Abstract
Suitable levels of interferon (IFN)-γ and interleukin (IL)-10 seem to favour the outcome of cutaneous leishmaniasis (CL), while high IFN-γ and low IL-10 production are associated with severity of mucosal leishmaniasis (ML). Considering that cytokine balance is important for the maintenance of protective responses in leishmaniasis, our aim was to investigate leishmanial antigens-induced IFN-γ and IL-10 levels maintained in healed individuals who had different clinical outcomes of Leishmania infection. Thirty-three individuals who recovered from L. braziliensis infection were studied: cured CL (CCL), cured ML (CML), spontaneous healing of CL (SH) or asymptomatic individuals (ASY). Cytokines were quantified by enzyme-linked immunosorbent assay (ELISA) in culture supernatants of L. braziliensis-stimulated peripheral blood mononuclear cells (PBMC). IFN-γ levels were higher in CML (7593 ± 5994 pg/ml) in comparison to SH (3163 ± 1526 pg/ml), ASY (1313 ± 1048 pg/ml) or CCL (1897 ± 2087 pg/ml). Moreover, cured ML cases maintained significantly lower production of IL-10 (127 ± 57.8 pg/ml) in comparison to SH (1373 ± 244 pg/ml), ASY (734 ± 233 pg/ml) or CCL (542 ± 375 pg/ml). Thus, a high IFN-γ/IL-10 ratio observed in CML can indicate unfavourable cytokine balance. On the other hand, no significant difference in the IFN-γ/IL-10 ratio was observed when CCL individuals were compared to SH or ASY subjects. In conclusion, even after clinical healing, ML patients maintained a high IFN-γ/IL-10 secretion profile in response to leishmanial antigens. This finding can explain a delayed down-modulation of exacerbated inflammatory responses, which can be related in turn to the necessity of prolonged therapy in ML management. Conversely, lower IFN-γ/IL-10 balance observed in CCL, SH and ASY individuals can represent a better-modulated immune response associated with a favourable prognosis.
Keywords: clinical cure, IFN-γ, IL-10, Leishmania (Viannia) braziliensis, regulatory immune responses
Introduction
American tegumentary leishmaniasis (ATL) is a vector-borne disease caused mainly by Leishmania (Viannia) braziliensis. The infection causes a wide spectrum of clinical presentation, ranging from self-healing or benign cutaneous leishmaniasis (CL) to more severe forms, such as mucosal leishmaniasis (ML). These different outcomes of infection occur in the presence of anti-Leishmania immune responses, and seem to be influenced profoundly by the balance between effector and regulatory specific T cell responses [1,2].
Cytokines such as interleukin (IL)-4, IL-5, IL-10 and transforming growth factor (TGF)-β favour parasite replication, while IL-10 also counterbalances the proinflammatory effect of IFN-γ and tumour necrosis factor (TNF)-α[3]. However, the levels of these cytokines are expressed differently depending on the clinical form of the disease [1,4–7]. Active leishmaniasis is characterized by mixed profiles in which type 1 and type 2 cytokines are observed [1,4–6,8,9]. On the other hand, ML patients present higher levels of inflammatory cytokines but low IL-10 production, which in turn can contribute to the exacerbated inflammatory immune response [1,10]. In contrast, lower levels of IFN-γ and TNF-α are observed in subjects who did not develop the disease after Leishmania infection (asymptomatic or subclinical individuals) [7]. However, CL patients who experienced spontaneous healing (SH) present a strong T cell response to leishmanial antigens with high capacity to produce IFN-γ[11].
Even so, immunological conditions associated with cure or disease control are still under-investigated. Resolution of CL and ML lesions is associated with a decrease of IL-4, IL-5 and TNF-α[5,12,13], although IFN-γ levels seem to be maintained after therapy [6,13]. These results indicate that cytokine modulation is a crucial factor in determining the fate of the disease. IL-10 is a potent antagonist of IFN-γ effects and has been considered an important regulatory cytokine in leishmaniasis [14], but its relevance in the maintenance of long-term immunity in ATL remains unknown. IL-10 production by CD4+CD25+ is required for maintenance of Leishmania after cure, which in turn preserves an adaptive immunity to L. major[14]. Considering that cytokine balance is important for the maintenance of protective responses to leishmaniasis, our aim was to investigate the leishmanial antigens-induced IFN-γ and IL-10 levels that persist in individuals who had different clinical outcomes of Leishmania infection but were able to control the parasite.
Material and methods
Casuistic
Thirty-three subjects with past history of Leishmania infection were studied. These subjects were subdivided into four groups: cured cutaneous leishmaniasis (CCL, n = 12), cured mucosal leishmaniasis (CML, n = 11), spontaneous healed CL (SH, n = 3) and asymptomatic individuals (ASY, n = 7). All the subjects were from endemic areas for L. (V.) braziliensis. The group was composed of 18 males (mean age 46.2 years, range 15–73 years) and 15 females (mean age 52.3 years, range 15–78 years). Patients with a past history of cutaneous or mucosal leishmaniasis fulfilled the clinical, parasitological and/or immunological diagnosis criteria for the disease [6]. The patients achieved clinical cure after being treated with pentavalent antimony, according to the schedules recommended by the Brazilian Ministry of Health (15–20 mg/kg/day of Sb+5(V) for 20–30 days). These subjects were clinically followed after healing of lesions and were studied 1 year after the end of successful therapy. Patients classified as SH were diagnosed with leishmaniasis, but before therapeutic intervention these patients developed spontaneous epithelization of the lesion without requiring drug administration, leading to full cicatrization. They were re-evaluated 1 year after the lesion was considered clinically healed. Asymptomatic individuals were possibly exposed to leishmanial infection because they were neighbours of confirmed cases of leishmaniasis. Moreover, they had no skin ulcers characteristic of ATL, and subclinical infection was determined by in vivo (delayed-type hypersensibility − Montenegro skin test (MST)) or in vitro (lymphocyte activation − proliferation and/or IFN-γ production) evidence of induction of cellular responses to leishmanial antigens. In addition, 11 patients with active cutaneous leishmaniasis (eight males and three females; mean age 42.3 ± 18 years) were also studied prior to the therapy. This work was approved by the Ethical Committee for Human Research from Fundação Oswaldo Cruz. Informed consent was obtained from all participants.
Peripheral blood mononuclear cells (PBMC) culture and cytokine measurement
PBMC were separated by centrifugation over a gradient of Ficoll-Hypaque (Histopaque 1077; Sigma Chemical Company, St Louis, MO, USA), as described elsewhere [6]. Briefly, PBMC (3 × 106 cells per ml) cultures were incubated for 3 days and 5 days at 37°C in a humidified atmosphere of 5% CO2 in air, in the presence of the equivalent of 5 × 106 disrupted L. braziliensis (MHOM/BR75/M2903) promastigotes per well as antigens or medium alone. The supernatant of each culture was collected on day 3 to quantify IL-10 and on day 5 to test IFN-γ concentrations. The supernatants were aliquoted and stored at −20°C until use. IFN-γ and IL-10 cytokines were measured by ELISA. Monoclonal antibodies and recombinant cytokines were purchased from BD Biosciences Pharmingen (San Diego, CA, USA). The procedures were performed according to the manufacturer's instructions. The samples were tested in duplicate and concentration was analysed using the SOFTmax®PRO 4.0 program (Life Sciences Edition; Molecular Devices Corporation, Sunnyvale, CA, USA). Results were expressed as picograms per millilitre. The minimum cytokine levels detected were 62.5 pg/ml for IFN-γ and 31.2 pg/ml for IL-10.
Statistical analysis
The results were expressed as mean ± standard deviation and median and were analysed by one-way analysis of variance (anova), Kruskal–Wallis test, using GraphPad Prism software. Statistical significance was assigned to P < 0.05.
Results
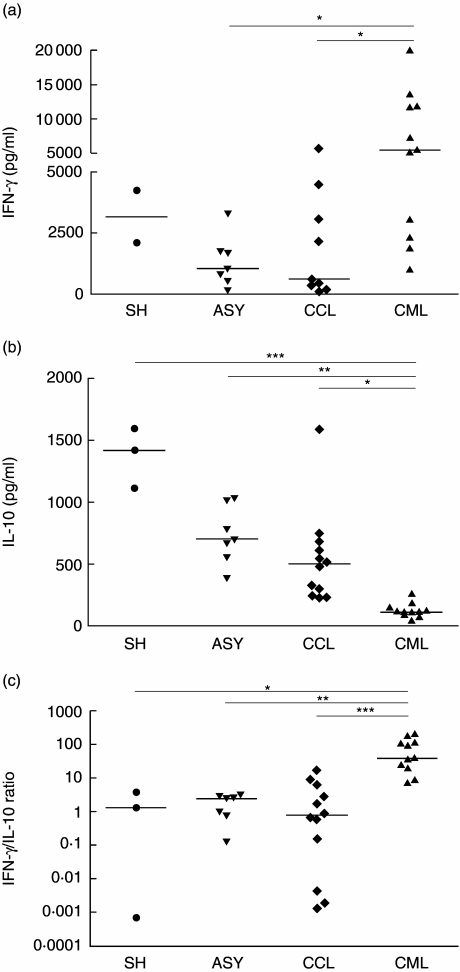
The levels of IFN-γ or IL-10 induced by leishmanial antigens were compared in subjects who controlled leishmanial infection either spontaneously or after antimonial therapy. Clinically cured subjects included in this study had no clinical sign of active disease and had no history of leishmaniasis reactivation. The mean levels of IFN-γ were significantly higher in CML (7593 ± 5994 pg/ml, median = 5582 pg/ml, n = 11) in comparison to SH (3163 ± 1526 pg/ml, n = 2), ASY (1313 ± 1048 pg/ml, median = 1040 pg/ml, n = 7) or CCL (1897 ± 2087 pg/ml, median = 612 pg/ml, n = 9) (Fig. 1a). Three CCL and one SH had IFN-γ levels below the detection limits. On the other hand, the IL-10 production by CML was significantly lower (127 ± 57.8 pg/ml, median = 114 pg/ml, n = 11) in comparison to that observed for SH (1373 ± 244 pg/ml, median = 1417 pg/ml, n = 3), ASY (734 ± 233 pg/ml, median = 698 pg/ml, n = 7) and CCL (542 ± 375 pg/ml, median = 499 pg/ml, n = 12) (Fig. 1b).
Fig. 1.
Leishmania-induced interferon (IFN)-γ (a) and interleukin (IL)-10 (b) production by peripheral blood mononuclear cells (PBMC) from clinically cured American tegumentary leishmaniasis (cutaneous and mucosal disease) subjects and asymptomatic individuals. (c) IFN-γ/IL-10 ratio of cytokine production. PBMC were stimulated in vitro with Leishmania (Viannia) braziliensis antigens and supernatants were collected after 3 days (for IL-10) and 5 days (for IFN-γ) in culture. Cytokines were measured by enzyme-linked immunosorbent assay. Each point represents a subject. Medians are indicated by horizontal bars. SH: spontaneous healed cutaneous leishmaniasis; ASY: asymptomatic individuals; CCL: cured cutaneous leishmaniasis; CML: cured mucosal leishmaniasis. *P < 0.05; **P < 0.01; ***P < 0.001.
The inflammatory and regulatory cytokine balance was also investigated using the quotient of IFN-γ/IL-10 ratio. The mean ratio was compared in the different groups. It is noteworthy that these mean ratios were similar for CCL, ASY and SH subjects (3.3 ± 5.3, median = 0.8; 1.9 ± 1.2, median = 2.5; and 1.7 ± 1.9, median = 1.3, respectively) but differed substantially for CML individuals (77 ± 71, median = 39.1) (Fig. 1c). Different from the other groups analysed, CML individuals presented a high heterogeneity of in vitro IFN-γ production, in which a group of low producers were observed clearly. These low IFN-γ producers also presented low IL-10 levels. Despite this, these low IFN-γ producers still maintained an IFN-γ/IL-10 ratio much higher (15.4 ± 8.8, median = 14.6, n = 4) than CCL, ASY and SH (P < 0.05). There was no correlation between IFN-γ/IL-10 ratio and other clinical parameters as a response to therapy or period of cure.
In an attempt to determine if IFN-γ/IL-10 balance can be modulated during the clinical evolution of leishmaniasis we compared cured CL subjects to patients with active disease. The mean levels of IFN-γ were significantly higher during active CL (3459 ± 1796 pg/ml, median = 2975 pg/ml, n = 11) than in CCL (P < 0.05). In addition, an increase of IL-10 was observed long-term after the clinical cure of CL when compared to the levels observed during active disease (244.7 ± 241.4 pg/ml, median = 206 pg/ml, n = 7, P < 0.05). Consequently, an elevated IFN-γ/IL-10 ratio was associated with active CL (38.4 ± 36.9, median = 12.6, n = 7), while a low IFN-γ/IL-10 ratio is a cytokine profile observed in individuals who healed the disease.
Discussion
Several studies have demonstrated that deregulation of Leishmania-induced immune response is involved in the pathogenesis of ATL [1,2]. However, the mechanisms that lead to an adequate equilibrium between inflammatory mediators and their counter-regulatory molecules, enabling restraint of the parasite without inducing tissue damage, are poorly understood. Herein, clinically cured ATL patients and asymptomatic individuals were the target subjects because of their sustained capacity to control parasite infection, possibly by the induction of a proper immune response. Our results showed that even after controlling the infection or disease, subjects with a benign prognosis of Leishmania infection (ASY, SH and CCL) presented a clear difference in capacity to produce IFN-γ and IL-10 upon Leishmania antigens stimuli in comparison to cured ML patients, a more severe clinical form of the disease.
The cured ML individuals in this study produced lower levels of IL-10 in comparison to CCL or to individuals naturally able to control the infection (SH and ASY). In fact, this low pattern of IL-10 production by ML was associated previously with a poor ability to down-modulate the exacerbated inflammatory tissue-mediated damage [1]. The addition of IL-10 only slightly inhibits IFN-γ production by mononuclear cells from ML, although it can suppress IFN-γ secretion in CL patients [1]. Accordingly, the low expression of IL-10 receptor observed in ML patients could account for damping the regulatory effect of IL-10 [2]. Also, the levels of IL-10 detected in our cured ML subjects were higher than those reported in active ML patients [1], which can suggest that an increase of IL-10 levels should occur after clinical cure. On the other hand, in spite of maintaining an elevated IFN-γ production, these cured ML subjects did not reactivate the disease, suggesting that they are under adequate immune regulatory control [6]. Nevertheless, this control seems to be somewhat subtle, as it is known that ML cases are at high risk of relapse [15]. Whether or not a low increase in IL-10 production is able to counter-regulate the IFN-γ effects is still an open question. Indeed, other regulatory mechanisms resulting, for instance, in a decrease of TNF-α or IL-4 production [6,12,13], are also likely to be involved in the healing process of ML. In attempt to address whether IL-10 levels are important to the maintenance of protective immune response, these patients are still under clinical follow-up in order to survey relapses.
The majority of Leishmania-infected individuals should develop a balanced T cell immunity responsible for the favourable prognosis, as observed in cutaneous leishmaniasis [5] or in subjects that controlled the infection without evolving to an ulcerated lesion [7]. Herein, the good prognosis observed in asymptomatic individuals or CL patients may be associated with the ability to produce optimal levels of IL-10 and IFN-γ. A dual role of IL-10, either in inhibiting macrophages functions or exerting regulatory effects on type 1 responses, is attributed to different facts [10,14]. It is known that IL-10 allows the parasite to survive and multiply, thus favouring the progression of infection [14]. It is conceivable that a possible harmful effect due to high IFN-γ induction can be modulated by an increase of IL-10 [16]. In accordance with this argument, an increase of IL-10 levels in association with a reduction of IFN-γ/IL-10 ratio were observed when CCL subjects were compared with active CL patients. A similar profile can also be suggested for ML cases.
Several studies either in humans [9,17,18] or in mice models [14,19] have shown unequivocally that IFN-γ and IL-10 are molecules involved chiefly in the fate of leishmaniasis and other intracellular infections [10]. In visceral leishmaniasis, a lack of IFN-γ activity was considered to be related to the simultaneous presence of elevated levels of IL-10 [18,20]. However, it has to be considered that other cytokines such as TGF-β[1,21], IL-4 [4,5] or IL-13 [22] can also influence parasite persistence or inflammatory regulation. Notwithstanding, it was shown that IL-10 produced by CD4+CD25+ T cell is also required for parasite persistence in mice, which in turn would preserve an adaptive immunity to Leishmania[14].
Another important point to be addressed concerns the cell sources of IFN-γ and IL-10. CD4+ T cells have been considered to be the main producers of IFN-γ both in CL [8] and in ML patients [1]. This T cell subtype is expanded preferentially upon leishmanial stimuli both in long-term cured subjects [6] and asymptomatic individuals [23]. The main source of IL-10 has not yet been determined in tegumentary leishmaniasis. However, besides macrophages, lymphocytes such as gamma-delta double-negative T cells [24] and T CD4 regulatory lymphocytes [25,26] are likely to exert a regulatory profile, probably involving IL-10 secretion.
Our results reveal that the IFN-γ/IL-10 ratio could be a useful parameter to predict the clinical evolution or response to therapy. Even after cure, ML individuals maintain a higher IFN-γ/IL-10 ratio, probably mirroring an impaired capacity to down-modulate the exacerbated inflammatory reaction. Thus, it is reasonable to propose that an equilibrium in the production of these cytokines, as observed in CCL, ASY and SH, enables parasite killing without producing skin tissue damage. The definition of immunological parameters correlated with cure and long-term immune response in ATL would help to understand more clearly the mechanisms associated with protection in human leishmaniasis.
Acknowledgments
We are grateful to Ms Rosangela Pellegrino for secretarial assistance. This work was supported by Instituto Oswaldo Cruz/FIOCRUZ: internal funds; PAPES/VPPDT/FIOCRUZ, FAPERJ (grant number E-26/170.844/2003), FAPERJ/SUS: Ministério da Saúde and Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico − CNPq (grant number E-26/170.614/2005). AMC is a research fellow of CNPq.
References
- 1.Bacellar O, Lessa H, Schriefer A, et al. Up-regulation of th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–40. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faria DR, Gollob KJ, Barbosa J, Jr, et al. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun. 2005;73:7853–9. doi: 10.1128/IAI.73.12.7853-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott P. Development and regulation of cell-mediated immunity in experimental leishmaniasis. Immunol Res. 2003;27:489–98. doi: 10.1385/IR:27:2-3:489. [DOI] [PubMed] [Google Scholar]
- 4.Pirmez C, Yamamura M, Uyemura K, Paes-Oliveira M, Conceição-Silva F, Modlin RL. Cytokine patterns in the pathogenesis of human leishmaniasis. J Clin Invest. 1993;91:1390–5. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coutinho SG, Oliveira MP, Da-Cruz AM, et al. T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure. Exp Parasitol. 1996;84:144–55. doi: 10.1006/expr.1996.0100. [DOI] [PubMed] [Google Scholar]
- 6.Da-Cruz AM, Bittar R, Mattos M, et al. T-cell-mediated immune responses in patients with cutaneous or mucosal leishmaniasis: long-term evaluation after therapy. Clin Diagn Lab Immunol. 2002;9:251–6. doi: 10.1128/CDLI.9.2.251-256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Follador I, Araújo C, Bacellar O, et al. Epidemiologic and immunologic findings for the subclinical form of Leishmania braziliensis infection. Clin Infect Dis. 2002;34:E54–8. doi: 10.1086/340261. [DOI] [PubMed] [Google Scholar]
- 8.Bottrel RLA, Dutra WO, Martins FA, et al. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis. Infect Immun. 2001;69:3232–9. doi: 10.1128/IAI.69.5.3232-3239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toledo VPCP, Mayrink W, Gollob KJ, et al. Immunochemotherapy in American cutaneous leishmaniasis: immunological aspects before and after treatment. Mem Inst Oswaldo Cruz. 2001;96:89–98. doi: 10.1590/s0074-02762001000100010. [DOI] [PubMed] [Google Scholar]
- 10.Trinchieri G. Regulatory role of T cells producing both interferon γ and interleukin 10 in persistent infection. J Exp Med. 2001;194:F53–7. doi: 10.1084/jem.194.10.f53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho EM, Correia Filho D, Bacellar O, Almeida RP, Lessa H, Rocha H. Characterization of the immune response in subjects with self-healing cutaneous leishmaniasis. Am J Trop Med Hyg. 1995;53:273–7. doi: 10.4269/ajtmh.1995.53.273. [DOI] [PubMed] [Google Scholar]
- 12.Da-Cruz AM, Oliveira MP, De Luca PM, Mendonça SCF, Coutinho SG. Tumor necrosis factor-α in human American tegumentary leishmaniasis. Mem Inst Oswaldo Cruz. 1996;91:225–9. doi: 10.1590/s0074-02761996000200019. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro-de-Jesus A, Almeida RP, Lessa H, Bacellar O, Carvalho EM. Cytokine profile and pathology in human leishmaniasis. Braz J Med Biol Res. 1998;31:143–8. doi: 10.1590/s0100-879x1998000100020. [DOI] [PubMed] [Google Scholar]
- 14.Belkaid Y, Hoffmann KF, Mendez S, et al. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J Exp Med. 2001;194:1497–506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zajtchuk JT, Casler JD, Netto EM, et al. Mucosal leishmaniasis in Brazil. Laryngoscope. 1989;99:925–39. doi: 10.1288/00005537-198909000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Rocha PN, Almeida RP, Bacellar O, et al. Down-regulation of Th1 type of response in early human American cutaneous leishmaniasis. J Infect Dis. 1999;180:1731–4. doi: 10.1086/315071. [DOI] [PubMed] [Google Scholar]
- 17.Bosque F, Saravia NG, Valderrama L, Milon G. Distinct innate and acquired immune responses to Leishmania in putative susceptible and resistant human populations endemically exposed to L. (Viannia) panamensis infection. Scand J Immunol. 2000;51:533–41. doi: 10.1046/j.1365-3083.2000.00724.x. [DOI] [PubMed] [Google Scholar]
- 18.Caldas A, Favali C, Aquino D, et al. Balance of IL-10 and interferon-γ plasma levels in human visceral leishmaniasis: implications in the pathogenesis. BMC Infect Dis. 2005;5:113–21. doi: 10.1186/1471-2334-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stober CB, Lange UG, Roberts MTM, Alcami A, Blackwell JM. IL-10 from regulatory T cells determines vaccine efficacy in murine Leishmania major infection. J Immunol. 2005;175:2517–24. doi: 10.4049/jimmunol.175.4.2517. [DOI] [PubMed] [Google Scholar]
- 20.Peruhype-Magalhães V, Martins-Filho OA, Prata A, et al. Mixed inflammatory/regulatory cytokine profile marked by simultaneous raise of interferon-γ and interleukin-10 and low frequency of tumour necrosis factor-α+ monocytes are hallmarks of active human visceral leishmaniasis due to Leishmania chagasi infection. Clin Exp Immunol. 2006;146:124–32. doi: 10.1111/j.1365-2249.2006.03171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barral A, Barral-Netto M, Yong EC, Brownell CE, Twardzik DR, Reed SG. Transforming growth factor β as a virulence mechanism for Leishmania braziliensis. Proc Natl Acad Sci USA. 1993;90:3442–6. doi: 10.1073/pnas.90.8.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourreau E, Prévot G, Gardon J, Pradinaud R, Launois P. High intralesional interleukin-10 messenger RNA expression in localized cutaneous leishmaniasis is associated with unresponsiveness to treatment. J Infect Dis. 2001;184:1628–30. doi: 10.1086/324665. [DOI] [PubMed] [Google Scholar]
- 23.Bittar RC, Nogueira RS, Vieira-Gonçalves R, Ribeiro VP, Mattos MS, Oliveira-Neto MP, Coutinho SG, Da-Cruz AM. T-cell responses associated with resistance to Leishmania infection in individuals from endemic areas for Leishmania braziliensis. Mem Inst Oswaldo Cruz. 2007 doi: 10.1590/s0074-02762007005000069. in press. [DOI] [PubMed] [Google Scholar]
- 24.Antonelli LRV, Dutra WO, Oliveira RR, et al. Disparate immunoregulatory potentials for double-negative (CD4– CD8–) αβ and γδ T cells from human patients with cutaneous leishmaniasis. Infect Immun. 2006;74:6317–23. doi: 10.1128/IAI.00890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji J, Masterson J, Sun J, Soong L. CD4+CD25+ regulatory T cells restrain pathogenic responses during Leishmania amazonensis infection. J Immunol. 2005;174:7147–53. doi: 10.4049/jimmunol.174.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campanelli AP, Roselino AM, Cavassani KA, et al. CD4+CD25+ T cells in skin lesions of patients with cutaneous leishmaniasis exhibit phenotypic and functional characteristics of natural regulatory T cells. J Infect Dis. 2006;193:1313–22. doi: 10.1086/502980. [DOI] [PubMed] [Google Scholar]