Abstract
IgM is one major type of B cell receptor (BCR) expressed on most of the B cells from immature to mature stages. During normal B cell ontogeny, signals transduced through the IgM BCR play an important role in regulating B cell maturation and survival at multiple checkpoints. In addition, IgM BCR is also required for antigen-dependent differentiation and activation of B cells. However, whether IgM BCR-mediated signalling is important for the pathogenesis of autoimmune diseases remains elusive. Using IgM-deficient mice, we examined the effect of absence of IgM on the development of collagen-induced arthritis (CIA), an animal model of rheumatoid arthritis (RA). Compared to their wild-type littermates, IgM-deficient mice were either resistant to arthritis induction or developed significantly less severe arthritis. There was a significant decrease of autoantibody production in IgM-deficient mice, particularly IgG2a antibodies, which is believed to be pathogenic in CIA. Thus, although IgM−/− mice have relatively normal B cell development with IgD BCR replacing IgM BCR, the absence of IgM-mediated signals has a profound impact on the development of CIA, indicating that IgM plays an important role in the development and pathogenesis of autoimmune arthritis and IgM-mediated signalling is critical in the generation of pathogenic autoreactive antibodies.
Keywords: arthritis, autoantibodies, autoimmunity, IgM
Introduction
During B cell development, the expression of IgM and IgD is tightly regulated and controlled. Newly generated B cells in the bone marrow express IgM, but not IgD, on their surface, whereas the vast majority of mature peripheral B cells co-express IgM and IgD. IgD expression is first up-regulated in transitional B cells in the spleen at the T2 stage; then the expression of IgD increases as B cells differentiate towards the mature stage [1]. Marginal-zone B cells express high levels of IgM but down-regulate IgD on the surface [2,3]. After activation, B cells rapidly down-regulate the expression of IgD, but not IgM [4,5]. To date, the biological function of the dual expression of IgM and IgD by most B cells and the differential expression of IgM and IgD during B cell development and differentiation is not fully understood.
It is now clear that B cells play an important role in some autoimmune diseases, including autoimmune arthritis. The presence of high levels of autoantibodies is a characteristic of rheumatoid arthritis (RA). Most patients with RA develop high titres of IgM- and IgG-types of rheumatoid factors (RF) [6], which are considered to be pathogenic. However, by studying mice deficient for secreted IgM, a recent report indicates that IgM autoantibodies may play a protective role in autoimmune diseases, as the absence of IgM antibodies accelerates the development of IgG autoantibodies and glomerulonephritis in lpr mice [7]. In the current study, we explored the roles of IgM in the development and progression of collagen-induced arthritis (CIA) using IgM-deficient mice that lack both membrane and secreted IgM but exhibit normal B cell development [8]. Interestingly, the results showed that IgM−/− mice were resistant to CIA induction compared to their wild-type littermates. The production of IgG2a antibodies was reduced significantly in IgM−/− mice. Taken together, IgM as secreted antibodies and as B cell receptors (BCRs) may play distinct roles in autoimmune responses.
Materials and methods
Mice
IgM-deficient mice [9] were back-crossed to DBA/1 background for six generations. Wild-type littermates were used as controls in all the experiments. All the mice were housed in autoclaved micro-isolators, provided with sterile bedding, food and water and maintained on a 12-h day/night cycle. Animal experimentation was performed in accordance with protocols approved by the Animal Research Committee of Baylor College of Medicine.
Induction of CIA and evaluation of arthritis
Mice were immunized with bovine type II collagen (CII) to induce CIA, as described previously [10]. Briefly, mice were injected subcutaneously (s.c.) at the base of the tail with 100 µg (in 100 µl) bovine CII (Sigma Chemical Co., St Louis, MO, USA) dissolved in 0.01 M acetic acid and emulsified in an equal volume of complete Freund's adjuvant (CFA) (Sigma). Three weeks after primary immunization, the mice were challenged intraperitoneally (i.p.) with 100 µg bovine CII/incomplete Freund's adjuvant (IFA). The scoring system was based on the degree of swelling and periarticular erythema. The scores of all four paws were summed to yield the arthritis index [10]. Clinical arthritis was assessed using the following scoring system: grade 0 = no swelling, grade 1 = paws with swelling in a single digit, grade 2 = paws with swelling in multiple digits and grade 3 = severe swelling and joint rigidity. Each limb was graded, and the maximum possible score per mouse was 12. Anteroposterior radiographs of the four limbs were obtained using a Faxitron machine. Each limb was assessed for the presence of periarticular osteopenia, bone erosion ankylosis and deformity, with a score range of 0–4 (0 = no involvement, 4 = extensive involvement comprising bone mineralization, erosion, periostitis, cartilage space loss, soft tissues, alignment changes and associated degenerative changes).
Detection of autoreactive antibodies
CII, dsDNA or histone-specific antibodies in mouse sera were determined by enzyme-linked immunosorbent assay (ELISA). Briefly, microplates were coated with bovine CII, dsDNA or histone overnight, respectively, and then blocked with 10% fetal calf serum (FCS). Samples were added and incubated for 1 h at 37°C and washed. Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG1 and IgG2a (Southern Biotechnology Associates, Birmingham, AL, USA) were used as secondary detection reagents. RF activity was measured using a modified ELISA method [11]. Plates were coated with NIP [(4-hydroxy-5-iodo-3-nitrophenyl)acetyl]-bovine serum albumin (BSA) (20 µg/ml). A NIP-specific monoclonal antibody (mAb) (IgG/λ) was added and incubated for 1 h, and bound anti-NIP mAb served as ligands for RF binding. Samples were then incubated at 4°C overnight and RFs bound to plates were detected by anti-mouse κ-HRP. HRP activity was visualized using a TMB peroxidase substrate kit (Bio-Rad Laboratories, Hercules, CA, USA) and optical densities were determined at 450 nm using an automated reader. CII-specific antibody titres were determined using pooled serum from CIA mice as standard on each plate.
Joint cell preparation and flow cytometry
Cells from inflamed joints of DBA/1 mice were isolated at peak time of CIA. Briefly, after the skin and muscles were removed, cells in the joints were flushed and collected. Cells were washed with phosphate-buffered saline (PBS)/0.08% sodium azide and first incubated with anti-CD16/CD32 antibody to block FcγR-mediated binding. Cells were then stained with antibodies to B220, CD19, CD138, IgM, IgG1 and IgG2a/2b (BD PharMingen, San Jose, CA, USA) and analysed using fluorescence activated cell sorter analysis (FACScalibur; Becton Dickinson, SanJose, CA, USA).
T cell priming and antigen-specific proliferation
Mice were immunized with either 100 µg bovine CII or chicken γ-globulin (CGG) emulsified in CFA. One week later, draining (inguinal) lymph nodes (LNs) were taken and single-cell suspension prepared; then 5 × 104 cells/well were cultured in 96-well flat-bottomed plates with various concentrations of CII or CGG for 3 days. Cells were pulsed with [3H]-thymidine (1 µCi/well) for the last 18 h and incorporation of [3H]-thymidine was measured with an LKB 1205 Betaplate liquid scintillation counter (Wallac, Gaithersburg, MD, USA).
Statistical analysis
Student's t-test was used to determine the significance of differences in means. A value of P < 0.05 was considered statistically significant.
Results
IgM-deficient mice are resistant to CIA induction
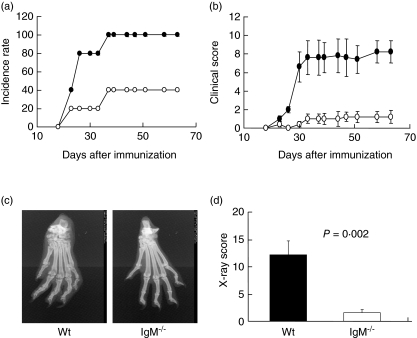
To investigate the role of IgM in the development and pathogenesis of CIA, we induced CIA in IgM−/− mice and wild-type littermates with CII and monitored the development and progression of the disease. Our results showed that IgM−/− mice were resistant to CIA induction. We observed a delayed onset and decreased incidence of articular disease in IgM−/− mice compared with wild-type mice (Fig. 1a). By day 40 after immunization all the wild-type mice had developed arthritis, whereas only about 40% of the IgM−/− mice developed the disease. The majority (60%) of the IgM−/− animals never developed arthritis when the experiment was completed at day 63 after primary immunization. In addition, among the IgM−/− mice that developed articular inflammation, the severity of the disease was reduced significantly (Fig. 1b). This reduced disease severity was sustained throughout the chronic phase of the disease. Thus, our results indicate the that lack of IgM-mediated signalling not only suppresses the initiation of arthritis, but also attenuates the progression of the disease. We further examined the joint morphology by X-ray. The representative photographs of arthritic joints from control wild-type and IgM−/− mice are shown in Fig. 1c. Marked joint destruction with severe disfiguration, osteolysis and osteophyte production can be observed in the paws of wild-type mice; whereas the paws of IgM−/− mice exhibited relative normal structure at this late phase of the disease. Consistent with clinical scores, the X-ray scores of joint pathology in IgM−/− mice were minimal compared to that of wild-type mice (Fig. 1d).
Fig. 1.
IgM-deficient mice were resistant to collagen-induced arthritis (CIA) induction. IgM-deficient mice and wild-type littermates were immunized with bovine type II collagen in CFA on days 0 and boosted on day 21. Mice were examined for the development (a) and severity (b) of arthritis. Disease severity was scored by visual inspection of paws on a scale of 0–3 as indicated in Methods. Data [mean ± standard error (s.e.)] are representative of two independent experiments with eight to 10 mice in each group. (c) Representative pathological changes of arthritic paws revealed by X-ray. A hind paw from a wild-type control mouse shows severe disfiguration, osteolysis and osteophyte production, whereas a hind paw from an IgM-deficient mouse shows relatively normal structure. (d) X-ray scores (mean ± s.e.) of wild-type (black bar) or IgM mutant (open bar) mice at day 63 post-primary immunization.
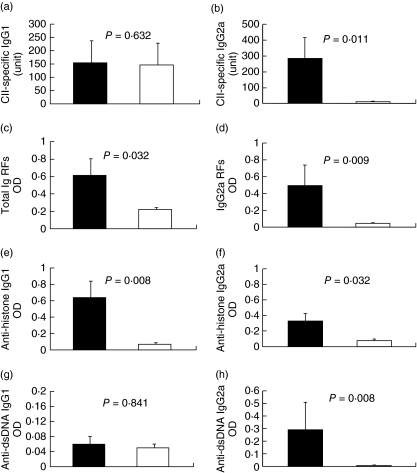
Production of CII-specific IgG2a antibodies was reduced significantly in IgM−/− mice
Because it has been shown that anti-CII antibodies play an important role in the pathogenesis of CIA [12–14], we determined whether the absence of IgM-mediated signalling affected the levels of other isotypes of CII-specific antibodies. The serum levels of IgG1 and IgG2a CII-specific antibodies were evaluated by ELISA. Our results showed that wild-type and IgM−/− mice produced comparable levels of CII-specific IgG1 antibodies (Fig. 2a). However, there was a dramatic reduction of CII-specific IgG2a antibodies in IgM−/− mice compared with wild-type controls (Fig. 2b). As it is known that, in mice, antibodies of IgG2a isotype are the most efficient antibodies to mediate effector functions such as activating complement [15–17], reduced IgG2a antibody production is likely to be one of the mechanisms contributing to the decreased disease pathology in IgM−/− mice.
Fig. 2.
Decreased production of autoantibodies in IgM-deficient mice. Six weeks after secondary immunization with CII, sera from wild-type (black bars) or IgM-deficient (open bars) mice were tested for autoreactive antibody production. The levels of CII-specific IgG1 (a) or IgG2a (b) antibodies were measured by enzyme-linked immunosorbent assay against a standard serum. Levels of rheumatoid factors (c, d), anti-histone (e, f) or anti-dsDNA (g, h) antibodies were expressed as OD value at 1:100 dilution. Data (mean ± standard error) are representative of two independent experiments with eight to 10 mice in each group. P-values are indicated for each type of autoreactive antibodies between the two groups.
Production of rheumatoid factors (RFs) and other autoreactive antibodies was also diminished in IgM−/− mice
To characterize further the autoimmune mechanisms underlying CIA resistance in IgM−/− mice, we investigated whether there was a general reduction of antibodies to other self-components, including IgG (RFs), dsDNA and histone. We found that there was a significant reduction in the levels of total RFs and IgG2a type RFs in IgM−/− mice (Fig. 2c,d). Remarkably, IgG2a antibodies specific for histone and dsDNA were also decreased to a level that was barely detectable (Fig. 2f,h). In addition, anti-histone IgG1 antibodies were also reduced in IgM−/− mice (Fig. 2e). Thus, our findings demonstrated that, in IgM−/− mice, production of autoreactive antibodies, particularly IgG2a antibodies, was generally diminished.
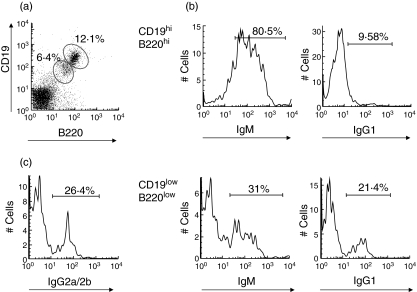
High numbers of IgM-expressing B cells and IgM-producing plasma cells are present in the arthritic joints
It is known that the contribution of B cells to autoimmune arthritis is far beyond autoantibody production. B cells may exert their role via antigen presentation and cytokine production at the inflammatory sites. To understand the significance of IgM BCR-mediated signals in the affected joints, we examined the frequency of IgM-expressing B cells in the inflamed joints of wild-type mice with CIA. The infiltrating cells in the joints of wild-type DBA/1 mice with CIA were isolated and analysed by flow cytometry. The results showed that B cells in the arthritic joints of wild-type mice can be divided into two populations: CD19highB220high and CD19lowB220low cells (Fig. 3a). It is known that plasma cells down-regulate CD19 and B220. Indeed, these CD19lowB220low cells are mainly CD138+ (data not shown). Surprisingly, most of the B cells infiltrating in the joints express IgM. When CD19highB220high B cells were gated, vast majority of them were IgM+ and only ∼10% were IgG1+ (Fig. 3b). However, in the CD19lowB220low plasma cells, only one-third expressed IgM and about 20% express IgG1 (Fig. 3b). These results suggest that most of the B cells that migrated into joints had not undergone class switching. Predominant class switching may occur locally in the joints during the inflammatory response, rather than in lymphoid tissues. Thus, the presence of high numbers of IgM positive B cells and IgM-producing plasma cells may suggest a role of IgM as membrane BCR and antibodies in this chronic inflammation. In addition, a substantial number of IgG2a/2b-expressing plasma cells are present in the arthritic joints (Fig. 3c), which supports the importance of IgG2a in the pathogenesis of CIA.
Fig. 3.
IgM+ B cells are largely present in the arthritic joints. Joint cells were flushed from arthritic joints of DBA/1 mice at day 20 after secondary immunization. (a) The percentages of CD19high B220high and CD19low B220low cells in lymphocyte gate were shown. (b) CD19high B220high and CD19low B220low cells were gated and further analysed for expression of IgM and IgG1. (c) B220lowCD138+ cells were gated and analysed for IgG2a/2b expression.
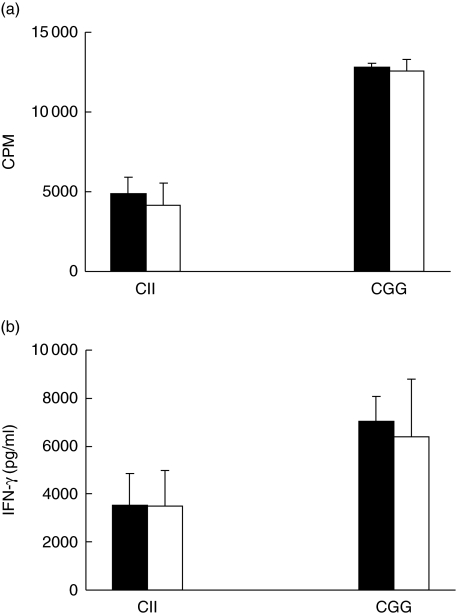
Normal T cell priming and cytokine production in IgM−/− mice
Because T helper 1 (Th1) type and/or proinflammatory cytokines such as interferon (IFN)-γ play a dominant role in the pathogenesis of RA and CIA, and tipping the balance between Th1/Th2 cytokines will affect the development and outcome of the disease [18–21], we examined whether T cell activation and Th1/Th2 cytokine production was altered in the absence of IgM. Our results showed that CII-specific proliferation of draining LN cells was similar between IgM−/− mice and wild-type controls (Fig. 4a). Production of IFN-γ was comparable between LN cells from IgM−/− mice and wild-type controls (Fig. 4b). The levels of IL-4 or IL-10 were very low (< 31 pg/ml) in cell cultures from either wild-type or IgM−/− mice (data not shown). Similarly, when mice were immunized with CGG, cellular proliferation and IFN-γ production from draining LN cells of wild-type and IgM−/− mice were not significantly different (Fig. 4a,b). We obtained similar results with either CFA or RIBI as adjuvant, indicating that lack of IgM-mediated signalling does not affect T cell priming in vivo.
Fig. 4.
Comparable T cell priming and interferon (IFN)-γ production between IgM-deficient and wild-type mice. Wild-type (black bars) or IgM-deficient (open bars) mice were immunized with CII or chicken γ-globulin (CGG). Draining lymph node cells were isolated 7 days after immunization and stimulated with 10 µg/ml CII or CGG for 3 days. (a) Antigen-specific cellular proliferation in vitro. Cells were harvested 72 h later in the presence of [3H]-thymidine for the last 18 h. (b) IFN-γ production in supernatant was analysed by enzyme-linked immunosorbent assay at 3 days after in vitro re-stimulation with antigen. Data (mean ± standard error) are representative of three independent experiments with three to six mice in each group.
Discussion
To explore the role of IgM in the development and pathogenesis of autoimmune arthritis, we have investigated CIA induction and disease outcome in IgM-deficient mice in which B cell development and maturation proceed normally, with IgD replacing IgM [8]. Here we report that IgM-deficient mice exhibited significantly reduced incidence of CIA and attenuated disease severity. In IgM−/− mice, not only was the incidence rate of joint inflammation suppressed, but also the degree of arthritogenic responses was decreased, suggesting that IgM deficiency inhibits both the initiation and progression of the disease.
Because B cells exert their immunological functions through acting as direct effector cells to produce antibodies, and/or as regulatory cells to modulate immune responses, IgM-mediated signals may mediate the arthritogenic response by modulating generation of autoreactive antibodies, cytokine production and antigen presentation. In the current study, the first apparent abnormality of IgM deficient mice is that they do not produce IgM antibodies. Production of high levels of autoantibodies is associated with many autoimmune diseases [6]. In rheumatoid arthritis (RA), more than 70% of the patients develop high titres of serum IgM and IgG RFs. However, a recent study has shown that lpr mice in the absence of secreted IgM develop elevated levels of IgG autoantibodies and more severe glomerulonepritis, indicating that IgM autoantibodies may lessen the severity of autoimmune pathology [7]. The authors postulated that the overall levels of autoreactive IgM and/or its local concentration may determine which pathway the immune complexes may follow. In the absence of autoreactive IgM, autoantibody response may be promoted because autoantigens are not cleared efficiently, such as in the secreted IgM-deficient mice [7]. If, indeed, the secreted IgM autoantibodies play a feedback or protective role in CIA, then the resistance of IgM−/− mice, which lack both membrane IgM and secreted IgM, to CIA induction may be predominantly caused by the absence of membrane IgM-mediated signalling. Thus, our current study, together with the previous report showing IgM autoantibody as a protective mechanism [7], suggest that membrane IgM and secreted IgM may play very distinct roles in regulating autoimmune responses.
One of the major deficiencies in IgM-deficient mice is that, in contrast to the elevation of IgG autoantibodies in lpr mice that lack secreted IgM, there is a significant reduction in production of antibodies to various self-components, particularly IgG2a autoantibodies. Because IgG2a autoantibodies are considered pathogenic in autoimmune diseases, including human RA and murine CIA, diminished production of IgG2a autoantibodies is at least partially responsible for the attenuated CIA in IgM-deficient mice.
It is known that B cells play multiple roles in autoimmune arthritis, far beyond autoantibody production [22]. As activated B cells are among the most efficient antigen-presenting cells (APCs), another possible mechanism to explain the resistance to CIA in IgM−/− mice is that lack of IgM-mediated signals may influence the T cell response. However, our data demonstrated that antigen-specific proliferation of T cells from IgM mutant mice was comparable to that of T cells from wild-type mice. In addition, production of IFN-γ was similar between T cells from IgM-deficient mice and wild-type controls. Similar results were obtained from experiments with either self-antigen (CII) or conventional antigen (CGG) in CFA or RIBI. Therefore, modulation of T cell activation and function is unlikely to contribute to the disease resistance in IgM mutant mice under the current immunization protocol.
To understand the significance of IgM BCR-mediated signals at the inflammatory sites, we further examined the frequency of IgM+ B cells in the inflamed joints. Interestingly, we found that in wild-type mice, the infiltrating cells in the arthritic joints contained a large percentage of IgM+ B cells. Most CD19high and B220high B cells in the joints express IgM. In CD19lowB220low plasma cells, the percentage of IgM+ cells reduced significantly. This observation suggests that plasma cell differentiation and class switching may take place significantly in the inflammatory sites. Because the rheumatoid synovitis is associated with the formation of complex lymphoid microstructures, such as lymphoid follicles with germinal centre (GC) reaction [23], the microenvironment in the inflamed joints may support B cell differentiation towards plasma cells and memory cells. Therefore, when permitted, activated or even naive IgM+ B cells migrate into the joints, along with other mononuclear cells, and participate in the local inflammatory response. During this process, by interacting with T cells or other cells, B cells undergo differentiation as in the lymphoid tissues. In IgM−/− mice, IgD expressing only B cells may not have the same properties to function in a similar manner to IgM-expressing B cells.
Taken together, the absence of IgM-mediated signals renders the mice resistant to CIA induction. One of the mechanisms is perhaps the reduced production of IgG2a autoantibodies in IgM−/− mice. In addition, lack of membrane IgM may also contribute to the outcome, which is supported by the presence of large numbers of IgM-expressing B cells at the local inflammatory sites.
Acknowledgments
This work was supported by National Institute of Health grant AI051532 and AI055554 (to S. Han).
References
- 1.Loder F, Mutschler B, Ray RJ, et al. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J Exp Med. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliver AM, Martin F, Gartland GL, Carter RH, Kearney JF. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol. 1997;27:2366–74. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- 3.Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999;162:7198–207. [PubMed] [Google Scholar]
- 4.Bourgois A, Kitajima K, Hunter IR, Askonas BA. Surface immunoglobulins of lipopolysaccharide-stimulated spleen cells. The behavior of IgM, IgD and IgG. Eur J Immunol. 1977;7:151–3. doi: 10.1002/eji.1830070307. [DOI] [PubMed] [Google Scholar]
- 5.Monroe JG, Havran WL, Cambier JC. B lymphocyte activation: entry into cell cycle is accompanied by decreased expression of IgD but not IgM. Eur J Immunol. 1983;13:208–13. doi: 10.1002/eji.1830130306. [DOI] [PubMed] [Google Scholar]
- 6.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–10. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 7.Boes M, Schmidt T, Linkemann K, Beaudette BC, Marshak-Rothstein A, Chen J. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci USA. 2000;97:1184–9. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutz C, Ledermann B, Kosco-Vilbois MH, et al. IgD can largely substitute for loss of IgM function in B cells. Nature. 1998;393:797–801. doi: 10.1038/31716. [DOI] [PubMed] [Google Scholar]
- 9.Han S, Zhang X, Xu R, Finkelman FD, Brombacher F, Zheng B. IgD+IgM– B cells mount immune responses that exhibit altered antibody repertoire. Eur J Immunol. 2004;34:661–8. doi: 10.1002/eji.200324493. [DOI] [PubMed] [Google Scholar]
- 10.Han S, Cao S, Bheekha-Escura R, Zheng B. Germinal center reaction in the joints of mice with collagen-induced arthritis: an animal model of lymphocyte activation and differentiation in arthritis joints. Arthritis Rheum. 2001;44:1438–43. doi: 10.1002/1529-0131(200106)44:6<1438::AID-ART239>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Wolfowicz CB, Sakorafas P, Rothstein TL, Marshak-Rothstein A. Oligoclonality of rheumatoid factors arising spontaneously in lpr/lpr mice. Clin Immunol Immunopathol. 1988;46:382–95. doi: 10.1016/0090-1229(88)90057-8. [DOI] [PubMed] [Google Scholar]
- 12.Holmdahl R, Rubin K, Klareskog L, Larsson E, Wigzell H. Characterization of the antibody response in mice with type II collagen-induced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum. 1986;29:400–10. doi: 10.1002/art.1780290314. [DOI] [PubMed] [Google Scholar]
- 13.Wooley PH, Luthra HS, Krco CJ, Stuart JM, David CS. Type II collagen-induced arthritis in mice. II. Passive transfer and suppression by intravenous injection of anti-type II collagen antibody or free native type II collagen. Arthritis Rheum. 1984;27:1010–7. doi: 10.1002/art.1780270907. [DOI] [PubMed] [Google Scholar]
- 14.Terato K, Hasty KA, Reife RA, Cremer MA, Kang AH, Stuart JM. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992;148:2103–8. [PubMed] [Google Scholar]
- 15.Nimmerjahn F, Ravetch JV. Fcgamma receptors: old friends and new family members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–2. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- 17.Nimmerjahn F, Bruhns P, Horiuchi K, Ravetch JV. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Dolhain RJ, van der Heiden AN, ter Haar NT, Breedveld FC, Miltenburg AM. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996;39:1961–9. doi: 10.1002/art.1780391204. [DOI] [PubMed] [Google Scholar]
- 19.Maini RN, Taylor PC. Anti-cytokine therapy for rheumatoid arthritis. Annu Rev Med. 2000;51:207–29. doi: 10.1146/annurev.med.51.1.207. [DOI] [PubMed] [Google Scholar]
- 20.Cooper SM, Sriram S, Ranges GE. Suppression of murine collagen-induced arthritis with monoclonal anti-Ia antibodies and augmentation with IFN-gamma. J Immunol. 1988;141:1958–62. [PubMed] [Google Scholar]
- 21.Mauritz NJ, Holmdahl R, Jonsson R, Van der Meide PH, Scheynius A, Klareskog L. Treatment with gamma-interferon triggers the onset of collagen arthritis in mice. Arthritis Rheum. 1988;31:1297–304. doi: 10.1002/art.1780311012. [DOI] [PubMed] [Google Scholar]
- 22.Weyand CM, Seyler TM, Goronzy JJ. B cells in rheumatoid synovitis. Arthritis Res Ther. 2005;7(Suppl. 3):S9–12. doi: 10.1186/ar1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takemura S, Braun A, Crowson C, et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol. 2001;167:1072–80. doi: 10.4049/jimmunol.167.2.1072. [DOI] [PubMed] [Google Scholar]