Abstract
Several reports have demonstrated a possible association of periodontal infections with coronary heart disease (CHD) by elevated antibody titre to periodontopathic bacteria in CHD patients compared with non-diseased controls. Although each periodontopathic bacterium may vary in virulence for periodontitis and atherosclerosis, antibody response to multiple bacteria in CHD patients has not been understood fully. Therefore, serum levels of antibody to 12 periodontopathic bacteria together with other atherosclerotic risk markers were compared among 51 patients with CHD, 55 patients with moderate to severe chronic periodontitis and 37 healthy individuals. The antibody response was the most prevalent for Porphyromonas gingivalis, a major causative organism, in CHD as well as periodontitis patients. However, antibody positivity was different between CHD and periodontitis if the response was analysed for two different strains of P. gingivalis, namely FDC381 and Su63. While periodontitis patients were positive for both P. gingivalis FDC381 and Su63, a high frequency of antibody positivity for P. gingivalis Su63 but not for FDC381 was observed in CHD patients. The results indicate that the presence of particular periodontopathic bacteria with high virulence may affect atherogenesis. Identifying the virulence factors of P. gingivalis Su63 may gain insight into the new therapeutic modality for infection-induced deterioration of atherosclerosis.
Keywords: CHD, periodontitis, Porphyromonas gingivalis, serum antibody
Introduction
Coronary heart disease (CHD) is the leading cause of death in Japan as well as other developed countries. The main underlying pathological pathway for the disease is atherosclerosis. Although hypertension, elevated serum cholesterol, smoking, diabetes and obesity are classical risk factors for atherosclerosis [1], a substantial proportion of patients have no such traditional risk factors. The general hypothesis that chronic infections can contribute to the development of atherosclerosis has come from (i) direct effects of infectious agents on cellular components of the vessel wall; (ii) increased expression of cytokines, chemokines and cellular adhesion molecules resulting in local endothelial dysfunction; and (iii) immune responses targeted to self-proteins located in the vessel wall mediated by molecular mimicry [2]. Recent epidemiological studies have suggested a link between atherosclerosis and infection/inflammation. Associations have been reported with Chlamydia pneumoniae, Helicobacter pylori, cytomegalovirus [3,4] and dental infections; in particular, those associated with periodontal disease [5]. With respect to inflammation, serum high-sensitivity C-reactive protein (hs-CRP) has been used as a risk marker for CHD [6] and a number of reports have demonstrated that hs-CRP is also elevated in periodontitis patients [7–13]. However, other systemic inflammatory markers such as tumour necrosis factor (TNF)-α and interleukin (IL)-6 showed inconsistent results [8,14].
In vitro studies have suggested that Porphyromonas gingivalis may have a relation to atherogenesis, because this bacterium can invade endothelial cells [15]; lipopolysaccharide induces cell adhesion molecules and cytokine production in endothelial cells [16]; and autoimmune or cross-reactive response to heat shock protein 60 may also be involved in both periodontitis and CHD [17]. The association of this bacterium to atherosclerotic disease is documented by higher antibody titres in patients compared with non-diseased controls [18]. The apparent specificity of the antibody to P. gingivalis for incident CHD supports the hypothesis that infection with, or the host response to, this particular bacterium is particularly deleterious in terms of atherosclerotic complications [19].
Not all subjects infected with these infectious agents necessarily develop CHD. It is important to recognize that the results from these studies identify only an association, not causation, between periodontitis and CHD. In response to infection and inflammation, certain individuals may exhibit greater expression of local and systemic mediators, and consequently be at increased risk for atherosclerosis [20].
Because pathogens in periodontitis comprise several genetically and serologically heterogeneous bacterial species, it can be speculated that a single or a few species may be of particular importance in the development and progression of atherosclerosis due to having the relevant virulence to the pathogenesis. The aim of the present study, therefore, was to investigate whether particular periodontal pathogens are associated with CHD by measuring the serum antibody levels to various periodontopathic bacteria. The difference of systemic inflammatory conditions and serum lipid profiles among patients with both CHD and periodontitis, with periodontitis and normally healthy subjects, was also compared.
Materials and methods
Patients
We studied 51 CHD patients who underwent percutaneous coronary intervention for chronic stable angina (CSA; n = 17) or acute coronary syndrome (ACS; n = 34) at the Coronary Care Unit of Niigata City General Hospital, and 55 patients with chronic periodontitis admitted to the Periodontal Clinic of Niigata University Medical and Dental Hospital. ACS and CSA were grouped together for biochemical and immunological analyses. Although all CHD patients demonstrated clinical signs of periodontitis, both the degree and extent of the disease were variable. As a control, 37 healthy individuals selected from the staff members of the university were included. The study protocol was approved by the review boards of both institutions. Written informed consent was obtained from each patient and control subject prior to entry into the study. The periodontal status of each of the subjects was assessed as described previously [21]. Briefly, the clinical attachment level and probing pocket depth were measured at six sites per tooth, and the alveolar bone levels were examined radiographically. Smoking status was defined as ‘ever smoker’ and ‘never smoker’. Fasting serum was obtained from periodontitis patients and control subjects. Sera of CHD patients were obtained after operations. The cholesterol and triglyceride profiles in terms of serum lipoproteins were analysed at Skylight Biotech Inc. (Akita, Japan).
None of the periodontitis patients or healthy control individuals had self-reported overt atherosclerotic disease at their most recent regular medical check.
Serum IgG antibody titres to periodontopathic bacteria and Chlamydia pneumoniae
Antibody responses to antigens of periodontopathic bacteria were assessed by enzyme-linked immunosorbent assay (ELISA) and evaluated according to the method as described previously [22]. Values ≥ 1 represent more than 2 standard deviations (s.d.) of the mean in controls and is considered to be antibody positive. Absolute measures of serum antibody were categorized into positive or negative groups. Serum IgG antibodies to C. pneumoniae was determined by enzyme immunoassay (SRL Inc., Tokyo, Japan).
Measurement of CRP
Serum high-sensitivity CRP (hs-CRP) was measured with nephelometry, a latex particle-enhanced immunoassay (NA Latex CRP kit; Dade Behring, Tokyo, Japan) on a commercial basis (SRL Inc.). Only one sample from a control subject demonstrated a value lower than the limit of the assay (50 ng/ml). Undetectable CRP values were recorded as 25 ng/ml, halfway between zero and the threshold of detection.
Measurement of serum interleukin (IL)-6 and tumour necrosis factor (TNF)-α
Serum levels of IL-6 and TNF-α were determined by sensitive ELISA using commercial kits (R&D Systems Inc., Minneapolis, MN, USA), according to the manufacturer's instructions. The lower limit of detection was 0.016 pg/ml for IL-6 and 0.06 pg/ml for TNF-α.
Statistical analysis
Clinical and biochemical parameters were compared using unpaired t-test and Mann–Whitney U-tests, respectively. A value of P < 0.05 was considered significantly different.
For anti-bacterial antibodies, the association of antibody positivity and disease type, e.g. periodontitis, or CHD with periodontitis, was determined by χ2 and adjusted further for multiple comparisons using Bonferroni's correction, where the significance was accepted at P < 0.025. A logistic regression analysis was utilized to assess the relationship between antibody positivity and disease status while adjusting for potential confounding factors. We carried out this analysis with CHD patients with periodontitis versus periodontitis patients without CHD as dependent variable and age, gender, smoking and antibody positivity as independent variables. Odds ratios (OR) were calculated with 95% confidence intervals (CI). Correlation coefficient was analysed for antibody levels between either C. pneumonia and P. gingivalis FDC381 or C. pneumonia and P. gingivalis Su63 in each serum sample from CHD patients. Statistical analyses were performed by using the standard statistical software (StatView J-4.5 application program; SAS Institute Inc., Cary, NC, USA). Significance was set at 5% (P < 0.05).
Results
Clinical status of the patients
The clinical profile of the study population is shown in Table 1. The mean age of the CHD patients was higher than the periodontitis patients and control. The percentages of having ever smokers were 77.8% for the CHD patients and 34.5% for the periodontitis patients, whereas none of the controls had ever been smokers. Seventeen of 51 CHD patients were taking atorvastatin calcium. Although the CHD patients manifested apparent symptom of periodontitis, the severity of the disease was significantly greater than that of control subjects but significantly less than periodontitis patients. However, CHD patients had significantly less teeth than periodontitis patients, suggesting that CHD patients have had severe periodontitis previously and several teeth had been extracted due to the disease. Because the severely involved teeth had been extracted and the periodontal status of remaining teeth is less severe than in the periodontitis patients, overall periodontal infection in CHD patients is considered to be more serious than in periodontitis patients. P. gingivalis was detected in the dental plaque samples obtained from all CHD patients by using polymerase chain reaction (PCR); however, the strains could not be differentiated (data not shown).
Table 1.
Study population and periodontal disease status.
| CHD (n = 51) | Periodontitis (n = 55) | Control (n = 37) | |
|---|---|---|---|
| Age | 62.4 ± 1.71,a | 47.2 ± 1.7 | 48.6 ± 1.5 |
| Male/female | 46/5 | 24/31 | 18/19 |
| Smoking status (never smoker/ever smoker) | 10/355 | 36/19 | 37/0 |
| PD2 (mm) | 2.8 ± 0.1b | 3.8 ± 0.1c | 2.0 ± 0.0 |
| CAL3 (mm) | 3.4 ± 0.2d | 4.5 ± 0.2e | 2.1 ± 0.1 |
| Mean bone loss (%) | 34.7 ± 2.5 | 40.0 ± 2.0 | n.d.4 |
| Number of teeth | 19.6 ± 1.4 | 24.5 ± 0.7f | 27.9 ± 0.4g,h |
Data are expressed as mean ± s.e.
PD: pocket depth
CAL: clinical attachment level
n.d.: not determined
smoking status of some of the patients was not checked.
P < 0.0001 versus periodontitis and controls
P < 0.0001 versus controls
P < 0.0001 versus coronary heart disease (CHD) and controls
P < 0.0001 versus controls
P < 0.0001 versus CHD and controls
P = 0.0011 versus CHD
P < 0.0001 versus CHD
P = 0.0002 versus periodontitis.
The high density lipoprotein (HDL) cholesterol and triglyceride levels of CHD patients were significantly lower than those of periodontitis patients and controls (P < 0.0001). The HDL cholesterol level of periodontitis patients was also significantly lower than that of controls (P = 0.024). However, the total cholesterol level of periodontitis patients and controls were significantly higher than that of CHD patients (P = 0.0007 for periodontitis versus CHD; P < 0.0001 control versus CHD). The very low density lipoprotein (VLDL) level of CHD patients was significantly lower than that of periodontitis patients and controls (P = 0.0002 and P < 0.0001, respectively). No difference was observed for the low density lipoprotein (LDL) level (Table 2).
Table 2.
Serum lipid profiles of the study population.
| CHD (n = 51) | Periodontitis (n = 55) | Control (n = 37) | |
|---|---|---|---|
| Total cholesterol (mg/dl) | 158.9 ± 4.8 | 180.8 ± 4.6a | 198.2 ± 5.7b |
| HDL cholesterol (mg/dl) | 38.2 ± 1.9 | 57.5 ± 1.9c | 63.9 ± 1.9c,d |
| LDL cholesterol (mg/dl) | 96.1 ± 3.7 | 89.3 ± 2.9 | 94.2 ± 4.0 |
| VLDL cholesterol (mg/dl) | 24.4 ± 1.5 | 33.5 ± 1.8e | 39.6 ± 2.7f |
| Triglyceride (mg/dl) | 36.1 ± 2.0 | 113.5 ± 9.0g | 96.9 ± 9.2g |
P = 0.0007 versus coronary heart disease (CHD)
P < 0.0001 versus CHD
P < 0.0001 versus CHD
P = 0.024 versus periodontitis
P = 0.0002 versus CHD
P < 0.0001 versus CHD
P < 0.0001 versus CHD. HDL: high density lipoprotein; LDL: low density lipoprotein; VLDL: very low density lipoprotein.
Serum antibody levels
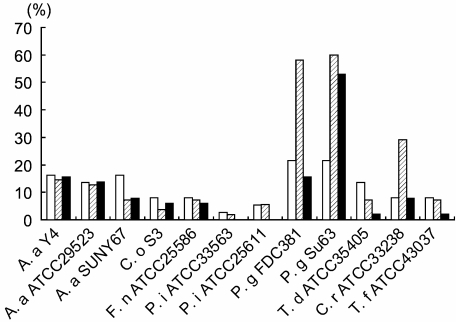
Serum IgG positivity to 12 periodontal pathogens is shown in Fig. 1. The prevalence of antibody positivity for P. gingivalis FDC381 and P. gingivalis Su63 was higher than the other bacteria. Furthermore, the antibody positivities for these bacteria were higher in periodontitis patients compared with control subjects. These two strains of P. gingivalis are serologically different but are isolated more frequently from Japanese periodontitis patients than the other strains, such as W50 and W83 [23]. There was no difference of antibody positivity to these two pathogens within periodontitis patients. In addition, no difference was observed for any other pathogens tested between these two patients. In CHD patients, on the other hand, a significant antibody response was observed only for P. gingivalis Su63 and not for P. gingivalis FDC381. As the characteristic response was observed for P. gingivalis Su63 and P. gingivalis FDC381, the antibody positivity to these strains was analysed further. The prevalence of antibody positive subjects for both strains in CHD patients and periodontitis patients was 13.7% and 50.9%, respectively. The prevalence of antibody positive subjects for only P. gingivalis Su63 in CHD patients and periodontitis patients was 41.2% and 9.8%, respectively, while that for P. gingivalis FDC381 was 0% and 7.3%, respectively. The distribution of single-positive subjects for P. gingivalis Su63 was significantly different between the CHD patients and periodontitis patients (CHD versus periodontitis: χ2 = 13.3, P = 0.0006). This difference in distribution was not seen between periodontitis patients and controls (χ2 = 1.07, P = 0.50).
Fig. 1.
Prevalence of antibody-positive subjects in each group. Serum antibody levels were evaluated against listed periodontopathic bacteria by enzyme-linked immunosorbent assay (ELISA). Values ≥ 1 represent more than 2 s.d. of the mean in controls and is considered to be antibody positive. Absolute measures of serum antibody were categorized into positive or negative groups. Data are expressed as percentages of antibody-positive subjects within each group. Control: open bar, periodontitis: hatched bar, coronary heart disease: closed bar. Actinobacillus actinomycetemcomitans: A. a., Capnocytophaga ochracea: C. o., Fusobacterium nucleatum: F. n., Prevotella intermedia: P. i., Porphyromonas gingivalis: P. g., Treponema denticola: T. d., Campylobacter rectus: C. r., Tannerella forsythia: T. f.
In order to evaluate the association of the pattern of antibody positivity for P. gingivalis with an increased frequency of CHD while adjusting for possible confounding factors, we further performed a logistic regression analysis. As age is a strong confounding factor for CHD, the analyses were performed for 46 CHD patients (mean age, 60.7 ± 10.1 years; range, 36–73 years) and 22 periodontitis patients (mean age, 59.4 ± 5.4 years; range, 52–70 years) to minimize age difference between two groups. Smoking status could not be adjusted because of the very low frequency of ever smokers in periodontitis patients. As can be seen in Table 3, the logistic regression analysis indicated that only the antibodies positive for P. gingivalis Su63, but not for both P. gingivalis Su63 and P. gingivalis FDC381 or negative for either as well as age, smoking and HDL-c, were significant factors of an increased frequency of CHD. Interestingly, antibody positivity was found to be a highly significant factor in the model (P = 0.022; OR 18.26, 95% CI = 1.53–218.48) and was even higher than smoking (P = 0.016; OR 8.56, 95% CI = 1.49–49.17). On the other hand, mBL and gender were not statistically significant.
Table 3.
Logistic regression analysis for coronary heart disease (CHD) (n = 46) with periodontitis (n = 22).
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| Age | 1.08 | 0.988–1.17 | n.s. |
| Gender | 0.34 | 0.042–1.61 | n.s. |
| Smoking | 8.56 | 1.49–49.17 | 0.016 |
| HDL-c | 0.93 | 0.87–0.99 | 0.023 |
| mBL | 0.99 | 0.95–1.04 | n.s. |
| Antibody | 18.26 | 1.53–218.48 | 0.022 |
Logistic regression with disease status (0: periodontitis without CHD, 1: periodontitis with CHD) as dependent variable, independent variables: ages as continuous, gender (0: male, 1: female), smoking (0: never smoker, 1: ever smoker), HDL-c (mg/dl) as continuous, mBL (%) as continuous and antibody positivity (0: either positive or negative for both Porphyromonas gingivalis Su63 and P. gingivalis FDC381, or negative for P. gingivalis Su63 and positive for P. gingivalis FDC381, 1: positive for P. gingivalis Su63 only); n.s.: not significant.
For IgG antibody to C. pneumonia, 42.9% of the CHD patients were antibody-positive. However, there were no correlations between antibody levels to either P. gingivalis FDC381 and C. pneumoniae (r = − 0.142, P = 0.33) or P. gingivalis Su63 and C. pneumoniae (r = − 0.175, P = 0.24), suggesting that there is no cross-reactivity between these two bacteria.
Systemic inflammatory markers
Serum levels of hs-CRP, IL-6 and TNF-α are shown in Table 4. The values of hs-CRP and IL-6 in the CHD patients were significantly higher compared with those of the periodontitis patients and control subjects (P < 0.0001). The value of hs-CRP of the periodontitis patients was also significantly higher than that of controls (P = 0.014). In contrast to hs-CRP and IL-6, the CHD patient TNF-α level was significantly lower than that of periodontitis patients and control subjects (P < 0.0001). The TNF-α level of periodontitis patients tended to be lower compared with that of control subjects, but did not reach statistical significance.
Table 4.
Level of the serum inflammatory markers in each group.
| CHD (n = 51) | Periodontitis (n = 55) | Control (n = 37) | |
|---|---|---|---|
| CRP (ng/ml) | 3958.9 ± 1855.4 (918.0)a | 765.0 ± 148.1b (349.0) | 426.9 ± 121.5 (217.0) |
| IL-6 (pg/ml) | 4.00 ± 1.41c (1.56) | 0.64 ± 0.08 (0.57) | 0.82 ± 0.32 (0.44) |
| TNF-α (pg/ml) | 0.86 ± 0.18 (0.57) | 1.36 ± 0.08d (1.32) | 1.96 ± 0.22d (1.42) |
Data are expressed as mean ± s.e. and median values are indicated in the parentheses.
P < 0.0001 versus periodontitis and controls
P = 0.014 versus controls
P < 0.0001 versus periodontitis and controls
P < 0.0001 versus coronary heart disease. CHD: coronary heart disease; CRP: C-reactive protein; IL: interleukin; TNF: tumour necrosis factor.
Discussion
In spite of epidemiological evidence for an association between CHD and infection, and the effect of periodontal infection on the systemic inflammation, the contribution of periodontal disease to such an infectious burden is largely unknown. Here, we first report a relationship between the systemic immune response to a particular strain of periodontopathic bacteria (e.g. P. gingivalis Su63) and clinical manifestation of CHD. Although the association of infectious agents such as C. pneumoniae with CHD has been explored previously by serological testing, only a few studies have been performed specifically for the association with periodontal infection. In the present study, it was demonstrated that there was no correlation of antibody levels between both strains of P. gingivalis and C. pneumoniae.
Pussinen et al. reported that antibodies to selected periodontal pathogens were associated with CHD. They used a mixture of three serotypes of P. gingivalis as antigens in their multi-serotype ELISA [18]. In a prospective follow-up study by the same group, it was demonstrated that high-serum IgA antibody to P. gingivalis and Actinobacillus actinomycetemcomitans were associated with future incidence of CHD [24]. Beck et al. also demonstrated an association of elevated IgG antibodies to oral microorganisms and atherosclerosis [25]. In addition, Dye et al. showed that an elevated IgG titre to P. gingivalis was associated independently with high serum CRP [26]. However, these studies did not take into account the difference in the strains of each bacterium. In addition, none of these studies showed the antibody levels to periodontopathic bacteria in CHD patients without periodontal disease as a control. Our study was also unable to set such control groups. Age is a crucial risk factor for both CHD and periodontal disease, and a number of epidemiological studies have demonstrated that periodontal disease could be a risk for cardiovascular diseases [27]. According to the fact-finding survey of dental diseases conducted by the Ministry of Health, Labour and Welfare of Japan in 1999, more than 88% of the population whose ages are similar to those of patients analysed in this study had periodontal disease. Therefore, although the control data are important, it was difficult to include such patients in this study.
Because peripheral blood T cells from CHD patients with gingivitis and those with periodontitis showed a distinct proliferative response to bacterial HSP65 but not to human HSP60, the immune response may be different between CHD patients with periodontitis and those without periodontitis [28]. None the less, the antibody response in CHD patients without periodontitis remains to be determined.
It is reported that the different strains of P. gingivalis vary in their ability to invade human coronary artery endothelial cells [15]. These results therefore suggest that several species of periodontopathic bacteria are of particular importance in the association with CHD. In the present study, 12 putative periodontal pathogens were used as antigens. Of these, antibody positivity to two strains of P. gingivalis was much higher compared with the other bacteria. Several studies have demonstrated a clonal heterogeneity in virulence among various P. gingivalis strains [29]. P. gingivalis can be classified into six genotypes based on the genomic diversity of the fimA gene [30] in addition to serological classification. The fimA genotypes of P. gingivalis FDC381 and Su63 are type I and type IV (Amano A, personal communication), respectively. The abscess formation caused by fimA type II and type IV organisms in mice leads to higher levels of systemic inflammation compared to the other fimAgenotypes [31]. Although the virulence of P. gingivalis Su63 has not been characterized fully, it is assumed to have equivalent pathogenic activity to other strains with fimA type IV, such as P. gingivalis W50 and W83. The pathogenicity of these strains is mediated at least in part by the cysteine proteinases called gingipains. It is shown that the biological effects of gingipains are related directly to the pathogenesis of atheroma formation and atherosclerotic plaque rupture [32–35]. P. gingivalis can be found in approximately 64% of the periodontal pocket of Japanese patients with periodontitis [36]. However, the prevalence of each strain of P. gingivalis in the periodontal pockets is not known. In this context, Amano et al. investigated the relationship between the prevalence of fimA genotypes of P. gingivalis and periodontal status and demonstrated that occurrence of fimA types I and IV within P. gingivalis-positive patients was 2.5% and 16.5%, respectively [37].
It is interesting that the antibody positivity to P. gingivalis Su63 was similar among the CHD and periodontitis patients, whereas that to P. gingivalis FDC381 was much lower in the CHD patients compared to the periodontitis patients. The logistic regression model showed clearly a strong correlation between the antibody positive for P. gingivalis Su63, not for P. gingivalis FDC381, and an increased frequency of CHD (P = 0.022; OR 18.26). This suggests that in spite of the similar infectious capability of these P. gingivalis strains, in the periodontitis lesion only P. gingivalis Su63-susceptible patients are at risk for CHD. Alternatively, it is possible that antibodies against P. gingivalis FDC381 cross-react with P. gingivalis Su63, whereas antibody to P. gingivalis Su63 does not cross-react with P. gingivalis FDC381. If this is the case, P. gingivalis Su63 is considered to be more probably involved in CHD than P. gingivalis FDC381. In the present study, we used sonic extracts of the bacteria as antigens instead of purified antigen(s). Although we have not determined the specificity of the antigens, as with other studies in which similar bacterial preparations are used [38,39], variable patterns of antibody response to different bacteria or to different species of the same bacteria in each patient suggest that the antibody response to each bacterium can be considered as specific, even though the reactivity may be reflected by some cross-reactive response. In addition, the number of false negatives undoubtedly increases when only one antigen has been chosen as a source of antigen [38]. Nevertheless, the differential antibody response to different strains of P. gingivalis needs to be clarified further.
Treponema denticola, Tannerella forsythia and Campylobacter rectus as well as P. gingivalis FDC381 are considered periodontal pathogens as the bacteria possess several virulence factors such as fimbriae, proteases, haemagglutinins and lipopolysaccharide [40]. However, antibody response to these bacteria was low in CHD patients. Although the precise reason is not known, prevalence of these bacteria in the plaque may be low compared with P. gingivalis Su63 in CHD patients, or the immune response to P. gingivalis FDC381 in CHD patients may be different from that in periodontitis patients.
In the present study, intergroup variation of the serum lipid profile was found and the variation in HDL cholesterol was of particular interest. HDL cholesterol is considered to be an anti-atherogenic lipoprotein [41,42], and a low HDL cholesterol concentration is one of the established independent CHD risk factors [43].
HDL cholesterol concentration was the lowest in CHD patients, the highest in controls and with periodontitis patients between these two. It has been reported that although the concentration of total cholesterol and triglyceride was higher than that of controls, no difference in HDL cholesterol was observed [44,45]. In support of our results, Pussinen et al. demonstrated that high combined serum antibody levels against A. actinomycetemcomitans and P. gingivalis were associated significantly with low HDL cholesterol concentrations [18]. Furthermore, the same group demonstrated that periodontitis decreases the serum HDL cholesterol by comparing the levels before and after treatment [46]. Although it is well established that infection and inflammation are associated with a reduction in serum HDL cholesterol, the exact mechanism has not yet been established. Nevertheless, our study suggests that periodontal infection exerts effects on both systemic inflammation and the serum lipid profile towards pro-atherogenesity.
In spite of huge diversity of microflora in periodontal pockets, only a small number of species are involved in the pathogenesis of periodontal disease. Each individual species of bacteria has a different genotype with a different virulence. We show here for the first time that a particular genotype of P. gingivalis is probably involved in the mechanisms linking periodontitis and CHD. Further study will enable us to identify the particularly important virulence factors in both the development and progression of atherosclerosis.
Acknowledgments
The authors thank Professor Atsuo Amano (Osaka University Graduate School of Dentistry, Osaka, Japan) for the analysis of the fimA genotype. This study was supported by grants from the Ministry of Education, Science Sports and Culture of Japan (16390613, 17659655 and 19390536), 8020 Promotion Foundation and Medtronic Japan Co. Ltd, Kanagawa, Japan.
References
- 1.Anderson KM, Castelli WP, Levy D. Cholesterol and mortality: 30 years of follow-up from the Framingham study. JAMA. 1987;257:2176–80. doi: 10.1001/jama.257.16.2176. [DOI] [PubMed] [Google Scholar]
- 2.Epstein SE, Zhou YF, Zhu J. Infection and atherosclerosis: emerging mechanistic paradigms. Circulation. 1999;100:e20–8. doi: 10.1161/01.cir.100.4.e20. [DOI] [PubMed] [Google Scholar]
- 3.Chiu B, Viira E, Tucker W, Fong IW. Chlamydia pneumoniae, cytomegalovirus, and herpes simplex virus in atherosclerosis of the carotid artery. Circulation. 1997;96:2144–8. doi: 10.1161/01.cir.96.7.2144. [DOI] [PubMed] [Google Scholar]
- 4.Mendall MA, Goggin PM, Molineaux N, et al. Relation of Helicobacter pylori infection and coronary heart disease. Br Heart J. 1994;71:437–9. doi: 10.1136/hrt.71.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck JD, Pankow J, Tyroler HA, Offenbacher S. Dental infection and atherosclerosis. Am Heart J. 1999;138:S528–33. doi: 10.1016/s0002-8703(99)70293-0. [DOI] [PubMed] [Google Scholar]
- 6.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 7.Ebersole JL, Machen RL, Steffen MJ, Willmann DE. Systemic acute-phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. Clin Exp Immunol. 1997;107:347–52. doi: 10.1111/j.1365-2249.1997.270-ce1162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000;71:1528–34. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- 9.Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001;72:1221–7. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- 10.Craig RG, Yip JK, So MK, Boylan RJ, Socransky SS, Haffajee AD. Relationship of destructive periodontal disease to the acute-phase response. J Periodontol. 2003;74:1007–16. doi: 10.1902/jop.2003.74.7.1007. [DOI] [PubMed] [Google Scholar]
- 11.Slade GD, Ghezzi EM, Heiss G, Beck JD, Riche E, Offenbacher S. Relationship between periodontal disease and C-reactive protein among adults in the Atherosclerosis Risk in Communities Study. Arch Intern Med. 2003;163:1172–9. doi: 10.1001/archinte.163.10.1172. [DOI] [PubMed] [Google Scholar]
- 12.D'Aiuto F, Parkar M, Andreou G, et al. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83:156–60. doi: 10.1177/154405910408300214. [DOI] [PubMed] [Google Scholar]
- 13.Salzberg TN, Overstreet BT, Rogers JD, Califano JV, Best AM, Schenkein HA. C-reactive protein levels in patients with aggressive periodontitis. J Periodontol. 2006;77:933–9. doi: 10.1902/jop.2006.050165. [DOI] [PubMed] [Google Scholar]
- 14.Ide M, McPartlin D, Coward PY, Crook M, Lumb P, Wilson RF. Effect of treatment of chronic periodontitis on levels of serum markers of acute-phase inflammatory and vascular responses. J Clin Periodontol. 2003;30:334–40. doi: 10.1034/j.1600-051x.2003.00282.x. [DOI] [PubMed] [Google Scholar]
- 15.Dorn BR, Dunn WA, Jr, Progulske-Fox A. Invasion of human coronary artery cells by periodontal pathogens. Infect Immun. 1999;67:5792–8. doi: 10.1128/iai.67.11.5792-5798.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham MD, Bajorath J, Somerville JE, Darveau RP. Escherichia coli and Porphyromonas gingivalis lipopolysaccharide interactions with CD14: implications for myeloid and nonmyeloid cell activation. Clin Infect Dis. 1999;28:497–504. doi: 10.1086/515158. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki K, Ohsawa Y, Tabeta K, et al. Accumulation of human heat shock protein 60-reactive T cells in the gingival tissues of periodontitis patients. Infect Immun. 2002;70:2492–501. doi: 10.1128/IAI.70.5.2492-2501.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pussinen PJ, Jousilahti P, Alfthan G, Palosuo T, Asikainen S, Salomaa V. Antibodies to periodontal pathogens are associated with coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:1250–4. doi: 10.1161/01.ATV.0000072969.71452.87. [DOI] [PubMed] [Google Scholar]
- 19.Haynes WG, Stanford C. Periodontal disease and atherosclerosis: from dental to arterial plaque. Arterioscler Thromb Vasc Biol. 2003;23:1309–11. doi: 10.1161/01.ATV.0000087144.24654.71. [DOI] [PubMed] [Google Scholar]
- 20.Beck JD, Offenbacher S, Williams R, Gibbs P, Garcia R. Periodontitis: a risk factor for coronary heart disease? Ann Periodontol. 1998;3:127–41. doi: 10.1902/annals.1998.3.1.127. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki K, Ueki-Maruyama K, Oda T, et al. Single-nucleotide polymorphism in the CD14 promoter and periodontal disease expression in a Japanese population. J Dent Res. 2003;82:612–6. doi: 10.1177/154405910308200808. [DOI] [PubMed] [Google Scholar]
- 22.Murayama Y, Nagai A, Okamura K, et al. Serum immunoglobulin G antibody to periodontal bacteria. Adv Dent Res. 1988;2:339–45. doi: 10.1177/08959374880020022401. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa E, Kumada H, Umemoto T. Serological classification of Bacteroides gingivalis and purification of group specific antigens. Shika Kiso Igakkai Zasshi. 1989;31:647–55. doi: 10.2330/joralbiosci1965.31.647. [DOI] [PubMed] [Google Scholar]
- 24.Pussinen PJ, Nyyssönen K, Alfthan G, Salonen R, Laukkanen JA, Salonen JT. Serum antibody levels to Actinobacillus actinomycetemcomitans predict the risk for coronary heart disease. Arterioscler Thromb Vasc Biol. 2005;25:833–8. doi: 10.1161/01.ATV.0000157982.69663.59. [DOI] [PubMed] [Google Scholar]
- 25.Beck JD, Eke P, Lin D, et al. Associations between IgG antibody to oral organisms and carotid intima-medial thickness in community-dwelling adults. Atherosclerosis. 2005;183:342–8. doi: 10.1016/j.atherosclerosis.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Dye BA, Choudhary K, Shea S, Papapanou PN. Serum antibodies to periodontal pathogens and markers of systemic inflammation. J Clin Periodontol. 2005;32:1189–99. doi: 10.1111/j.1600-051X.2005.00856.x. [DOI] [PubMed] [Google Scholar]
- 27.Behle JH, Papapanou PN. Periodontal infections and atherosclerotic vascular disease: an update. Int Dent J. 2006;56:256–62. doi: 10.1111/j.1875-595x.2006.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 28.Hasan A, Sadoh D, Palmer R, Foo M, Marber M, Lehner T. The immune responses to human and microbial heat shock proteins in periodontal disease with and without coronary heart disease. Clin Exp Immunol. 2005;142:585–94. doi: 10.1111/j.1365-2249.2005.02953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neiders ME, Chen PB, Suido H, et al. Heterogeneity of virulence among strains of Bacteroides gingivalis. J Periodont Res. 1989;24:192–8. doi: 10.1111/j.1600-0765.1989.tb02005.x. [DOI] [PubMed] [Google Scholar]
- 30.Amano A. Molecular interaction of Porphyromonas gingivalis with host cells: implication for the microbial pathogenesis of periodontal disease. J Periodontol. 2003;74:90–6. doi: 10.1902/jop.2003.74.1.90. [DOI] [PubMed] [Google Scholar]
- 31.Sugano N, Ikeda K, Oshikawa M, Sawamoto Y, Tanaka H, Ito K. Differential cytokine induction by two types of Porphyromonas gingivalis. Oral Microbiol Immunol. 2004;19:121–3. doi: 10.1046/j.0902-0055.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 32.Imamura T, Pike RN, Potempa J, Travis J. Pathogenesis of periodontitis: a major arginine-specific cysteine proteinase from Porphyromonas gingivalis induces vascular permeability enhancement through activation of the kallikrein/kinin pathway. J Clin Invest. 1994;94:361–7. doi: 10.1172/JCI117330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imamura T, Potempa J, Pike RN, Travis J. Dependence of vascular permeability enhancement on cysteine proteinases in vesicles of Porphyromonas gingivalis. Infect Immun. 1995;63:1999–2003. doi: 10.1128/iai.63.5.1999-2003.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wingrove JA, DiScipio RG, Chen Z, Potempa J, Travis J, Hugli TE. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J Biol Chem. 1992;267:18902–7. [PubMed] [Google Scholar]
- 35.Ross R. Atherosclerosis − an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 36.Yano-Higuchi K, Takamatsu N, He T, Umeda M, Ishikawa I. Prevalence of Bacteroides forsythus, Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans in subgingival microflora of Japanese patients with adult and rapidly progressive periodontitis. J Clin Periodontol. 2000;27:597–602. doi: 10.1034/j.1600-051x.2000.027008597.x. [DOI] [PubMed] [Google Scholar]
- 37.Amano A, Kuboniwa M, Nakagawa I, Akiyama S, Morisaki I, Hamada S. Prevalence of specific genotypes of Porphyromonas gingivalis fimA and periodontal health status. J Dent Res. 2000;79:1664–8. doi: 10.1177/00220345000790090501. [DOI] [PubMed] [Google Scholar]
- 38.Pussinen PJ, Vilkuna-Rautiainen T, Alfthan G, Mattila K, Asikainen S. Multiserotype enzyme-linked immunosorbent assay as a diagnostic aid for periodontitis in large-scale studies. J Clin Microbiol. 2002;40:512–8. doi: 10.1128/JCM.40.2.512-518.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rams TE, Listgarten MA, Slots J. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis subgingival presence, species-specific serum immunoglobulin G antibody levels, and periodontitis disease recurrence. J Periodont Res. 2006;41:228–34. doi: 10.1111/j.1600-0765.2005.00860.x. [DOI] [PubMed] [Google Scholar]
- 40.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 41.Levine DM, Parker TS, Donnelly TM, Walsh A, Rubin AL. In vivo protection against endotoxin by plasma high density lipoprotein. Proc Natl Acad Sci USA. 1993;90:12040–4. doi: 10.1073/pnas.90.24.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackness MI, Durrington PN, Mackness B. How high-density lipoprotein protects against the effects of lipid peroxidation. Curr Opin Lipidol. 2000;11:383–8. doi: 10.1097/00041433-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Gordon DJ, Rifkind BM. High-density lipoprotein − the clinical implications of recent studies. N Engl J Med. 1989;321:1311–16. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 44.Lösche W, Karapetow F, Pohl A, Pohl C, Kocher T. Plasma lipid and blood glucose levels in patients with destructive periodontal disease. J Clin Periodontol. 2000;27:537–41. doi: 10.1034/j.1600-051x.2000.027008537.x. [DOI] [PubMed] [Google Scholar]
- 45.Katz J, Flugelman MY, Goldberg A, Heft M. Association between periodontal pockets and elevated cholesterol and low density lipoprotein cholesterol levels. J Periodontol. 2002;73:494–500. doi: 10.1902/jop.2002.73.5.494. [DOI] [PubMed] [Google Scholar]
- 46.Pussinen PJ, Jauhiainen M, Vilkuna-Rautiainen T, et al. Periodontitis decreases the antiatherogenic potency of high density lipoprotein. J Lipid Res. 2004;45:139–47. doi: 10.1194/jlr.M300250-JLR200. [DOI] [PubMed] [Google Scholar]