Abstract
Cytokines are involved in regulating HIV-1 infection. They are also placental environment major components. We assessed the potential impact of HIV-1 infection and/or anti-retroviral drugs on the placental cytokine profiles that may be involved in controlling HIV-1 placental dissemination. Placental explants were obtained after elective caesarean section from anti-retroviral-treated HIV-1-infected pregnant women and from HIV-1 non-infected pregnant women. The main placental cytokines were assessed for protein secretion in the supernatants of 24-h placental culture explants and/or in uncultured placental explants for mRNA expression levels. The cytokine profiles were different between the HIV-1-infected and the non-infected groups. Higher medians of leukaemia inhibiting factor (LIF), tumour necrosis factor (TNF)-α and interleukin (IL)-8 secretion were found in the 24-h culture supernatant of term placenta from HIV-1-infected women. High median levels of IL-16 and regulated upon activation normal T cell expressed and secreted (RANTES) levels were found in both groups. The mRNA expression medians were lower for TNF-α and IL-8 and higher for stromal cell-derived factor-1 (SDF-1) in uncultured placental explants from HIV-1-infected women. In the HIV-1-infected group, but not in the non-infected group, the secretion levels of TNF-α and IL-8, as well as their mRNA expression levels, were highly positively correlated; furthermore, their secretion levels were correlated positively with LIF and IL-10 secretion levels. We found no correlation between the cytokine levels and the immunovirological status of the HIV-1-infected mothers or the type or duration of treatment. These results highlight the potential impact of HIV-1 and of the anti-retroviral treatments on the placental cytokines pattern, independently of their anti-viral activity.
Keywords: anti-retroviral treatment, cytokines, HIV-1, mother-to-child transmission, placenta
Introduction
Mother-to-child-transmission (MTCT) of HIV-1 has decreased from 15% to 20% to less than 2% in Northern countries since the widespread use of anti-retroviral prophylaxis during pregnancy [1–4]. However, at the same time, the relative proportion of in utero MTCT to intrapartum MTCT has increased from less than 25% to 80%, showing that in utero transmission still remains an issue [5].
Even in the absence of anti-retroviral drugs, 90% of fetuses seem to be protected from in utero HIV-1 transmission [6–9]. Despite placental cells and, in particular, trophoblasts being in close contact with the maternal blood, the placenta acts as a materno–fetal barrier that prevents the in utero MTCT of various pathogens, including HIV-1 [10–12].
The placental environment contains many soluble factors, including cytokines and hormones, which have regulatory activities [13,14]. Several cytokines and chemokines present in the placental environment are involved in establishing and maintaining pregnancy [13,15–20]. The principal placental cytokines are also known to have different effects on HIV-1 replication: leukaemia inhibiting factor (LIF), interferon (IFN)-γ, interleukin (IL)-16, regulated upon activation normal T cells expressed and secreted (RANTES) and stromal cell-derived factor-1 (SDF-1) inhibit replication; tumour necrosis factor (TNF)-α and IL-8 increase replication; and IL-10 can both inhibit and increase replication depending on the HIV-1 cell targets [21–30].
Several studies have suggested that cytokines and chemokines may be major regulators of transplacental transmission of HIV-1 [8,21,31–35].
After infection by HIV-1, the immune system is activated in the lymphoid compartment and there is increased expression of various cytokines, particularly proinflammatory cytokines, which leads to immunodeficiency [36–38]. Anti-retroviral drugs can decrease HIV-1 induced activation at the onset of HIV-1 infection and also HIV-1 plasma viral replication, both leading to an increase of T CD4+ cell numbers.
Until now, the impact of HIV-1 infection and/or of anti-retroviral drugs on the principal cytokine expression patterns within the local placental compartment has not been evaluated clearly and extensively. As highly active anti-retroviral therapy (HAART) is now used widely during pregnancy, the decrease in maternal viral load is one of the primary determinants of MTCT reduction. An anti-retroviral monotherapy such as zidovudine does not decrease the plasma HIV RNA viral load of the infected mothers significantly, despite reducing MTCT efficiently [39,40], suggesting that anti-retrovirals may decrease MTCT through mechanisms other than reducing maternal viral load.
This study aimed to assess the impact on the placental cytokine environment of HIV-1 infection and of the anti-retroviral drugs used to prevent MTCT. We evaluated, in a group of HIV-1-infected pregnant women being treated with different preventive anti-retroviral regimens in France, the level of production and/or mRNA expression in the placental environment of several major cytokines/chemokines involved in HIV-1 regulation. We compared these profiles with those of non-infected non-treated pregnant women. Finally, modulation of the placental cytokine pattern in HIV-1-infected pregnant women by both the virus and the anti-retroviral drugs independently of their anti-viral action is discussed.
Materials and methods
Placentas
Human term placentas were obtained aseptically immediately after elective caesarean section at the maternity unit of the Antoine Béclère Hospital, Clamart, France for HIV-1 non-infected women (n = 15) and at the maternity units of the Bichat Hospital, Paris, France (n = 18), the Jean Verdier Hospital, Bondy, France (n = 3) and the Tenon Hospital, Paris, France (n = 1) for HIV-1-infected women. Clinical data concerning the age, co-infections, term at delivery, anti-retroviral treatment during pregnancy, HIV RNA viral load (log copies/ml, Roche®) and CD4 cell count (cells per mm3) at delivery for HIV-1-infected women were collected anonymously. The study was conducted according to French ethics procedures.
Twenty-four-hour culture of placental explants
Placental chorionic villi were isolated, minced and extensively washed three times with phosphate-buffered saline (PBS) (Dulbecco, Invitrogen, Paisley, Scotland, UK) within the 2–3 h following collection of each placenta. We cultured 3 g of placental chorionic villi in 20 ml RPMI-1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 1% l-glutamine, 1% penicillin–streptomycin (all from Gibco ltd, Paisley, Scotland, UK). Cultures were maintained at 37°C in a 5% CO2 humid incubator. Supernatants were collected after 24 h of culture, centrifuged at 1000 g for 10 min, separated into aliquots and frozen at −80°C.
Evaluation of cytokine levels in the supernatants of 24-h cultured placental explants
The supernatants of the 24-h cultured placental explants were analysed by enzyme-linked immunosorbent assays (ELISAs) using commercial kits according to the manufacturer's instructions. Quantification kits for TNF-α, IL-10 and IFN-γ were from Immunotech (Beckman Coulter, Marseille, France) (sensitivity: 10 and 5 pg/ml and 0.08 IU/l, respectively); for IL-16 from Biosource International, Nivelles, Belgium (sensitivity: 5 pg/ml); and for IL-8 and RANTES from Quantikine R&D Systems Europe Ltd (Abingdon, Oxon, UK) (sensitivity: 3.5 and 8 pg/ml, respectively). We measured human LIF using a non-commercial sandwich ELISA using two anti-human LIF-specific monoclonal antibodies, 1F10 and 7D2, as described previously [41] (sensitivity: 19 pg/ml). We also measured IFN-α in a limited number of placentas (six placentas from HIV-1-infected pregnant women and six from non-infected pregnant women) using a kit from RDI (Flanders, NJ, USA) (sensitivity: 5 pg/ml).
Detection of cytokine and chemokine mRNA expression in the placental explants by quantitative real-time reverse transcription–polymerase chain reaction (RT–PCR)
For each collected placenta, about 100–200 mg of extensively washed placental villi were frozen immediately at −80°C in RNA stabilization reagent (RNA later®; Qiagen, Hilden, Germany). After thawing, the tissue was homogenized using a homogenizer (Ultraturax®, IKA-Werk, Staufen, Germany) and columns (QIAshredder®; Qiagen). Total RNA from each sample was extracted using an RNeasy® mini kit (Qiagen) followed by DNase treatment (Qiagen) according to the manufacturer's recommendations. We obtained cDNA with the Taqman® reverse transcription kit (Applied Biosystems, Branchburg, NJ, USA) by adding 90 µl of reaction mix (1× RT buffer, 5.5 mM MgCl2, 500 µM deoxyribonucleoside triphosphate (dNTP), 2.5 µM random hexamer, 0.4 U/µl RNase inhibitor, 1.25 U/µl Multiscribe® RT enzyme, Applied Biosystems) to 0.1–1 µg RNA in 10 µl RNase DNase free water (Bio101 Systems, Q.Biogene, Illkirch, France). After 10 min incubation at 25°C, reverse transcription was carried out for 30 min at 48°C followed by a reverse transcriptase inactivation for 5 min at 95°C. The cDNAs were then amplified by quantitative real-time PCR using the ABI-PRISM 7700 Sequence Detector (PE Applied Biosystems, Foster City, CA, USA). The specific oligonucleotide primer sets and Taqman® probes used to amplify SDF-1 and IL-10 have been described previously [42]. For detection of TNF-α, IL-8 and IFN-γ mRNA expression, we used the specific oligonucleotide primer sets and the Taqman® probes of the Taqman® predeveloped kit (Applied Biosystems). RNA levels were normalized by including 18S RNA quantification (20×, Applied Biosystems) in the same reaction. Each sample was analysed in triplicate. For SDF-1 and IL-10 mRNA detection, real-time PCR was carried out as described previously [42,43]. We quantified gene expression levels in each sample using the comparative threshold cycle (CT) method according to the manufacturer's recommendations. The levels of SDF-1, IL-10, TNF-α, IL-8 and IFN-γ were reported to a reference term placental tissue (reference calibration: cal) similar for each experiment and determined in parallel for each analysis. Target gene levels in both the sample and the calibration sample were normalized to the endogenous reference (18S RNA). Thus, all measured quantities were expressed as fold reference value to the calibration sample.
Statistical analysis
The characteristics of the HIV-1-infected and non-infected groups are given as proportion for qualitative items and as median [interquartile range (IQR)] for quantitative items. The levels of cytokines/chemokines production and mRNA expression are presented for each group as the median (IQR) and as the proportion of results above the detection limit when the median was below the detection limit in the HIV-1-infected or non-infected groups.
Their medians were compared between the two groups using the Mann–Whitney non-parametric test and the proportion of undetectable values using the χ2 test or Fisher's exact test when necessary. Statistical significance was declared for P-values less than 0.05 and a trend for P-values between 0.05 and 0.15.
Interrelations between the levels of different cytokines/chemokines production and/or mRNA expression were tested using Spearman's non-parametric correlation (r) test separately for infected and non-infected women. In order to take into account the multiplicity of the performed tests, statistically significant correlation was declared only if the P-value was inferior to 0.01. A trend was envisaged for a P-value between 0.01 and 0.05.
In HIV-1-infected pregnant women, interrelations between the levels of cytokines/chemokines production or mRNA expression and clinical data were assessed using a χ2 test for qualitative clinical variables, such as the type of anti-retroviral treatment. To study their interrelation with quantitative clinical variables, such as viral load, first Spearman's correlation test was used. In addition, quantitative variables were categorized using approximately the 33 and 67 percentiles or the median, and their interrelations with the levels of cytokines/chemokines production or mRNA expression were tested using the Mann–Whitney (two groups) or Kruskal–Wallis (three groups) tests. Here again, only a P-value inferior to 0.01 leads to statistical significance and a trend was envisaged for P-values between 0.01 and 0.05.
Results
Characteristics of the studied population
We obtained 22 placentas from elective caesarean section of HIV-1-infected pregnant women and 15 from non-infected pregnant women. The elective caesarean sections were carried out between 37 and 39 weeks of amenorrhoea for the HIV-1-infected women. The elective caesarean sections for non-infected pregnant women were carried out after 38 weeks of amenorrhoea for obstetric reasons in women in a stable condition with no acute intercurrent infection.
The median age at delivery was 31 years (IQR 27–35). For HIV-1-infected women, the median HIV RNA viral load was 2.5 log copies/ml (IQR 2.3–3.4) and the median CD4 counts were 368 cells/mm3 (IQR 323–445). These values were determined during the last month of pregnancy. None of the HIV-1-infected women experienced fever during delivery or had any other viral (hepatitis, cytomegalovirus), parasitic (toxoplasmosis) or bacterial active co-infection. Of the 22 women, nine were treated with zidovudine (ZDV) alone during pregnancy to prevent mother-to-child transmission, three were treated with ZDV and lamivudine (3TC), eight with a triple therapy, including two nucleoside reverse transcriptase inhibitors (NRTI) and a protease inhibitor (nelfinavir), and one with two NRTI and a non-NRTI (nevirapine). The median treatment duration during pregnancy was 70 days (IQR 61–92). One HIV-1-infected pregnant woman received no treatment during pregnancy but received a ZDV infusion just before the elective caesarean section. All HIV-1-infected women received a ZDV infusion at the start of the caesarean section, as described in the ACTG 076/ANRS 024 protocol [44]. None of the babies born to these women were infected by HIV-1 at 24 months of age.
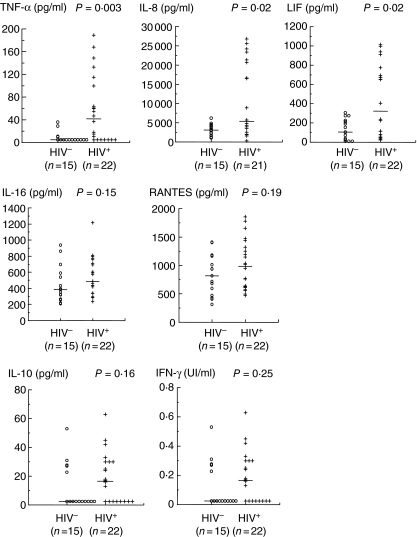
High production of LIF, TNF-α and IL-8 in the supernatant of 24-h explant culture of term placentas from HIV-1-infected women
We measured the levels of proinflammatory cytokines/chemokines (TNF-α and IL-8) and LIF, as well as IL-10, IFN-γ, IL-16 and RANTES in the supernatant of 24-h placental explant cultures from non-infected and HIV-1-infected women (Fig. 1). The median levels of TNF-α, IL-8 and LIF were significantly higher in the placentas of HIV-1-infected women than in those of HIV-1 non-infected women (P = 0.003, P = 0.02, P = 0.02, respectively). For TNF-α, this result was confirmed by a higher proportion of values above the detection limit in HIV-1-infected women (68% versus 27% in HIV-1 non-infected women, P = 0.01). In addition, a trend was observed for a higher proportion of observed values above the detection limit of IL-10 in HIV-1-infected women (64% versus 33% in HIV-1 non-infected women, P = 0.07) and of IFN-γ in HIV-1 non-infected women (27% versus 60% in HIV-1-infected women, P = 0.05). High levels of secreted IL-16 and RANTES were observed in placenta culture supernatants from both HIV-1-infected women and non-infected women.
Fig. 1.
Levels of the major cytokines/chemokines in the supernatants of 24-h cultures of explants from placentas of HIV-1-infected (+) and non-infected (○) women. The levels of the different cytokines were determined by enzyme-linked immunosorbent assay. The values for each placenta are plotted and the medians are indicated (—).
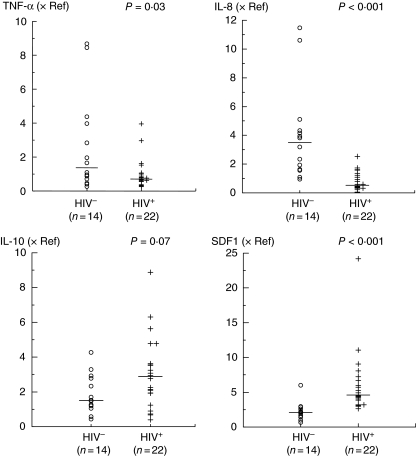
Low mRNA expression of TNF-α and IL-8 and high expression of SDF-1 in the explants of term placentas from HIV-1-infected women
We evaluated the levels of mRNA expression of the proinflammatory cytokines (TNF-α and IL-8) and SDF-1, as well as of IL-10 and IFN-γ, which were expressed at low levels in the supernatants of term placentas, in placental explants of the HIV-1-infected women and non-infected women (Fig. 2). The median levels of TNF-α and IL-8 mRNA expression were lower in the placentas of HIV-1-infected women than in the placentas of non-infected women (P = 0.03 and P < 0.001, respectively). The median level of mRNA expression of the chemokine SDF-1 was higher in the HIV-1-infected group than in the HIV-1 non-infected group (P < 0.001). There was a trend for higher median level of IL-10 mRNA expression in the HIV-1-infected group than in the non-infected group (P = 0.07). Levels of IFN-γ mRNA expression were very low in both groups.
Fig. 2.
mRNA expression levels of the major cytokines/chemokines in the placental explants of the HIV-1-infected (+) and non-infected (○) women. The mRNA expression levels are referenced to a term placental tissue (reference calibrator: cal). The amount of target gene in the sample and in the calibrator was normalized to the endogenous reference (18S RNA). The values for each placenta are plotted and the medians are indicated (—).
Correlations between the levels of the different cytokines/chemokines
We then analysed all the interrelations between the levels of the different cytokines/chemokines. The results are shown in Table 1 for production and Table 2 for mRNA expression except for RANTES, due to the absence of any interrelation and for IFN-γ, the values of which were either close to the detection limit (production) or very low (mRNA expression).
Table 1.
Correlations between the levels of the different cytokines/chemokines production in the placentas of HIV-1 non-infected women (n = 15) and in the placentas of HIV-1-infected women (n = 22) (shown in bold type).
| HIV- | TNF-α | IL-8 | LIF | IL-10 | IL-16 | |
|---|---|---|---|---|---|---|
| HIV + | ||||||
| TNF-α | −0.495 | −0.557 | 0.128 | 0.275 | ||
| P = 0.06 | P = 0.03 | P = 0.65 | P = 0.32 | |||
| IL-8 | 0.832 | 0.416 | −0.576 | −0.014 | ||
| P < 0.001 | P = 0.12 | P = 0.03 | P = 0.96 | |||
| LIF | 0.624 | 0.717 | −0.274 | 0.294 | ||
| P = 0.002 | P < 0.001 | P = 0.32 | P = 0.29 | |||
| IL-10 | 0.672 | 0.655 | 0.234 | −0.219 | ||
| P = 0.001 | P = 0.001 | P = 0.29 | P = 0.43 | |||
| IL-16 | −0.634 | −0.527 | −0.416 | −0.595 | ||
| P = 0.002 | P = 0.01 | P = 0.05 | P = 0.004 |
IL: interleukin; LIF: leukaemia inhibiting factor; TNF: tumour necrosis factor.
Table 2.
Correlations between the levels of the different cytokines/chemokines mRNA expression in the placentas of HIV-1 non-infected women (n = 14) and in the placentas of HIV-1-infected women (n = 22) (shown in bold type).
| HIV- | TNF-α | IL-8 | IL-10 | SDF-1 | |
|---|---|---|---|---|---|
| HIV + | |||||
| TNF-α | 0.248 | 0.433 | 0.767 | ||
| P = 0.39 | P = 0.12 | P = 0.001 | |||
| IL-8 | 0.582 | −0.231 | 0.090 | ||
| P = 0.005 | P = 0.43 | P = 0.76 | |||
| IL-10 | 0.447 | 0.238 | 0.345 | ||
| P = 0.04 | P = 0.29 | P = 0.230 | |||
| SDF-1 | 0.222 | 0.261 | 0.380 | ||
| P = 0.32 | P = 0.24 | P = 0.08 |
IL: interleukin; TNF: tumour necrosis factor; SDF-1: stromal cell-derived factor-1.
The levels of TNF-α, IL-10, IL-8 and LIF production were highly positively correlated 2 × 2 (r between 0.62 and 0.83, P = 0.002), except for IL-10 and LIF, in only the placentas of HIV-1-infected women, but not in the placentas of HIV-1 non-infected women. Moreover, among the latter group, there was a trend for a negative correlation between TNF-α and LIF levels and between IL-8 and IL-10 levels. The level of IL-16 production was correlated negatively with the levels of TNF-α, IL-8 and IL-10 in only the placentas of HIV-1-infected women (r between – 0.53 and – 0.63, P < 0.01).
The levels of TNF-α and IL-8 mRNA expression were correlated positively (r = 0.58, P = 0.005) and a trend was observed for a positive correlation between the levels of TNF-α and IL-10 mRNA expression (r = 0.45, P = 0.04), in only the placentas of the HIV-1-infected women. The levels of TNF-α and SDF-1 mRNA expression were highly positively correlated (r = 0.77, P = 0.001) in only the placentas of the HIV-1 non-infected women.
Finally, a trend for a positive correlation between the levels of TNF-α production and mRNA expression was observed (r = 0.590, P = 0.03) in the placentas of HIV-1 non-infected women and not of HIV-1-infected women (r = 0.386, P = 0.08).
Lack of correlation between the levels of cytokines/chemokines and the immunovirological status or anti-retroviral regimen of the mothers
We tried to correlate the levels of the different cytokines/chemokines (secretion of LIF, TNF-α, IL-8, IFN-γ, IL-10, IL-16, RANTES and mRNA expression of TNF-α, IL-8, SDF-1, IL-10 and IFN-γ) with either characteristics of the HIV-1-infected mothers − age, plasma viral load and CD4 cell count at delivery − or characteristics of mothers' preventive treatment: type of treatment, mother's gestational age at treatment initiation, duration of treatment.
The levels of the different cytokines/chemokines secretion or mRNA expression were not significantly correlated to mothers' age, viral load and CD4 cell count. Similarly, their median levels did not vary significantly according to mothers' age (relative to 27 and 35, or 31 years), viral load (relative to 200 and 1000, or 300 copies/ml), CD4 cell count (relative to 325 and 425, or 375/mm3).
The medians of the different cytokines/chemokines secretion or mRNA expression did not vary significantly according to preventive anti-retroviral regimen (mono-, bi- and tri-therapy, or mono-, bi- or tri-therapy). Their levels were not correlated significantly with gestational age at treatment initiation or duration of treatment. Similarly, their median levels did not vary significantly according to gestational age at treatment initiation (relative to 27 and 30, or 29 weeks) or duration of this treatment (relative to 8 and 12, or 10 weeks).
Discussion
The most important findings of this study are the differences between the major cytokine patterns observed in the placental environment of the HIV-1-infected women treated with anti-retroviral drugs and the patterns observed in non-infected women. Furthermore, the interrelations between the levels of the cytokines/chemokines are highly different in both groups.
Our results might suggest that the anti-retroviral drugs used in mother-to-child transmission prevention could modulate the placental cytokine pattern independently of their direct anti-HIV activity and that the placental environment might have an effect on the control of the HIV-1 dissemination at the placental level.
We detected high levels of IL-8, LIF, IL-16 and RANTES in the supernatants of 24-h cultured placental explants from both HIV-1-infected and non-infected mothers.
LIF has been shown to inhibit HIV-1 replication in vitro and has been associated in vivo with control of HIV-1 infection [21,22,45]. Here, we show for the first time that the levels of LIF secreted in the supernatants of 24-h placental cultures were higher in the treated non-transmitting HIV-1-infected women than in the non-infected women. These higher levels of LIF may help to control HIV infection within the placenta.
High levels of IL-16 and RANTES secretion have also been described in the supernatants of placental explants studied previously [43,46]. IL-16 is a CD4 ligand and RANTES is a ligand of the CCR5 HIV-1 co-receptor, and both inhibit HIV-1 replication in vitro, thus contributing to an HIV-1 ‘protective’ cytokine pattern within the placental environment [47–49]. Finally, the low levels of IFN-γ, IFN-α (data not shown) and IL-10 secretion in the supernatants of 24-h cultured placental explants from both HIV-1-infected and non-infected groups are consistent with other studies [43,46]. The levels of IFN-γ mRNA expression were also very low. These results are consistent with interferons not playing a major role in the inhibition of HIV-1 MTCT, as it has been suggested previously [50,51].
The higher, but not far from significance, IL-10 mRNA expression levels found in the placental explants of HIV-1-infected non-transmitting mothers versus non-infected mothers are consistent with a previous study, showing that IL-10 levels were higher in HIV-1 non-transmitting mothers than in transmitting mothers [34]. This suggests that high placental levels of IL-10 may contribute to the protective cytokine pattern of the placental environment.
We detected lower levels of mRNA expression of TNF-α and IL-8 in the placental explants of the HIV-1-infected women than in non-infected women, despite detecting higher secretion levels of these cytokines. We cannot exclude that this apparent discrepancy may be due to the fact that culturing the placenta could induce the secretion of these cytokines within the first 24 h of culture. However, as this is not observed in the placenta of HIV-1 non-infected women, this is due probably more to the levels of cytokine mRNA expression being evaluated in tissue fragments obtained immediately after delivery. Therefore, these fragments have still been under the influence of the anti-retroviral drugs given to the HIV-1-infected pregnant women, whereas we evaluated the secretion levels in the supernatants of the explants after 24 h of culture, with no addition of such drugs in the culture media. The anti-retroviral drugs may have reduced the level of the proinflammatory cytokine mRNA by repressing their gene transcription. Consistent with this, we have shown previously in an in vitro model of placental histocultures that ZDV decreases the levels of TNF-α mRNA expression in placental microexplants [42]. Furthermore, the levels of secreted TNF-α correlated only with their mRNA expression levels in the non-infected mothers and not in the HIV-1-infected mothers, supporting further the hypothesis that the anti-retroviral drugs may affect the level of TNF-α mRNA expression. TNF-α is one of the major cytokines that enhances HIV-1 replication [26,27,52,53] and, like IL-8, it also increases the passage of the virus across the trophoblast barrier [9]. Therefore, a decrease in the expression of those cytokines in the placental environment would limit HIV-1 placental dissemination.
We found that in the HIV-1-infected, contrary to what was observed in the non-infected mothers, the levels of secreted TNF-α were correlated positively with those of IL-8, LIF and IL-10; equally, the levels of secreted LIF were correlated positively with those of IL-8 and IL-10; we also observed that the levels of IL-16 were correlated negatively with those of TNF-α, IL-8 and IL-10 and the levels of mRNA expression of TNF-α were correlated positively with those of IL-8 and IL-10. These correlations may reflect the impact of HIV-1 and/or the anti-retroviral drugs on the placental environment leading to the concomitant variation in the expression levels of the major cytokines in the HIV-1-infected mothers but not in the HIV-1 non-infected mothers.
In a previous study, no significant differences of TNF-α and IL-8 secretion were observed in the placental explants of HIV-1-infected women compared to the non-infected women [46]. This discrepancy is due to higher medians of secretion levels of TNF-α and IL-8 in the control group of HIV-1 non-infected women in the Moussa et al. study compared to our study.
Finally, the higher levels of SDF-1, the major chemokine that interacts with the CXCR4 HIV-1 co-receptor, observed in the placental explants of HIV-1-infected women, may help to limit the spread of X4 HIV-1 across the placental barrier [33].
Altogether, our data show that the high levels of secreted LIF, IL-16 and RANTES, the low levels of TNF-α and IL-8 mRNA expression and the high levels of SDF-1 in the placentas of HIV-1-infected mothers treated with anti-retroviral drugs may promote the ‘placental barrier’ effect by creating a protective cytokine/chemokine local environment that can control HIV-1 spread within the placenta [9].
The lack of correlation between the observed cytokine pattern and the maternal viral load or CD4 cell count reinforce the hypothesis that anti-retroviral drugs modulate cytokine/chemokine expression independently of the systemic immunovirological status of the mother. However, we did not observe any correlation between the cytokine/chemokine patterns and the type (i.e. monotherapy versus bi-therapy or multiple therapy) or the duration of the anti-retroviral treatment. However, our sample sizes for each treatment group were not large enough to lead to any definitive conclusions. This study is part of a multi-centre analysis in which other centres administered different anti-retroviral regimens to prevent HIV-1 MTCT: in Thailand, ZDV monotherapy started at 28 weeks of pregnancy or as soon as possible with or without nevirapine at delivery [54], and in Cameroon no treatment during pregnancy, but nevirapine just before delivery, as described in the HIVNET 012 study [55]. Results from the Thai study support our findings that anti-retroviral drugs may have an impact on the placental cytokine profile (Pornprasert et al., manuscript submitted).
The modulation of the placental environment by anti-retroviral drugs may be explained by their direct impact on the placental tissue. The potential impact of anti-retroviral drugs, and particularly nucleoside reverse transcriptase inhibitors such as ZDV, on cell function is well documented [56,57]. Among other effects, ZDV increases the level of reactive oxygen species, decreases cell proliferation and induces cell death and mitochondrial dysfunction in the human placenta [58]. Such a mitochondrial dysfunction has also been observed in HIV-1 non-infected infants exposed to anti-retroviral drugs [59,60]. These effects on cell function may modify the expression of several major placental cytokines.
This study shows that a better understanding of the impact of anti-retroviral drugs on the placental cytokine/chemokine pattern is essential for assessing future anti-retroviral or therapeutic approaches, such as immunotherapy, in HIV-1-infected pregnant women.
Acknowledgments
We thank the staff of the maternity wards of A. Béclère, Bichat, Jean Verdier and Bondy Hospitals. We acknowledge Dr E. Lachassinne and Dr F. Hervé for their help in collecting the placentas, Dr Jean-Luc Taupin for the LIF dosages and Mrs Jacqueline Regnault for technical assistance. We also thank Dr Anna-Laura Ross for the English correction of the manuscript and Dr Marie-Thérèse Nugeyre and Romain Marlin for their technical help. Fellowship: AF (Sidaction), SP (Thai Ministry of University Affairs), GD (ANRS), MD (Sidaction). Financial support was from ANRS, INSERM and Institut Pasteur.
ANRS 1267 study group and HIV-1 PMTCT-PlaNet
Ahidjo Ayouba (Institut Pasteur, Paris, France), Françoise Barré-Sinoussi (Institut Pasteur, Paris, France), Sanupong Chailert (IRD URI 174/PHPT, Chiang Mai Thailand), Gérard Chaouat (INSERMU782, Clamart, France), Muriel Derrien (ICGM, Paris, France), Guillermina Dolcini (Facultad de Medicina, Universidad de Buenos Aires, Argentine), Nicole Eteki (Maternité Principale, Hôpital Central de Yaoundé, Cameroon), Albert Faye (Institut Pasteur, Paris, France et INSERMU782, Clamart, France), Vorapin Gomuthbutra (Nakornping Hospital, Chiang Mai, Thailand), Anfumbom Jude Kfutwah (Centre Pasteur du Cameroun, Yaoundé, Cameroon), Odette Kouo (Centre de Santé Mgr J. ZOA de NKOLNDONGO, Yaoundé, Cameroon), Marc Lallemant (IRD URI 174/PHPT, Chiang Mai, Thailand), Pranee Leechanachai (Faculty of Associated Medical Sciences, CMU, Chiang Mai, Thailand), Sophie Lecoeur (Institut National d'Etudes Démographiques, Paris, France), Brigitte Lemen (Centre de Santé Mgr J. Zoa de Nkolndongo, Yaoundé, Cameroon), Aram Limtrakul (Health Promotion Center Region 10 Hospital, Chiang Mai, Thailand), Juan Maldonado-Estrada (Facultad de Ciencas Agrarias, Universidad de Antioquia, Medellin, Colombia), Jean-Yves Mary (INSERM Erm0321, Paris, France), Wanmanee Matanasaravoot (Lamphun Hospital, Lamphun, Thailand), Elisabeth Menu (Institut Pasteur, Paris, France), Eric Nerrienet (Institut Pasteur du Cambodge, Phnom Penh, Cambodia), Nicole Ngo-Giang-Huong (HSPH/IRD URI 174, Chiang Mai, Thailand), Bernadette Njinku (Centre Pasteur du Cameroun, Yaoundé, Cameroon), Sakorn Pornprasert (Faculty of Associated Medical Sciences, CMU, Chiang Mai, Thailand), Sungwal Rugpao (Maharaj Nakorn Chiang Mai Hospital, Thailand), Gabriella Scarlatti (DIBIT, San Raffaele Scientific Institute, Milano, Italy), Pannee Sirivatanapa (Suan Dok Hospital, Chiang Mai, Thailand), Mathurin Tejiokem (Centre Pasteur du Cameroun, Yaoundé, Cameroon), Gilbert Téné (Centre Mère et Enfant, Fondation C. Biya, Yaoundé, Cameroon).
References
- 1.Mandelbrot L, Landreau-Mascaro A, Rekacewicz C, et al. Lamivudine–zidovudine combination for prevention of maternal–infant transmission of HIV-1. JAMA. 2001;285:2083–93. doi: 10.1001/jama.285.16.2083. [DOI] [PubMed] [Google Scholar]
- 2.Cooper ER, Charurat M, Mofenson L, et al. Combination anti-retroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–94. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Dorenbaum A, Cunningham CK, Gelber RD, et al. Two-dose intrapartum/newborn nevirapine and standard anti-retroviral therapy to reduce perinatal HIV transmission: a randomized trial. JAMA. 2002;288:189–98. doi: 10.1001/jama.288.2.189. [DOI] [PubMed] [Google Scholar]
- 4.The European Collaborative Study. Mother-to-child transmission of HIV infection in the era of highly active anti-retroviral therapy. Clin Infect Dis. 2005. pp. 458–65. [DOI] [PubMed]
- 5.Magder LS, Mofenson L, Paul ME, et al. Risk factors for in utero and intrapartum transmission of HIV. J Acquir Immune Defic Syndr. 2005;38:87–95. doi: 10.1097/00126334-200501010-00016. [DOI] [PubMed] [Google Scholar]
- 6.Brossard Y, Aubin JT, Mandelbrot L, et al. Frequency of early in utero HIV-1 infection: a blind DNA polymerase chain reaction study on 100 fetal thymuses. AIDS. 1995;9:359–66. [PubMed] [Google Scholar]
- 7.Rouzioux C, Costagliola D, Burgard M, et al. Estimated timing of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission by use of a Markov model. Am J Epidemiol. 1995;142:1330–7. doi: 10.1093/oxfordjournals.aje.a117601. The HIV Infection in Newborns French Collaborative Study Group. [DOI] [PubMed] [Google Scholar]
- 8.Moussa M, Mognetti B, Dubanchet S, et al. Vertical transmission of HIV: parameters which might affect infection of placental trophoblasts by HIV-1: a review. Am J Reprod Immunol. 1999;41:312–9. doi: 10.1111/j.1600-0897.1999.tb00444.x. Biomed Group on the Study of In Utero Transmission of HIV 1. [DOI] [PubMed] [Google Scholar]
- 9.Derrien M, Faye A, Dolcini G, Chaouat G, Barre-Sinoussi F, Menu E. Impact of the placental cytokine–chemokine balance on regulation of cell–cell contact-induced human immunodeficiency virus type 1 translocation across a trophoblastic barrier in vitro. J Virol. 2005;79:12304–10. doi: 10.1128/JVI.79.19.12304-12310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton GJ, Watson AL. The structure of the human placenta: implications for initiating and defending against virus infections. Rev Med Virol. 1997;7:219–28. doi: 10.1002/(sici)1099-1654(199712)7:4<219::aid-rmv205>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Kilani RT, Chang LJ, Garcia-Lloret MI, Hemmings D, Winkler-Lowen B, Guilbert LJ. Placental trophoblasts resist infection by multiple human immunodeficiency virus (HIV) type 1 variants even with cytomegalovirus coinfection but support HIV replication after provirus transfection. J Virol. 1997;71:6359–72. doi: 10.1128/jvi.71.9.6359-6372.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menu E, M'Bopi Keou FX, Lagaye S, et al. Selection of maternal human immunodeficiency virus type 1 variants in human placenta. J Infect Dis. 1999;179:44–51. doi: 10.1086/314542. European Network for In Utero Transmission of HIV-1. [DOI] [PubMed] [Google Scholar]
- 13.Saito S. Cytokine network at the feto–maternal interface. J Reprod Immunol. 2000;47:87–103. doi: 10.1016/s0165-0378(00)00060-7. [DOI] [PubMed] [Google Scholar]
- 14.Fox H. Fox H, Pathology of the placenta. 2. London: WB Saunders Ltd; 1997. The development and structure of the placenta; pp. 1–42. [Google Scholar]
- 15.Delage G, Moreau JF, Taupin JL, et al. [In vitro modulation of the production of the cytokine HILDA/LIF, secreted by human endometrial explants: preliminary results] Contracept Fertil Sex. 1995;23:622–5. [PubMed] [Google Scholar]
- 16.Giess R, Tanasescu I, Steck T, Sendtner M. Leukaemia inhibitory factor gene mutations in infertile women. Mol Hum Reprod. 1999;5:581–6. doi: 10.1093/molehr/5.6.581. [DOI] [PubMed] [Google Scholar]
- 17.Meisser A, Chardonnens D, Campana A, Bischof P. Effects of tumour necrosis factor-alpha, interleukin-1 alpha, macrophage colony stimulating factor and transforming growth factor beta on trophoblastic matrix metalloproteinases. Mol Hum Reprod. 1999;5:252–60. doi: 10.1093/molehr/5.3.252. [DOI] [PubMed] [Google Scholar]
- 18.Bowen JM, Chamley L, Mitchell MD, Keelan JA. Cytokines of the placenta and extra-placental membranes: biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta. 2002;23:239–56. doi: 10.1053/plac.2001.0781. [DOI] [PubMed] [Google Scholar]
- 19.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 20.Chaouat G, Assal Meliani A, Martal J, et al. IL-10 prevents naturally occurring fetal loss in the CBA × DBA/2 mating combination, and local defect in IL-10 production in this abortion-prone combination is corrected by in vivo injection of IFN-tau. J Immunol. 1995;154:4261–8. [PubMed] [Google Scholar]
- 21.Patterson BK, Behbahani H, Kabat WJ, et al. Leukemia inhibitory factor inhibits HIV-1 replication and is upregulated in placentae from nontransmitting women. J Clin Invest. 2001;107:287–94. doi: 10.1172/JCI11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tjernlund A, Fleener Z, Behbahani H, et al. Suppression of leukemia inhibitor factor in lymphoid tissue in primary HIV infection: absence of HIV replication in gp130-positive cells. AIDS. 2003;17:1303–10. doi: 10.1097/00002030-200306130-00004. [DOI] [PubMed] [Google Scholar]
- 23.Cheret A, Le Grand R, Caufour P, et al. RANTES, IFN-gamma, CCR1, and CCR5 mRNA expression in peripheral blood, lymph node, and bronchoalveolar lavage mononuclear cells during primary simian immunodeficiency virus infection of macaques. Virology. 1999;255:285–93. doi: 10.1006/viro.1998.9558. [DOI] [PubMed] [Google Scholar]
- 24.Maciaszek JW, Parada NA, Cruikshank WW, Center DM, Kornfeld H, Viglianti GA. IL-16 represses HIV-1 promoter activity. J Immunol. 1997;158:5–8. [PubMed] [Google Scholar]
- 25.Berger EA, Murphy PM, Farber JM. Chemokines receptors as HIV-1 coreceptors: roles in viral entry, tropism and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 26.Clouse KA, Powell D, Washington I, et al. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989;142:431–8. [PubMed] [Google Scholar]
- 27.Folks TM, Clouse KA, Justement J, et al. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci USA. 1989;86:2365–8. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimoya K, Moriyama A, Matsuzaki N, et al. Human placental cells show enhanced production of interleukin (IL) -8 in response to lipopolysaccharide (LPS), IL-1 and tumour necrosis factor (TNF)-alpha, but not to IL-6. Mol Hum Reprod. 1999;5:885. doi: 10.1093/molehr/5.9.885. [DOI] [PubMed] [Google Scholar]
- 29.Weissman D, Poli G, Fauci AS. Interleukin 10 blocks HIV replication in macrophages by inhibiting the autocrine loop of tumor necrosis factor alpha and interleukin 6 induction of virus. AIDS Res Hum Retroviruses. 1994;10:1199–206. doi: 10.1089/aid.1994.10.1199. [DOI] [PubMed] [Google Scholar]
- 30.Weissman D, Poli G, Fauci AS. IL-10 synergizes with multiple cytokines in enhancing HIV production in cells of monocytic lineage. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:442–9. [PubMed] [Google Scholar]
- 31.Al-Harthi L, Guilbert LJ, Hoxie JA, Landay A. Trophoblasts are productively infected by CD4-independent isolate of HIV type 1. AIDS Res Hum Retroviruses. 2002;18:13–7. doi: 10.1089/088922202753394673. [DOI] [PubMed] [Google Scholar]
- 32.Menu E, Mognetti B, Moussa M, et al. Insights into the mechanisms of vertical transmission of HIV-1. Early Pregnancy. 1997;3:245–58. BIOMED2 Working Group on the in utero transmission of HIV-1. [PubMed] [Google Scholar]
- 33.Coulomb-L'Hermine A, Emilie D, Durand-Gasselin I, Galanaud P, Chaouat G. SDF-1 production by placental cells: a potential mechanism of inhibition of mother-to-fetus HIV transmission. AIDS Res Hum Retroviruses. 2000;16:1097–8. doi: 10.1089/08892220050075372. [DOI] [PubMed] [Google Scholar]
- 34.Behbahani H, Popek E, Garcia P, et al. Up-regulation of CCR5 expression in the placenta is associated with human immunodeficiency virus-1 vertical transmission. Am J Pathol. 2000;157:1811–8. doi: 10.1016/S0002-9440(10)64819-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee BN, Ordonez N, Popek EJ, et al. Inflammatory cytokine expression is correlated with the level of human immunodeficiency virus (HIV) transcripts in HIV-infected placental trophoblastic cells. J Virol. 1997;71:3628–35. doi: 10.1128/jvi.71.5.3628-3635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silvestri G, Feinberg MB. Turnover of lymphocytes and conceptual paradigms in HIV infection. J Clin Invest. 2003;112:821–4. doi: 10.1172/JCI19799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–8. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 38.Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to viral load. J Immunol. 2002;169:3400–6. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- 39.Sperling RS, Shapiro DE, Coombs RW, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. N Engl J Med. 1996;335:1621–9. doi: 10.1056/NEJM199611283352201. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. [DOI] [PubMed] [Google Scholar]
- 40.Mofenson LM, Lambert JS, Stiehm ER, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. N Engl J Med. 1999;341:385–93. doi: 10.1056/NEJM199908053410601. Pediatric AIDS Clin Trials Group Study 185 Team. [DOI] [PubMed] [Google Scholar]
- 41.Taupin JL, Gualde N, Moreau JF. A monoclonal antibody based elisa for quantitation of human leukaemia inhibitory factor. Cytokine. 1997;9:112–8. doi: 10.1006/cyto.1996.0144. [DOI] [PubMed] [Google Scholar]
- 42.Pornprasert S, Faye A, Mary JY, et al. Down modulation of TNF-alpha mRNA placental expression by AZT used for the prevention of HIV-1 mother-to-child transmission. Placenta. 2006;27:989–95. doi: 10.1016/j.placenta.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Faye A, Pornprasert S, Dolcini G, et al. Evaluation of the placental environment with a new in vitro model of histocultures of early and term placentae: determination of cytokines and chemokines expression profiles. Placenta. 2005;26:262–7. doi: 10.1016/j.placenta.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Sperling RS, Shapiro DE, McSherry GD, et al. Safety of the maternal–infant zidovudine regimen utilized in the Pediatric AIDS Clinical Trial Group 076 Study. Aids. 1998;12:1805–13. doi: 10.1097/00002030-199814000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Patterson BK, Tjernlund A, Andersson J. Endogenous inhibitors of HIV: potent anti-HIV activity of leukemia inhibitory factor. Curr Mol Med. 2002;2:713–22. doi: 10.2174/1566524023361817. [DOI] [PubMed] [Google Scholar]
- 46.Moussa M, Roques P, Fievet N, et al. Placental cytokine and chemokine production in HIV-1-infected women: trophoblast cells show a different pattern compared to cells from HIV-negative women. Clin Exp Immunol. 2001;125:455–64. doi: 10.1046/j.1365-2249.2001.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou P, Goldstein S, Devadas K, Tewari D, Notkins AL. Human CD4+ cells transfected with IL-16 cDNA are resistant to HIV-1 infection: inhibition of mRNA expression. Nat Med. 1997;3:659–64. doi: 10.1038/nm0697-659. [DOI] [PubMed] [Google Scholar]
- 48.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 49.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 50.Zachar V, Fazio-Tirrozzo G, Fink T, et al. Lack of protection against vertical transmission of HIV-1 by interferons produced during pregnancy in a cohort from East African republic of Malawi. J Med Virol. 2000;61:195–200. doi: 10.1002/(sici)1096-9071(200006)61:2<195::aid-jmv4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 51.Lee BN, Hammill H, Popek EJ, et al. Production of interferons and beta-chemokines by placental trophoblasts of HIV-1-infected women. Infect Dis Obstet Gynecol. 2001;9:95–104. doi: 10.1155/S1064744901000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zachar V, Fink T, Koppelhus U, Ebbesen P. Role of placental cytokines in transcriptional modulation of HIV type 1 in the isolated villous trophoblast PG-839-47. AIDS Res Hum Retroviruses. 2002;18:839–47. doi: 10.1089/08892220260190317. [DOI] [PubMed] [Google Scholar]
- 53.Vidricaire G, Tardif MR, Tremblay MJ. The low viral production in trophoblastic cells is due to a high endocytic internalization of the human immunodeficiency virus type 1 and can be overcome by the pro-inflammatory cytokines tumor necrosis factor-alpha and interleukin-1. J Biol Chem. 2003;278:15832–41. doi: 10.1074/jbc.M210470200. [DOI] [PubMed] [Google Scholar]
- 54.Lallemant M, Jourdain G, Le Coeur S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;35:217–28. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]
- 55.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 56.Poirier MC, Olivero OA, Walker DM, Walker VE. Perinatal genotoxicity and carcinogenicity of anti-retroviral nucleoside analog drugs. Toxicol Appl Pharmacol. 2004;199:151–61. doi: 10.1016/j.taap.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 57.Poirier MC, Divi RL, Al-Harthi L, et al. Long-term mitochondrial toxicity in HIV-uninfected infants born to HIV-infected mothers. J Acquir Immune Defic Syndr. 2003;33:175–83. doi: 10.1097/00126334-200306010-00010. [DOI] [PubMed] [Google Scholar]
- 58.Collier AC, Helliwell RJ, Keelan JA, Paxton JW, Mitchell MD, Tingle MD. 3′-azido-3′-deoxythymidine (AZT) induces apoptosis and alters metabolic enzyme activity in human placenta. Toxicol Appl Pharmacol. 2003;192:164–73. doi: 10.1016/s0041-008x(03)00274-6. [DOI] [PubMed] [Google Scholar]
- 59.Blanche S, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction and perinatal exposure to anti-retroviral nucleoside analogues. Lancet. 1999;354:1084–9. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- 60.Barret B, Tardieu M, Rustin P, et al. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. Aids. 2003;17:1769–85. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]