recognizing microbial components of P. acnes or M. tuberculosis are of interest. TLR-2 is involved in the immune response against both pathogens and seems to be a primary candidate for genetic and functional analysis [15,18,19]. TLR-2 signalling also seems important for the function of regulatory T cells, a population of T cells which might be functionally impaired in sarcoidosis [20,21]. We hypothesized that an aberrant innate immune response against TLR-2 agonists, such as P. acnes or M. tuberculosis, contributes to the development of sarcoidosis, or at least influences disease course. In the present study we tested the hypothesis that genetic variances altering TLR-2 function are more prevalent in (subgroups of) sarcoidosis patients.
Materials and methods
Patients and control subjects
One hundred and sixty-five unrelated and randomly selected Dutch Caucasian patients with sarcoidosis (92 men and 73 women) were included in the study cohort. In 108 patients, the diagnosis of sarcoidosis was established when clinical findings were supported by histological evidence, and after the exclusion of other known causes of granulomatosis. Fifty-seven patients presented with the classical Löfgren's syndrome of fever, erythema nodosum, bilateral hilar lymphadenopathy and joint symptoms. Verbal and written consent was obtained from all subjects, and authorization was given by the Ethics Committee of the St Antonius Hospital, Nieuwegein. The control subjects comprised 196 healthy Dutch Caucasian employees of the St Antonius Hospital in the Netherlands. By completing a questionnaire, relevant background information was provided by these volunteers and included medication, ethnicity and hereditary diseases. As a validation cohort, 254 unrelated and randomly selected Caucasian patients with sarcoidosis from a hospital located in a different geographical region in the Netherlands were used. Sarcoidosis was diagnosed as described above and written consent was obtained from these subjects as well as approval by the Ethics Committee of the University Hospital, Maastricht.
Evaluation of pulmonary disease severity
Chest radiographs at presentation, 2 years and 4 years were collected for each patient and assessed blind by a pulmonary physician for disease severity using standard radiographic staging for sarcoidosis. In brief, this comprises five stages: stage 0 = normal, stage I = bilateral hilar lymphadenopathy (BHL), stage II = BHL and parenchymal infiltration, stage III = parenchymal infiltration without BHL and stage IV = irreversible fibrosis with loss of lung volume. Radiographic evolution over a 4-year period was available for 165 patients and was categorized as follows: (A): normalization of improvement towards stage I (n = 50), (B): persistent stage II/III or progression in that direction (n = 35) and (C): stable stage IV or progression towards this stage (n = 23) [22]. Patients who had been diagnosed with Löfgren's syndrome at presentation (n = 57) were considered as a distinct group with radiographic evolution not exceeding stage I. In the validation cohort there were 42 patients with Löfgren's syndrome, 72 patients were categorized as stage A, 105 patients as stage B and 35 patients as stage C. Patients with Lögren's syndrome or stage A were defined as acute/self-remitting disease and patients with stages B or C as chronic disease.
Analysis of TLR-2 genetic single nucleotide polymorphisms
Genomic DNA extracted from all subjects and controls was genotyped for three different TLR-2 (SNPs) using sequence-specific primers (SSPs) and polymerase chain reaction (PCR), or Taqman genotyping assay when SSP-PCR was technically impossible (−16934 A/T). Three known SNPs and one short tandem repeat polymorphism in the TLR-2 gene were selected based on associations with Gram-positive infections and tuberculosis [23,24]. The three SNPs were located at the following nucleotide positions (including NCBI reference numbers): −16934 (rs4696480, promotor region) +1892 (rs5743704/Pro631His, exon 3) and +2258 (rs5743708/Arg753Gln, exon 3). Identification of the −16934 A/T polymorphism was performed using the dual-labelled allele-specific oligonucleotides 5′-(FAM)-ACCCTCTCCAGATGC-(NFQ) and 5′-(VICTM)CCCT CACCAGATGC-(NFQ) together with forward primer 5′-GGTTCTGGAGTCTG GGAAGTC and reverse primer 5′-TCTCACCATGTGATGCTTTCCATC, following the manufacturer's instructions (Custom TaqMan® SNP Genotyping Assay, Applied Biosystems, Foster City, CA, USA). For identification of the polymorphism at position +1892 the sequence-specific reverse primers 5′-CTGCTGGGAGCTTTCCTGG and 5′-CTGC TGGGAGCTTTCCTGT were combined with the forward primer 5′-AGCAAG CACTGGCCAAAGTCT, leading to an expected PCR product size of 268 base pairs (bp). For identification of the polymorphism at position +2258 the sequence-specific reverse primers 5′-AGGTCTTGGTGTTCATTATCTTCT and 5′-AGGTCTTGGTGTTC ATTATCTTCC were combined with the forward primer 5′-AGCAAGCACTGGC CAAAGTCT leading to an expected PCR product size of 406 bp. In all primer mixes we included control primers 5-ATGATGTTGACCTTTCCAGGG and 5′-GCAACT GATGAAAAGTTACAGAA, leading to an expected PCR product size of 720 bp. All PCR reactions were run under identical conditions as described previously [25].
Analysis of TLR-2 intron 1 microsatellite polymorphism
The TLR-2 GT-dinucleotide repeat is located 100 bp upstream of the translational start site in intron 1 (http://snpper.chip.org). A region of about 150 bp encompassing this repeat polymorphism was amplified using the primers 5′-ATGATATATATAG ATAT, labelled with FAM and primer 5′-ATATAGAGAGAGTA 15. The PCR reactions were run as described previously [26]. GeneScan was performed on an ABI PRISM® 3100-Avant Genetic Analyser (Applied Biosystems) and results were analysed with Gene Mapper™ 3.5 (Applied Biosystems).
TLR-2 surface expression
The following monoclonal antibodies (mAbs) were used in the flow cytometriTable 1c studies: anti-CD45 [peridinin chlorophyll (PerCP)], anti-CD3 [fluorescein isothiocyanate (FITC)] and anti-CD14 [allophycocyanin (APC)], all from BD Biosciences (Alpen aan den Rijn, the Netherlands). Anti-TLR-2 [phycoerythrin (PE), clone TL2.1] was purchased from eBioscience (San Diego, CA, USA). Fifty μl of ethylenediamine tetraacetic acid (EDTA) blood was incubated for 15 min at room temperature with 10 μl of the following mAbs: anti-CD45, anti-CD3, anti-CD14 and anti-TLR-2. The CD14+ population was gated after selecting the CD45+ population. The granulocyte population was selected based on forward scatter–side scatter (FSC–SSC) characteristics. Immunofluoresence was measured by flowcytometry (FACSCalibur, Becton Dickinson, Alpen aan den Rijn, the Netherlands) and data were analysed with CellQuest software (BD Biosciences). During each experiment a total of 20 000 cells were counted and experiments were performed in triplicate.
Quantification of cytokine production
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized venous blood using Ficoll-Paque density gradient centrifugation and seeded on 24-well plates at a density of 400 000 cells per well. PBMCs were stimulated with the TLR-2 agonist Pam3Cys-Ser-Lys4 (10 µg; EMC Microcollections, Tubingen, Germany) or with clinical isolates of either P. acnes or M. tuberculosis (100 000/well). P. acnes was identified based on morphology, Gram staining and both indolase and catalase producing characteristics and two different clinical isolates were pooled. Determination of M. tuberculosis was performed as described previously [27]. Prior to the experiments, M. tuberculosis was heat-inactivated at 100°C for 30 min. Phytohaemagglutinin (42 µg; Murex, Dartford, UK) and medium were used as positive and negative control, respectively. All stimulations were performed in a final volume of 1200 μl RPMI-1640 (Gibco, Breda, the Netherlands) containing 10% heat-inactivated fetal calf serum (FCS) (Gibco) and 1% clindamycin/streptomycin. After a 24-h incubation period at 37°C in humidified air containing 5% CO2, supernatants were collected and stored immediately at −80°C until further analysis. Five different cytokines [interleukin (IL)-12p70, interferon (IFN)-γ, tumour necrosis factor (TNF)-α, IL-4 and IL-6) were measured using Multiplex Immuno Assay (Biorad, Hercules, CA, USA) on a Bioplex 100 system (Biorad) according to the manufacturer's instructions. Calibration curves were constructed using the medium mentioned above. For the samples with IL-6 concentrations above the upper detection limit, IL-6 was determined in serial dilutions of culture supernatant by sandwich enzyme-linked immunosorbent assay (ELISA) (Sanquin, Amsterdam, the Netherlands).
Statistical analysis
Comparison of genotype prevalence between groups was performed using χ2 contingency table analysis with the appropriate number of degrees of freedom. Adjustment for multiple tests was made according to the Bonferroni method. The degree of correlation was determined using Pearson's correlation calculation. A P-value of < 0.05 was considered significant.
Results
Allelic distribution of TLR-2 single nucleotide polymorphisms and intron repeat polymorphism in patients and controls
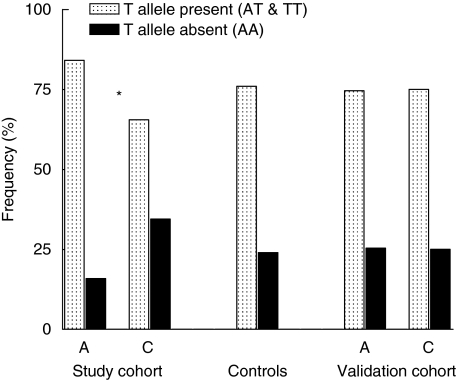
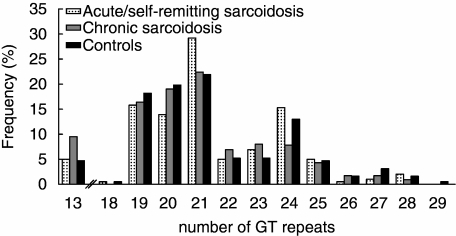
The genotype distribution of the investigated TLR-2 SNPs in our study cohort and controls are presented in Table 1. Genotype data from all populations conformed to Hardy–Weinberg equilibrium. When assigning all sarcoidosis patients to one group, no differences were found between patients and controls regarding the allelic distribution of the three SNPs and the number of GT tandem repeats. However, comparison of patients with acute/self-remitting disease with patients having chronic disease revealed that −16934 T allelic carriage was more prevalent in the first group (84.1% versus 65.5%, respectively, P = 0.006, Pc = 0.019, Fig. 1). Furthermore, we found that the distribution of the number of GT intron repeats also differed between patients with acute/self-remitting disease and patients having chronic disease (Fig. 2), with a higher prevalence of relative short alleles in the latter group.
Table 1.
Genotype distribution of the Toll-like receptor 2 single nucleotide polymorphisms in sarcoidosis patients from the study cohort and healthy controls.
| Radiographic evolution | |||||||
|---|---|---|---|---|---|---|---|
| Genotype | Controls (n = 196) | Patients (n = 165) | LOF (n = 57) | A (n = 50) | B (n = 35) | C (n = 23) | |
| −16934 | TT | 47 | 43 | 20 | 12 | 7 | 4 |
| TA | 102 | 85 | 29 | 29 | 15 | 12 | |
| AA | 47 | 37 | 8 | 9 | 13 | 7 | |
| Pro631His | CC | 181 | 151 | 55 | 46 | 28 | 22 |
| CA | 15 | 14 | 2 | 4 | 7 | 1 | |
| Arg753Trp | GG | 185 | 155 | 55 | 47 | 33 | 20 |
| GA | 11 | 10 | 2 | 3 | 2 | 3 | |
Fig. 1.
TLR-2 promotor polymorphism −16934 T allele carriage in sarcoidosis patients with acute/self remitting disease versus chronic disease. A = acute/self-remitting disease, C = chronic disease. *Pc = 0.019 (uncorrected P value = 0.006).
Fig. 2.
Allelic distribution of Toll-like receptor 2 intron 1 GT repeat polymorphism in sarcoidosis patients from the study cohort and healthy controls.
To confirm our genetic association, we determined the −16934 T allele carriage in a validation cohort consisting of 254 unrelated and randomly selected Caucasian patients with sarcoidosis from a separate geographical region within the Netherlands. In this cohort we did not find a difference in T allele carriage between patients with acute/self-remitting disease and chronic disease (acute/self-remitting: AA n = 29, AT n = 56, TT n = 29, chronic: AA n = 35, AT n = 69, TT n = 36, P = 0.92).
Linkage between TLR-2 promotor polymorphism −16934 A/T and intron 1 GT repeat polymorphism
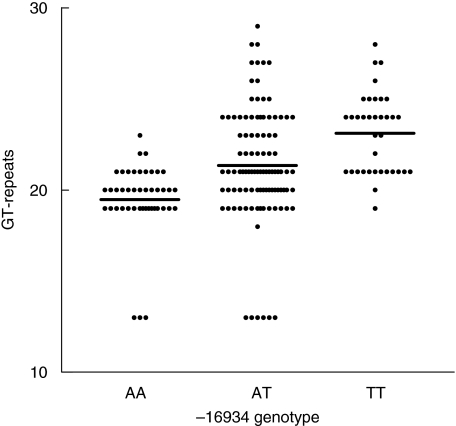
The distribution of the promotor polymorphism and the intron 1 repeat polymorphism differed between patients with an acute/self-remitting form of sarcoidosis and patients having a chronic disease form in the study cohort. Because single nucleotide polymorphisms and short tandem repeats occur frequently in the genome, closely linked pairs are common. We therefore tested whether these two polymorphisms were in linkage disequilibrium. Both polymorphisms were determined in 96 healthy Dutch controls. We found a highly significant association between the TLR-2 promotor polymorphism and the number of tandem repeats in the intron 1 polymorphism (Fig. 3). The A allele was linked mainly to GT repeat numbers 13, 19, 20 and 21, the T allele was linked mainly to repeat numbers 21–27. The mean number of GT tandem repeats was 19.5 in the AA genotype group, 21.3 in the AT genotype group and 23.1 in the TT genotype group (P < 0.0001). A similar association was also found in sarcoidosis patients (data not shown, P < 0.0001).
Fig. 3.
Linkage between the genotype of Toll-like receptor 2 promotor polymorphism −16934A/T and the number of intron 1 GT repeats; 192 GT alleles derived from 96 healthy donors are grouped according to −16934 genotype (P < 0.0001) Horizontal bars indicate the mean number of GT repeats.
Influence of TLR-2 promotor polymorphism −16934 A/T and intron 1 GT repeat polymorphism on TLR-2 expression and cytokine production
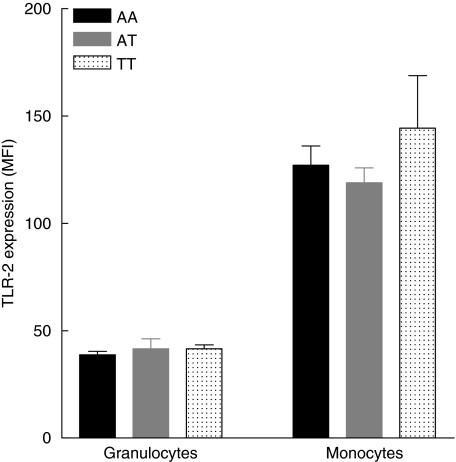
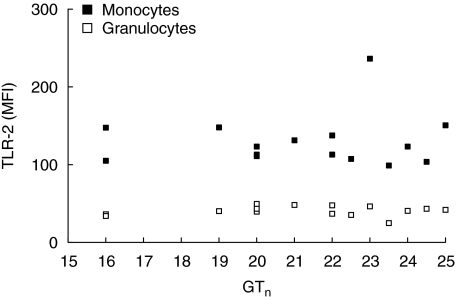
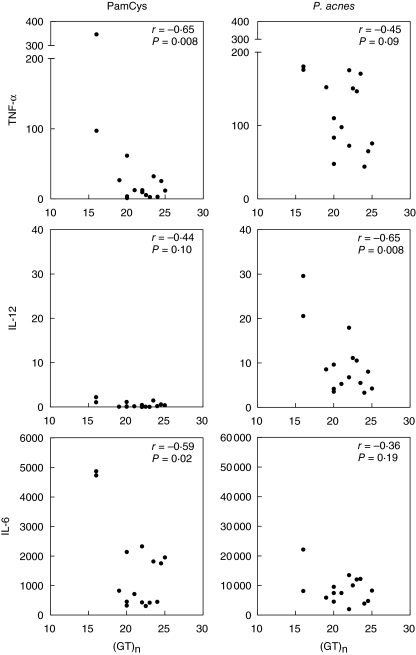
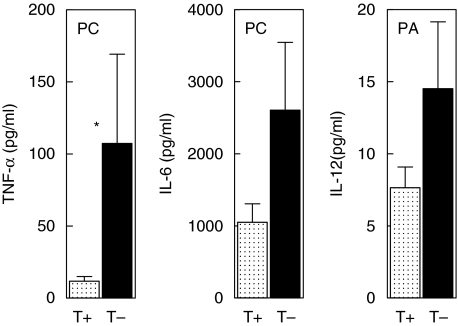
Considering the discrepant genetic results on the distribution of promotor polymorphism −16934 A/T and intron 1 repeat polymorphism in two independent groups of sarcoidosis patients, we decided to investigate the functional relevance of both polymorphisms. First, we measured basal TLR-2 expression on both granulocytes and CD14-positive monocytes in 15 healthy donors with either promotor genotype AA (n = 5), AT (n = 5) or TT (n = 5) and a corresponding variable number of GT repeats. We did not find a correlation between either of the two polymorphisms and TLR-2 expression on granulocytes or monocytes (Figs 4 and 5). Secondly, production of proinflammatory cytokines was studied in PMBCs from the same 15 healthy donors after stimulation with different TLR-2 agonists (Pam3Cys-Ser-Lys4, P. acnes and M. tuberculosis). We observed a correlation between the production of TNF-α and IL-12 as well as IL-6 and the number of GT repeats after stimulation with PamCys or P. acnes (Fig. 6). The effect of TLR-2 polymorphisms on production of proinflammatory cytokines was also found when data were grouped according to 16934 T allele carriage (Fig. 7). No correlation was found between IFN-γ or IL-4 and the number of GT repeats (data not shown). Also, as expected, no correlations were found after stimulation with phytohaemagglutinin (PHA). The use of heat-killed M. tuberculosis led to a small, insignificant induction of cytokines (data not shown).
Fig. 4.
Influence of the Toll-like receptor 2 (TLR-2) promotor polymorphism −16934A/T on the TLR-2 cell surface expression measured on both granulocytes and monocytes. No differences were found between TLR-2 expression and different promotor genotypes (granulocytes; P = 0.77, monocytes; P = 0.52). MFI = mean fluorescence intensity ± standard error of the mean.
Fig. 5.
Influence of the number of Toll-like receptor 2 (TLR-2) intron 1 GT repeats on TLR-2 surface expression measured on both granulocytes and monocytes. No correlation was found between TLR-2 expression and the number of GT repeats (granulocytes P = 0.70, monocytes P = 0.78). MFI = mean fluorescence intensity. GTn = mean number of GT repeats per donor.
Fig. 6.
Influence of the number of Toll-like receptor 2 (TLR-2) intron 1 GT repeats on the induction of pro-inflammatory cytokines (in pg/ml) after stimulation of PBMCs with different TLR-2 agonists. PamCys = Pam3Cys-Ser-(Lys)4, P. acnes = Propionibacterium acnes. TNF-α = tumour necrosis factor-alpha, IL-6 = interleukin-6, IL-12 = interleukin 12. GTn = mean number of GT repeats per donor.
Fig. 7.
Influence of the Toll-like receptor 2 (TLR-2) −16934 promotor T allele carriage on the induction of proinflammatory cytokines after stimulation of peripheral blood mononuclear cells (PBMCs) with different TLR-2 agonists. T+ = T allele present at location −16934, T– = T allele absent at location −16934. PC = stimulated with Pam3Cys-Ser-Lys4, PA = stimulated with Propionibacterium acnes. TNF-α = tumour necrosis factor-alpha, IL-12 = interleukin 12, IL-6 = interleukin 6. Values are in picograms per millilitre and shown as ±standard error of the mean. *P < 0.05.
Discussion
In the present study we investigated the genetic influence of the innate immunity receptor TLR-2 in Dutch sarcoidosis patients. TLR-2 is expressed primarily on monocytes, macrophages, dendritic cells, B cells and, to a lesser extent, on neutrophils [28]. Activation of TLR-2 results in the production of proinflammatory cytokines and up-regulation of co-stimulatory molecules and antigen presentation, thereby leading to priming of the ensuing adaptive immune response [29]. TLR-2 recognizes microbial components of P. acnes and M. tuberculosis, both possible causative agents for sarcoidosis, and is therefore of interest when postulating a role for an altered innate immune response in the pathogenesis of this disease.
The results did not show an association between TLR-2 polymorphisms Pro631His or Arg753Gln and disease susceptibility or influence on disease course in Dutch sarcoidosis patients. A major role for these functional polymorphisms therefore seems unlikely; however, in order to exclude definitively a role for these genetic variants, studies including greater number of patients together with cohorts of non-Caucasian ethnicity are required. Pertaining to polymorphism −16934 A/T, we found a significantly higher prevalence of the −16934-AA genotype in patients with chronic disease compared to patients with acute/self-remitting disease in the study cohort, partially confirming our hypothesis. To establish the importance of this genetic association, an unrelated validation cohort of 254 Dutch sarcoidosis patients was also genotyped. The allelic distribution of this polymorphism in both patients with acute/self-remitting disease and chronic disease were identical and comparable with the healthy controls (Fig. 1). These results make it unlikely that TLR-2 genetics plays a major role in sarcoidosis pathogenesis. On the other hand, however, assuming that sarcoidosis has multiple causative agents, it could be that TLR-2 agonists are triggering agents in only a small fraction of the entire population of Dutch patients, making it difficult to compare results from small independent patients groups. An elegant way to overcome this problem could be to subdivide patients by causative agents. In light of the cellular recognition of M. tuberculosis and P. acnes antigens in some sarcoidosis patients [30,31], future studies involving TLR-2 genetics in these particular subgroups should reveal more accurate data.
The present study also revealed a strong linkage between the promotor polymorphism and the GT repeat polymorphism in intron 1. Based on this linkage disequilibrium, the difference in allelic distribution of the GT repeat polymorphism between acute/self-remitting and chronic sarcoidosis found in the study cohort could not be confirmed in the validation cohort. The allelic distribution found in the control population was almost identical with the distribution found in a Caucasian–American cohort [32], reflecting the reliability of our method. The linkage between the TLR-2 promotor polymorphism −16934 A/T and the intron 1 GT repeat polymorphism is of interest not only in sarcoidosis research, but for all research focusing on one of these polymorphisms. A major question is to what extent do these two polymorphisms have functional implications on TLR-2 receptor expression and function? So far, no data are available on the functional implications of the −16934 A/T promotor polymorphism. Based on the location of this polymorphism far upstream in the promotor region and the lack of functional evidence of other distant promotor polymorphisms in the literature, functional implications seem unlikely. Two papers regarding the influence of the number of GT intron repeats on TLR-2 expression from the same group have been published, demonstrating strong conflicting results [15,32]. However, for other genes with variable number of tandem repeats it has been demonstrated that length variation in the number of repeats can influence transcription [33,34].
Therefore, based on the discrepant genetic data on the distribution of TLR-2 polymorphisms in two different cohorts of Dutch sarcoidosis patients and the indistinctness regarding their functional implications, we investigated a possible influence on TLR-2 expression and function. After stimulation with PamCys, the most specific TLR-2 agonist, we found a correlation between TNF-α production as well as IL-6 production and the number of GT repeats. After stimulation with P. acnes, which stimulates not only TLR-2 but also many other innate immune receptors, the same trend could still be observed regarding TNF-α production. Furthermore, stimulation with PamCys resulted in limited amounts of IL-12 production related to the number of GT repeats, although not statistically significant. P. acnes, however, is a more potent inducer of IL-12 compared with the peptide PamCys, and with this stimulus a significant correlation between IL-12 production and the number of GT repeats was found. We did not find a correlation between the number of GT repeats and the production of prototype T helper 1 (Th1) (IFN-γ) or Th2 (IL-4) cytokines. Toll-like receptors are associated predominantly with the production of proinflammatory cytokines, possibly explaining the absence of a correlation between production of specific Th cell-associated cytokines and number of GT repeats.
The number of GT repeats is not the only genetic variant in TLR-2 that influences cytokine production upon stimulation. Underhill and colleagues showed that the presence of TLR-2 polymorphism Pro631His results in a fivefold reduction of TNF-α production upon stimulation with M. tuberculosis[24]. Although using PamCys instead of M. tuberculosis, when we grouped our data according to −16934 T allele carriage we found a ninefold increase of TNF-α production in the group without a T allele (Fig. 7), indicating the importance of our findings.
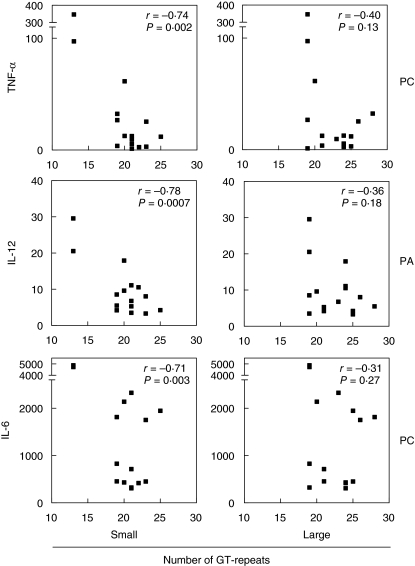
An obvious mechanism of how a variable number of GT repeats might affect TLR-2 function remains unknown. In our functional analysis we used the mean number of GT repeats per donor, based on the assumption that possible influences on TLR-2 function by a variable number of GT repeats would follow a linear relation. However, it remains to be seen if the impact of an allele with a small number of GT repeats in combination with an allele with a large number of GT repeats equals that of a combination consisting of two ‘medium-sized GT alleles’. For example, when the induced amounts of TNF-α, IL-12 or IL-6 were plotted against the presence of either the allele with the smallest number of GT repeats per donor or the allele containing the largest number of GT repeats per donor, we found correlations only in the first group (Fig. 8). The observed correlations using the smallest alleles proved to be stronger than the correlations using the mean number of GT repeats. This might be an indication that the presence of a small number of GT repeats could be dominant over the presence of a large number of GT repeats, presenting an interesting viewpoint for further studies.
Fig. 8.
Observed differences in correlation between the smallest and the largest number of Toll-like receptor 2 (TLR-2) intron 1 GT repeats per donor and cytokine induction (pg/ml) after stimulation with TLR-2 agonists. PC = stimulated with Pam3Cys-Ser-Lys4, PA = stimulated with Propionibacterium acnes. TNF-α = tumour necrosis factor-alpha, IL-12 = interleukin 12, IL-6 = interleukin 6. Small = per donor the smallest number of GT repeats present was selected for analysis. Large = per donor the largest number of GT repeats present was selected for analysis.
The genetic data from the study cohort, together with our functional data on cytokine induction, fitted the current model of sarcoidosis pathogenesis. TNF-α is important in the initiation and perpetuation of inflammation in sarcoidosis, contributing to progressive disease with or without development of fibrosis. Various clinical trials have demonstrated that blocking TNF-α using antagonists such as infliximab or etanercept [35] results in clinical improvement and reduces the requirement of corticosteroids. The amount of IL-12, the main macrophage-derived molecule involved in initiating a Th1 response, and IL-6, known for making T cells insensitive for suppression by regulatory T cells [36], are also correlated with persisting disease activity [37–40]. In our study cohort we found that in patients with chronic disease the prevalence of the TLR-2 −16934 AA genotype was significantly higher compared to patients with acute/self-remitting disease. It was also demonstrated that the AA genotype is linked to a significantly lower amount of TLR-2 intron GT repeats, implicating a higher amount of TNF-α, IL-6 and IL-12 production upon stimulation with TLR-2 agonists (Fig. 7). We speculate that patients with the −16934 AA genotype, based on the linkage with a low number of intron 1 GT repeats, produce higher amounts of these cytokines during continuous innate immunity stimulation by a persisting antigen, compared to patients with either the AT or TT genotype. This will increase their risk of developing chronic disease.
In summary, we found a difference in allelic distribution of a single nucleotide polymorphism in the promotor region of TLR-2 between sarcoidosis patients with acute/self-remitting disease and patients with chronic disease. However, this genetic association could not be confirmed in an independent cohort of sarcoidosis patients, suggesting a possible role in only a small percentage of patients. Furthermore, this promotor polymorphism is linked with the number of GT repeats in intron 1. The number of these GT repeats appears to influence the induction of TNF-α, IL-6 and IL-12 upon interaction with TLR-2 agonists, partly explaining the variation in cytokine pattern observed in different sarcoidosis phenotypes. Further genetic as well as functional studies are therefore required to confirm and explain these observations.
Acknowledgments
The authors would like to thank Mrs H. Alpar-Kili, Mr J. Broess (Department of Clinical Chemistry, St Antonius Hospital), Mrs D. van Ballegoy, Mrs A van Heugten-Roeling, Mr B. de Jong (Department of Medical Immunology and Microbiology, St Antonius Hospital), Mrs A. van Grootheest and Mrs K. Scheidel-Jacobse (Department of Pathology, St Antonius Hospital) for expert technical assistance. Mrs H. van Velzen-Blad and Dr B. de Jongh (Department of Medical Immunology and Microbiology, St Antonius Hospital) are acknowledged for valuable advice during the design of the study. This study was supported by an unrestricted grant from AstraZeneca®.
References
- 1.Dubaniewicz A, Kampfer S, Singh M. Serum anti-mycobacterial heat shock protein antibodies in sarcoidosis and tuberculosis. Tuberculosis (Edinb) 2006;86:60–7. doi: 10.1016/j.tube.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Fite E, Fernandez-Figueras MT, Prats R, Vaquero M, Morera J. High prevalence of Mycobacterium tuberculosis DNA in biopsies from sarcoidosis patients from Catalonia, Spain. Respiration. 2006;73:20–6. doi: 10.1159/000087688. [DOI] [PubMed] [Google Scholar]
- 3.Gazouli M, Ikonomopoulos J, Trigidou R, Foteinou M, Kittas C, Gorgoulis V. Assessment of mycobacterial, propionibacterial, and human herpesvirus 8 DNA in tissues of Greek patients with sarcoidosis. J Clin Microbiol. 2002;40:3060–3. doi: 10.1128/JCM.40.8.3060-3063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song Z, Marzilli L, Greenbee BM, et al. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med. 2005;201:755–67. doi: 10.1084/jem.20040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198–204. doi: 10.1128/JCM.40.1.198-204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley PJ, Mullighan CG, McGrath DS, et al. Mannose-binding lectin promoter and structural gene variants in sarcoidosis. Eur J Clin Invest. 2000;30:549–52. doi: 10.1046/j.1365-2362.2000.00656.x. [DOI] [PubMed] [Google Scholar]
- 7.Ho LP, Merlin F, Gaber K, Davies RJ, McMichael AJ, Hugot JP. CARD 15 gene mutations in sarcoidosis. Thorax. 2005;60:354–5. doi: 10.1136/thx.2004.036871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milman N, Nielsen OH, Hviid TV, Fenger K. CARD15 single nucleotide polymorphisms 8, 12 and 13 are not increased in ethnic Danes with sarcoidosis. Respiration. 2007;74:76–9. doi: 10.1159/000090638. [DOI] [PubMed] [Google Scholar]
- 9.Tanabe T, Ishige I, Suzuki Y, et al. Sarcoidosis and NOD1 variation with impaired recognition of intracellular Propionibacterium acnes. Biochim Biophys Acta. 2006;1762:794–801. doi: 10.1016/j.bbadis.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Striz I, Zheng L, Wang YM, Pokorna H, Bauer PC, Costabel U. Soluble CD14 is increased in bronchoalveolar lavage of active sarcoidosis and correlates with alveolar macrophage membrane-bound CD14. Am J Respir Crit Care Med. 1995;15:544–7. doi: 10.1164/ajrccm.151.2.7531099. [DOI] [PubMed] [Google Scholar]
- 11.Gazouli M, Koundorakis A, Ikonomopoulos J, et al. CARD15/NOD2, CD14, and Toll-like receptor 4 gene polymorphisms in Greek patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:23–9. [PubMed] [Google Scholar]
- 12.Marks DJ, Harbord MW, MacAllister R, et al. Defective acute inflammation in Crohn's disease: a clinical investigation. Lancet. 2006;367:668–78. doi: 10.1016/S0140-6736(06)68265-2. [DOI] [PubMed] [Google Scholar]
- 13.Bafica A, Scange CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202:1715–24. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogus AC, Yoldas B, Ozdemir T, et al. The Arg753GLn polymorphism of the human Toll-like receptor 2 gene in tuberculosis disease. Eur Respir J. 2004;23:219–23. doi: 10.1183/09031936.03.00061703. [DOI] [PubMed] [Google Scholar]
- 15.Yim JJ, Lee HW, Lee HS, et al. The association between microsatellite polymorphisms in intron II of the human Toll-like receptor 2 gene and tuberculosis among Koreans. Genes Immun. 2006;7:150–5. doi: 10.1038/sj.gene.6364274. [DOI] [PubMed] [Google Scholar]
- 16.Pabst S, Baumgarten G, Stremmel A, et al. Toll-like receptor (TLR) 4 polymorphisms are associated with a chronic course of sarcoidosis. Clin Exp Immunol. 2006;143:420–6. doi: 10.1111/j.1365-2249.2006.03008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veltkamp M, Grutters JC, van Moorsel CH, Ruven HJ, van den Bosch JM. Toll-like receptor (TLR) 4 polymorphism Asp299Gly is not associated with disease course in Dutch sarcoidosis patients. Clin Exp Immunol. 2006;145:215–18. doi: 10.1111/j.1365-2249.2006.03127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202:1715–24. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romics L, Jr, Dolganiuc A, Velayudham A, et al. Toll-like receptor 2 mediates inflammatory cytokine induction but not sensitization for liver injury by Propionibacterium acnes. J Leukoc Biol. 2005;78:1255–64. doi: 10.1189/jlb.0804448. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Komai-Koma M, Xu D, Liew FY. Toll-like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci USA. 2006;103:7048–53. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyara M, Amoura Z, Parizot C, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006;203:359–70. doi: 10.1084/jem.20050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grutters JC, Sato H, Pantelidis P, et al. Analysis of IL6 and IL1A gene polymorphisms in UK and Dutch patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:20–7. [PubMed] [Google Scholar]
- 23.Ogus AC, Yoldas B, Ozdemir T, et al. The Arg753GLn polymorphism of the human Toll-like receptor 2 gene in tuberculosis disease. Eur Respir J. 2004;23:219–23. doi: 10.1183/09031936.03.00061703. [DOI] [PubMed] [Google Scholar]
- 24.Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96:14459–63. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bunce M, O'Neill CM, Barnardo MC, et al. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355–67. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- 26.de Weger RA, Tilanus MG, Scheidel KC, van den Tweel JG, Verdonck LF. Monitoring of residual disease and guided donor leucocyte infusion after allogeneic bone marrow transplantation by chimaerism analysis with short tandem repeats. Br J Haematol. 2000;110:647–53. doi: 10.1046/j.1365-2141.2000.02233.x. [DOI] [PubMed] [Google Scholar]
- 27.Richter E, Weizenegger M, Rusch-Gerdes S, Niemann S. Evaluation of genotype MTBC assay for differentiation of clinical Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2003;41:2672–5. doi: 10.1128/JCM.41.6.2672-2675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muzio M, Bosisio D, Polentarutti N, et al. Differential expression and regulation of Toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 29.Hopkins PA, Sriskandan S. Mammalian Toll-like receptors: to immunity and beyond. Clin Exp Immunol. 2005;140:395–407. doi: 10.1111/j.1365-2249.2005.02801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drake WP, et al. Cellular recognition of Mycobacterium ESAT-6 and katG peptides in systemic sarcoidosis. Infect Immun. 2007;75:527–30. doi: 10.1128/IAI.00732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebe Y, Ikushima S, Yamaguchi T, et al. Proliferative response of peripheral blood mononuclear cells and levels of antibody to recombinant protein from Propionibacterium acnes DNA expression library in Japanese patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:256–65. [PubMed] [Google Scholar]
- 32.Yim JJ, Ding L, Schaffer AA, Park GY, Shim YS, Holland SM. A microsatellite polymorphism in intron 2 of human Toll-like receptor 2 gene: functional implications and racial differences. FEMS Immunol Med Microbiol. 2004;40:163–9. doi: 10.1016/S0928-8244(03)00342-0. [DOI] [PubMed] [Google Scholar]
- 33.Denschlag D, Marculescu R, Unfried G, et al. The size of a microsatellite polymorphism of the haem oxygenase 1 gene is associated with idiopathic recurrent miscarriage. Mol Hum Reprod. 2004;10:211–14. doi: 10.1093/molehr/gah024. [DOI] [PubMed] [Google Scholar]
- 34.Roberts J, Scott AC, Howard MR, et al. Differential regulation of the serotonin transporter gene by lithium is mediated by transcription factors, CCCTC binding protein and Y-box binding protein 1, through the polymorphic intron 2 variable number tandem repeat. J Neurosci. 2007;27:2793–801. doi: 10.1523/JNEUROSCI.0892-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baughman RP, Iannuzzi M. Tumour necrosis factor in sarcoidosis and its potential for targeted therapy. BioDrugs. 2003;17:425–31. doi: 10.2165/00063030-200317060-00005. [DOI] [PubMed] [Google Scholar]
- 36.Detournay O, Mazouz N, Goldman M, Toungouz M. IL-6 produced by type I IFN DC controls IFN-gamma production by regulating the suppressive effect of CD4+ CD25+ regulatory T cells. Hum Immunol. 2005;66:460–8. doi: 10.1016/j.humimm.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Kim DS, Jeon YG, Shim TS, et al. The value of interleukin-12 as an activity marker of pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:271–6. [PubMed] [Google Scholar]
- 38.Shigehara K, Shijubo N, Ohmichi M, et al. Increased circulating interleukin-12 (IL-12) p40 in pulmonary sarcoidosis. Clin Exp Immunol. 2003;132:152–7. doi: 10.1046/j.1365-2249.2003.02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bihl MP, Laule-Kilian K, Bubendorf L, et al. Progressive pulmonary sarcoidosis − a fibroproliferative process potentially triggered by EGR-1 and IL-6. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:38–50. [PubMed] [Google Scholar]
- 40.Takizawa H, Satoh M, Okazaki H, et al. Increased IL-6 and IL-8 in bronchoalveolar lavage fluids (BALF) from patients with sarcoidosis: correlation with the clinical parameters. Clin Exp Immunol. 1997;107:175–81. doi: 10.1046/j.1365-2249.1997.d01-905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]