Abstract
Our aim was to assess anti-inflammatory effects on the peripheral blood of subjects with inflammatory bowel disease (IBD) who consumed probiotic yogurt for 1 month. We studied 20 healthy controls and 20 subjects with IBD, 15 of whom had Crohn's disease and five with ulcerative colitis. All the subjects consumed Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 supplemented yogurt for 30 days. The presence of putative regulatory T (Treg) cells (CD4+ CD25high) and cytokines in T cells, monocytes and dendritic cells (DC) was determined by flow cytometry from peripheral blood before and after treatment, with or without ex vivo stimulation. Serum and faecal cytokine concentrations were determined by enzyme-linked immunosorbent assays. The proportion of CD4+ CD25high T cells increased significantly (P = 0.007) in IBD patients, mean (95% confidence interval: CI) 0.84% (95% CI 0.55–1.12) before and 1.25% (95% CI 0.97–1.54) after treatment, but non-significantly in controls. The basal proportion of tumour necrosis factor (TNF)-α+/interleukin (IL)-12+ monocytes and myeloid DC decreased in both subject groups, but of stimulated cells only in IBD patients. Also serum IL-12 concentrations and proportions of IL-2+ and CD69+ T cells from stimulated cells decreased in IBD patients. The increase in CD4+ CD25high T cells correlated with the decrease in the percentage of TNF-α- or IL-12-producing monocytes and DC. The effect of the probiotic yogurt was confirmed by a follow-up study in which subjects consumed the yogurt without the probiotic organisms. Probiotic yogurt intake was associated with significant anti-inflammatory effects that paralleled the expansion of peripheral pool of putative Treg cells in IBD patients and with few effects in controls.
Keywords: anti-inflammatory, inflammatory bowel disease, probiotics
Introduction
There is evidence to suggest that probiotic bacteria may have, in a species- or even strain-dependent manner, a potential use as anti-inflammatory agents in some chronic inflammatory diseases [1,2]. The most promising clinical results have been obtained in the prevention and management of atopic eczema and the management of inflammatory bowel disease (IBD) and post-operative pouchitis [3,4]. On the basis of experimental data, the anti-inflammatory effects of probiotics may be a consequence of antagonism against potentially pathogenic/proinflammatory endogenous microbiota; modulation of the balance between T helper 1 (Th1), Th2 and regulatory T (Treg) cells; down-regulation of proinflammatory [e.g. interleukin (IL)-12, tumour necrosis factor (TNF)-α] and/or stimulation of anti-inflammatory (e.g. IL-10) cytokine production; as well as effects seen such as enhanced elimination, modified degradation, permeation and presentation of proinflammatory antigens [3–5].
Data accumulated in the past few years have emphasized the central role of Treg cells in the formation and maintenance of tolerance to mucosally encountered antigens and down-regulation of ongoing inflammation [6,7]. Accordingly, their deficient activity, in contrast to enhanced Th1 or Th2 action, has been implicated in chronic inflammatory conditions, including allergic diseases and IBD [7–9]. Dendritic cells (DC) are thought to be the primary regulators in determining the balance between the different T cell subtype activities in a manner that is dependent upon multiple factors, including the DC subtype and local cytokine milieu, with the presence of IL-10 and IL-12 having particular importance [10–12]. Interestingly, a recent study in mice demonstrated that probiotic bacteria may confer protection against chemically induced intestinal inflammation by induction of Treg cells [5]. Whether any probiotic strains have this ability in humans is unknown, but is feasible based upon in vitro data and indirect evidence from clinical studies demonstrating that the intake of certain probiotics may enhance the production of IL-10 [13] and transforming growth factor (TGF)-β[14,15] which could, theoretically, promote the induction of Treg cells or be indicative of enhanced Treg function [16–18].
The aim of the current study was to assess whether intake of yogurt supplemented with two probiotic strains, Lactobacillus rhamnosus GR-1 and L. reuteri RC-14, with documented efficacy in controlling mucosal infections [19] and the ability to pass through the gastrointestinal tract alive, may promote an anti-inflammatory immunological milieu in subjects with active chronic inflammatory conditions, namely Crohn's disease or ulcerative colitis. These conditions are characterized by chronic intestinal inflammation that is thought to result from exaggerated effector T cell responses towards endogenous bacterial antigens [9]. There were two reasons for using a yogurt delivery system. First, patients with chronic inflammation are often receiving a range of pharmaceutical agents, some of which have side effects including diarrhoea and loss of appetite. Potentially, yogurt could provide an excellent nutritional supplement that reduced diarrhoeal problems. Secondly, we wanted to test the effect of probiotic yogurt on peripheral immunity as a step towards taking this food to populations that have little access to, and no buying power for the purchase of, pharmaceutical products. One such application is to developing countries, and to that end we have established active collaborations in Tanzania and Nigeria where, in the former case, local people have begun to produce the probiotic yogurt themselves in order to support the health of their families and community (http://www.westernheadseast.ca).
Materials and methods
Subjects
It should be noted that the principal aim of this project was not to cure IBD or study clinical outcomes, as the aim was to determine if any effects arose with a nutritional change that did not require any alteration in the standard medical management of the patient. To that end, no effort was made to control for use of steroids, although no subjects received antibiotics during the study. The study population comprised 20 subjects with IBD and 20 healthy controls with no known or suspected intestinal abnormalities. The mean age ± standard deviation (s.d.) of IBD patients was 44 ± 11.7 (range 26–63) years and that of controls 51 ± 6.4 (38–61) years. Of the IBD patients, 15 had Crohn's disease, five had ulcerative colitis and all had subjective symptoms, including liquid or very soft stools and/or abdominal pain, indicative of active IBD. To reduce patient-to-patient variability, all the subjects were women. Exclusion criteria included pregnancy, use of antibiotics, lactose intolerance and premature termination of the study (only three of 23 healthy subjects were excluded due to inability to comply with the study protocol). All subjects were asked to continue with their habitual diet but to refrain from taking any other yogurt or probiotic supplements 2 weeks before and during the study. The patient group did not alter any ongoing medication being given for their IBD. Informed consent was obtained from all subjects and the study was approved by the Review Board for Health Sciences Research involving Human Subjects, at the University of Western Ontario, London, Ontario, Canada.
Design
In this open-label study, all subjects consumed 125 g of probiotic yogurt per day for 30 days. The researchers were blinded regarding the study groups. To rule out the influence of yogurt alone, the treatment regimen was repeated in an exploratory study using unsupplemented yogurt with a subpopulation of the same IBD patients (n = 8; six with Crohn's disease, two with ulcerative colitis) after a washout period of 6 months.
The main outcome parameters measured were changes in the prevalence of putative Treg cells (CD4+ CD25high) and TNF-α- and IL-12-producing monocytes and DC in peripheral blood (PB) during treatment. Secondary outcome measures included changes in the presence of T cell surface activation markers, serum and faecal cytokine concentrations and ex vivo proliferative responses of PB mononuclear cells (PBMC). Individual stool and blood samples were collected before (day 0) and after (day 30) the treatment period.
The patients were asked to note in a diary any changes in symptoms, including bloating, gas, abdominal pain and constipation/loose stools throughout the study as possible side effects of the yogurt consumption.
Preparation of probiotic yogurt
To prepare a probiotic mother culture, dried L. rhamnosus GR-1 (GR-1) and L. reuteri RC-14 (RC-14) were added to Man, Rogosa and Sharpe broth (EM Science, Gibbstown, NJ, USA) at a rate of 1.5% and grown anaerobically at 37°C overnight. Then a mixture of milk (1% fat), 0.33% yeast extract and 0.4% inulin was autoclaved for 15 min, cooled to 37°C, and inoculated with the probiotic culture at a rate of 1% and incubated anaerobically at 37°C overnight.
To prepare probiotic yogurt, a mixture with milk (1% fat) and 5% sugar was heat-treated at 87°C for 30 min, cooled to 37°C, inoculated with 4% of the probiotic mother culture and 2% of standard plain yogurt containing L. delbreukii ssp. bulgaricus and Streptococcus thermophilus, fermented at 37°C for 6 h and stored at 4°C. After 2 days 11% strawberry flavouring (Sensient, Rexdale, ON, Canada) was added and the yogurts were packaged. Viable counts and quality assurance was tested at regular intervals. A new batch of yogurt was produced every 2 weeks to ensure consistency in viable counts of probiotic bacteria, especially as those of RC-14 decreased rapidly with time. After 2 weeks at 4°C the total counts were consistently at 1 × 103 for RC-14 and 2 × 107 colony-forming units (cfu)/ml for GR-1. No contaminants were isolated at any time in the study.
Analysis of intracellular cytokine production
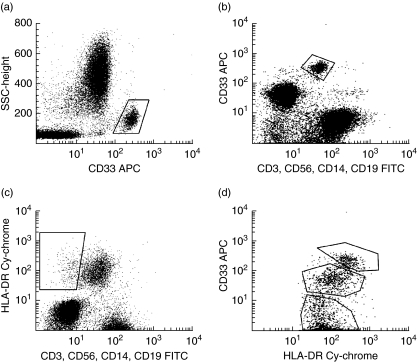
Intracellular cytokine detection was performed by flow cytometry as described previously, with some modifications [20,21]. PB samples in lithium heparin were supplemented one-to-one with RPMI-1640 medium (Invitrogen, Burlington, ON, Canada), incubated at 37°C in a 5% CO2 humidified atmosphere with brefeldin A (10 μg/ml, Sigma, St Louis, MO, USA) in the presence or absence of lipopolysaccharide (LPS, 100 ng/ml; from Escherichia coli, serotype 055:B5, Sigma) plus interferon (IFN)-γ (100 units/ml; R&D Systems, Inc., Minneapolis, MN, USA) for stimulation (6 h) of cytokine production by monocytes and DC; ionomycin (1 μg/ml, Sigma) plus phorbol 12-myristate 13-acetate (PMA, 25 ng/ml, Sigma) for stimulation (4 h) of cytokine production by T cells. For identification of the whole DC population [major histocompatibility complex (MHC) II+/lineage-/CD33+/–], their highly and intermediately CD33-expressing myeloid (CD33high, CD33intermed) and no or weakly CD33-expressing plasmocytoid (CD33–/low) subsets and monocytes (MHC II+/CD14+/CD33+), PB cells were then incubated for 15 min at room temperature (RT) with anti-human leucocyte antigen D-related (HLA-DR)-Cy-chrome, anti-CD33-allophycocyanin (APC) and each of the following fluorescein isothiocyanate (FITC)-labelled lineage marker antibodies: anti-CD3, anti-CD19, anti-CD56 and anti-CD14 (BD Biosciences, San Diego, CA, USA). Stained cells were washed with phosphate-buffered saline (PBS, pH 7.5) and centrifugation (5 min at 540 g), fixed, permeabilized and stained with anti-TNF-α-phycoerythrin (PE, clone MAb11) and anti-IL-12-PE (C11.5) using the Fix & Perm reagent (Caltag, Burlingame, CA, USA) following the manufacturer's instructions. T cell cytokines were analysed accordingly, but the cells were identified with anti-CD3-FITC and their cytokines detected with anti-IL-2-PE (clone MQ1-17H12), anti-IFN-γ-PE (B27), anti-IL-4-PE (8D4-8) and anti-IL-10-PE (JES3-19F1). Data acquisition was performed in two consecutive steps with a flow cytometer (FACSCalibur™, BD Biosciences). First, 30 000 events/test corresponding to the whole PB cellularity were collected for analysis of cytokines produced by T cells and monocytes. Secondly, only events in a HLA-DR+/CD3–/CD19–/CD56–/CD14– live gate were stored and a minimum of 300 000 events from the total PB cellularity were acquired in order to obtain at least 1000 MHC II+/lineage– cells for the analysis of cytokines produced by DC subsets. CellQuest™ software (BD Biosciences) was used for data acquisition and analysis. Representative acquisition dot plots demonstrating the identification of monocytes and DC are presented in Fig. 1.
Fig. 1.
Identification of monocytes and dendritic cells (DC) by flow cytometry. A representative example of the identification of monocytes based on the expression of (a) CD33 and (b) CD14 antigen. (c) Identification of DC as a human leucocyte antigen D-related (HLA-DR)+ lineage– (CD3–, CD56–, CD14–, CD19–) population. (d) After acquiring a higher number of cells within the HLA-DR+ lineage– live gate, three different dendritic cell subsets were identified on the basis of CD33 expression: myeloid CD33high, CD33intermed and plasmocytoid CD33–/low.
Analysis of T cell surface markers
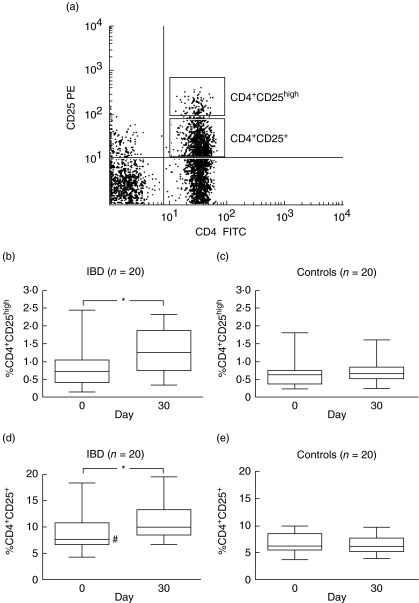
For the expression of early activation marker CD69 on T cells, RPMI-1640 diluted PB was incubated with or without PMA and ionomycin as described above, whereas only the unstimulated sample was used for Treg cell analysis. The percentage of CD4+ CD25+ Treg cells are enriched within the 1–2% of PB CD4+ T cells expressing high levels of CD25, while the population expressing lower levels of CD25 is thought to consist mainly of activated effector T cells [22]. Thus, using flow cytometry we gated on small lymphocytes and CD4+ T cells were subdivided into bright (CD4+ CD25high/Treg) and intermediate (CD4+ CD25+/activated T cell) populations based on their CD25 expression (Fig. 2a). The stimulated and/or unstimulated samples (200 μl each) were stained with 3 μl of anti-CD3-FITC in combination with anti-CD69-PE or anti-CD4-FITC plus anti-CD25-PE (BD Biosciences) for 15 min at RT. Data were acquired with a flow cytometer (30 000 events/test) and analysed as described above.
Fig. 2.
(a) A representative example of the analysis of CD4+ CD25+ cells by flow cytometer. Lymphocytes were gated according to their particular small forward-/side-scatter profile and the CD4+ CD25high and CD4+ CD25+ cells identified based on the concentration of CD25 expression. (b–e) Percentage of CD4+ CD25high and CD4+ CD25+ cells in peripheral blood before (0 day) and after (30 days) probiotic yogurt treatment in inflammatory bowel disease (IBD) patients and controls. Data are shown as box plots with median and 10th, 25th, 75th and 90th percentiles. *Significant (P < 0.05) increase in CD4+ CD25+ and CD4+ CD25high cells in IBD patients following treatment. #The basal (0 day) percentage of CD4+ CD25+ cells was significantly higher in IBD patients than in controls (P = 0.04).
Enzyme-linked immunosorbent assays (ELISA)
Faecal extracts were prepared by mixing 3 g of stool with 3 ml of PBS followed by centrifugation (30–45 min at 20 000 g) at 4°C and filtration of the supernatant through a 0.45-μm pore-size filter [23]. Serum samples and faecal extract aliquots were stored at −70°C until analysis. The concentrations of TNF-α, IL-12 and IL-10 were measured with BD OptEIA™ ELISA sets (BD Biosciences) according to the manufacturer's instructions.
Proliferation assay
Cell-free extracts (CFE) of RC-14 and GR-1 were prepared from capsules containing 1 × 109 cfu of RC-14 and GR-1 [24,25]. The bacteria were washed twice and suspended in PBS (1 ml) and then bead-beaten with 300 mg of zirconium beads (0.1 mm) (3 min at 2300 g) using a mini-bead beater (Biospec Products, Bartlesville, OK, USA). Particulates were removed by centrifugation (10 min at 12 000 g) and the protein concentration in the supernatants (CFE) determined with the bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL, USA), with bovine serum albumin as the protein standard. PBMC were isolated from PB in sodium heparin by Ficoll-Hypaque (Pharmacia Biotech,Uppsala, Sweden) gradient centrifugation. PBMC (0.5 × 106/ml) were cultured in RPMI-1640 with 2 mM l-glutamine, penicillin (100 U/ml), streptomycin (100 μg/ml) and 10% fetal bovine serum supplemented with CFE in the presence or absence of ionomycin (100 ng/ml) plus PMA (100 ng/ml) for 4 days at 37°C in a 5% CO2 humidified atmosphere. Cultured cells were then incubated further on 96-well plates (200 μl/well in triplicate) for 4 h at 37°C with 20 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma) (2.5 mg/ml in PBS) per well. The plates were centrifuged (5 min at 500 g) and supernatants were removed. Hydrochloric acid (HCL) (0.04 N) in isopropanol (100 μl) was added to each well and absorbance measured at 575 nm (reference wavelength 650 nm) with a microplate reader (Bio-Rad Model 550).
Statistics
Statistical analysis was performed with GraphPad Prism® version 4 (GraphPad, Software, Inc., San Diego, CA, USA) and StatView® version 4.57 (Abacus Concepts, Inc., Berkeley, CA, USA) with the exception of the Exact unconditional test for 2 × 2 tables, which was used for comparing frequency of symptoms before and after treatment [26]. Changes in immunological measurements between two time-points within a subject group were compared with the paired two-tailed t-test if the data were parametric with or without natural logarithmic transformation and by the Wilcoxon signed-rank test if the data were nonparametric and non-transformable. Differences between subject groups were compared with the unpaired two-tailed t-test if the datawere parametric and with Mann–Whitney U-test if the data were nonparametric and non-transformable. Correlations between two continuous variables were analysed by Spearman's rank correlation test. P-values < 0.05 were considered statistically significant.
Results
Effect of probiotic yogurt intake on T cells
The percentage of CD4+ CD25high cells increased significantly following treatment with probiotic yogurt from the group mean (95% confidence interval, CI) of 0.84% (95% CI 0.55–1.12) to 1.25% (95% CI 0.97–1.54) (P = 0.007, Fig. 2b). In controls the response was significantly different (P = 0.03) with little increase from before, 0.69% (95% CI 0.50–0.87%95% CI), to after, 0.73% (95% CI 0.59–0.87) the treatment (P = 0.09) (Fig. 2c). Similarly, the change in the percentage of CD4+ CD25+ T cells was significantly different between the groups (P = 0.01) with an increase from 9.1% (95% CI 7.2–11.0%95% CI) to 11.0% (95% CI 9.5–13.1) (P = 0.003, Fig. 2c) in IBD patients and no change from before, 6.68% (95% CI 5.78–7.59), to after, 6.47% (95% CI 5.69–7.24) the treatment in controls (P = 0.36, Fig. 2e).
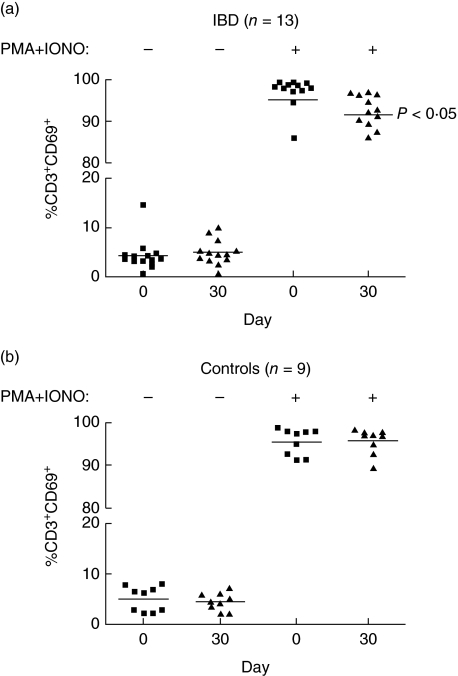
In IBD patients, but not in controls, the treatment was followed by a reduced percentage of CD3+ T cells responding to polyclonal ex vivo stimulation by production of IL-2. In IBD patients the mean percentage of IL-2+ CD3+ T cells was 42.3% (95% CI 35.4–49.2) before and 38.2% (32.2–44.2%) (P = 0.03) after the treatment, while the respective values for controls were 42.4% (95% CI 36.7–48.0) and 44.4% (95% CI 39.3–49.4) (P = 0.50). The difference in the change between the groups was not significant (P = 0.20). No other significant effects were observed in the intracellular cytokine production by CD3+ T cells (data not shown). However, the percentage of stimulated T cells expressing CD69 decreased in the IBD patients (P = 0.02), but again, not in the control group (P = 0.77, Fig. 3a,b). This difference between the groups approached statistical significance (P = 0.07).
Fig. 3.
The percentage of CD3+ CD69+ T cells in peripheral blood before (day 0) and after (day 30) probiotic yogurt treatment in inflammatory bowel disease (IBD) patients and controls with (+) or without (–) ex vivo stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin. The horizontal bars represent mean values.
Effect of probiotic yogurt intake on monocytes and DC
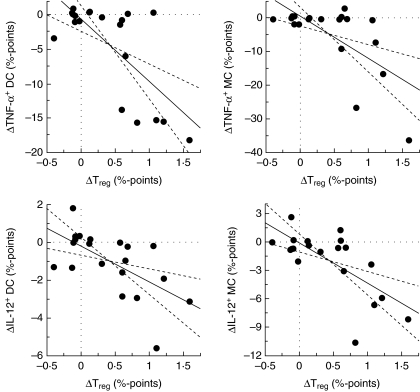
The basal proportion (before treatment) of monocytes and DC which produced TNF-α or IL-12 was higher in the IBD patients compared to controls, with some differences reaching statistical significance (P < 0.05; Table 1). The proportion of monocytes or DC populations in PB per se did not change following treatment with probiotic yogurt, whereas significant decreases were observed in the percentages of unstimulated TNF-α- and IL-12-producing monocytes and myeloid DC subsets in both IBD patients and controls, as summarized in Table 1. In unstimulated and/or stimulated plasmocytoid DC subset the production of these cytokines was very low or undetectable with no significant changes during the treatment (data not shown). Significant correlations were observed between the change in the proportion of Treg cells (increase) following the treatment and the change (decrease) in the proportion of unstimulated TNF-α- and IL-12-producing monocytes (ρ = −0.59, P = 0.01 and ρ = −0.58, P = 0.01, respectively) and DC (ρ = −0.53, P = 0.02 and ρ = −0.61, P = 0.008, respectively) (Fig. 4).
Table 1.
The ex vivo intracellular production of tumour necrosis factor (TNF)-α and interleukin (IL)-12 by unstimulated and stimulated peripheral blood (PB) monocytes and dendritic cells (DC) from inflammatory bowel disease (IBD) patients and controls before and after treatment with probiotic yogurt.
| % Cells in total in PB (mean ± s.e.)/% cytokine-producing cells (mean ± s.e.) | ||||
|---|---|---|---|---|
| IBD patients (n = 20) | Controls (n = 20) | |||
| Cell type/cytokine | Before treatment | After treatment | Before treatment | After treatment |
| Monocytes | 4.9 ± 0.4 | 4.4 ± 0.4 | 4.0 ± 0.3 | 3.9 ± 0.4 |
| TNF+ basala | 6.4 ± 2.4* | 1.6 ± 0.5‡ | 2.7 ± 0.4 | 1.5 ± 0.3§ |
| TNF+ stimulatedb | 58.1 ± 4.7 | 49.7 ± 3.3‡ | 50.7 ± 4.2 | 49.6 ± 3.7 |
| IL-12+ basal | 3.4 ± 0.7 | 1.5 ± 0.3‡ | 2.1 ± 0.2 | 1.2 ± 0.2‡ |
| IL-12+ stimulated | 21.7 ± 2.5 | 16.3 ± 2.0† | 17.2 ± 2.5 | 14.2 ± 2.5 |
| Dendritic cells (all) | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 |
| TNF+ basal | 5.9 ± 1.7* | 1.4 ± 0.3‡ | 2.2 ± 0.3 | 1.2 ± 0.2§ |
| TNF+ stimulated | 35.9 ± 3.5 | 27.3 ± 2.0‡ | 26.8 ± 3.7† | 29.3 ± 2.4 |
| IL-12+ basal | 2.1 ± 0.5 | 1.1 ± 0.2‡ | 1.2 ± 0.2 | 0.8 ± 0.1 |
| IL-12+ stimulated | 15.5 ± 1.9 | 9.6 ± 1.4§ | 11.5 ± 2.1 | 10.6 ± 2.4 |
| DC CD33high | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.05 | 0.3 ± 0.05 |
| TNF+ basal | 7.8 ± 2.5 | 1.9 ± 0.5‡ | 5.0 ± 1.2 | 1.2 ± 0.2§ |
| TNF+ stimulated | 46.5 ± 4.4* | 42.5 ± 3.5 | 33.4 ± 4.0 | 37.1 ± 3.7 |
| IL-12+ basal | 3.0 ± 0.8 | 1.4 ± 0.3 | 2.0 ± 0.4 | 1.0 ± 0.6§ |
| IL-12+ stimulated | 22.6 ± 3.3 | 14.7 ± 2.1‡ | 15.8 ± 2.1 | 9.7 ± 1.1 |
| DC CD33intermed | 0.2 ± 0.03 | 0.2 ± 0.03 | 0.3 ± 0.03 | 0.5 ± 0.03 |
| TNF+ basal | 5.4 ± 1.4* | 1.5 ± 0.4§ | 2.2 ± 0.4 | 1.5 ± 0.3‡ |
| TNF+ stimulated | 24.8 ± 4.0 | 22.3 ± 3.9 | 25.1 ± 4.9 | 24.4 ± 3.4 |
| IL-12+ basal | 3.3 ± 1.0 | 1.3 ± 0.3§ | 1.1 ± 0.4 | 0.6 ± 0.2 |
| IL-12+ stimulated | 11.9 ± 2.5 | 7.7 ± 1.7‡ | 7.4 ± 1.5 | 7.4 ± 1.5 |
s.e. = Standard error
unstimulated PB culture (6h)
lipopolysaccharide + IFN-γ-stimulated PB culture (6 h)
concentration before treatment significantly different (P < 0.05) from that of controls
change significantly different (P < 0.05) between IBD patients and controls
significant change during treatment at significance concentration of 5% (P < 0.05)
significant change during treatment at significance concentration of 1% (P < 0.01).
Fig. 4.
Correlation between the changes in the percentage of putative regulatory T cells (Treg, CD4+ CD25high) and in the percentage of tumour necrosis factor (TNF)-α- or interleukin (IL)-12-producing dendritic cells (DC) and monocytes (MC) in peripheral blood of inflammatory bowel disease (IBD) patients following treatment with the probiotic yogurt.
Effect of probiotic yogurt intake on serum and stool cytokines
The serum IL-12 concentration decreased significantly in both IBD patients and controls following the intake of probiotic yogurt, the group mean (95% CI) decreasing from 51.6 (95% CI 38.4–64.8) to 44.9 (95% CI 34.5–55.4) pg/ml (P = 0.02) in IBD patients and from 50.1 (95% CI 41.5–58.8) to 46.1 (95% CI 38.9–53.3) pg/ml in controls (P = 0.03). The concentrations of TNF-α and IL-10 were variable in IBD patients and no significant changes were observed (data not shown). In controls, the serum concentrations of TNF-α decreased from group mean (95% CI) 7.6 (95% CI 4.7–10.5) to 5.6 (95% CI 3.4–7.8) pg/ml (P = 0.002), while the faecal concentrations increased from 9.3 (95% CI 3.6–15.0) to 14.2 (95% CI 5.6–22.9) pg/ml (P = 0.006) after treatment.
Patient diaries
Analysis of patient diaries revealed two findings. One of 20 IBD patients reported excess intestinal gas at the time of recruitment and six at the end of the treatment period (P = 0.02), while one of 20 reported subjectively low abdominal pain at the recruitment and six at the end of the treatment period (P = 0.02). These latter six patients had significantly lower mean (95% CI) faecal concentrations of IL-12, 9.1 (95% CI 0.65–17.5) than the remaining IBD patients (n = 14), 13.0 (95% CI 8.9–17.0) pg/ml (P = 0.04) at the end of the treatment period. No other significant changes or correlations with immunological variables were noted regarding the subjective symptoms.
In vitro proliferative responses of PBMC to CFE of RC-14 and GR-1
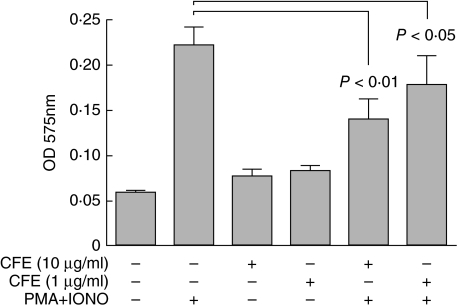
Addition of RC-14/GR-1 CFE to PBMC cultures induced only a marginal increase in proliferation compared to unstimulated PBMC from healthy controls, whereas it appeared to inhibit the PMA + ionomycin-induced proliferation (Fig. 5). Similar results were seen with PBMC from IBD patients and controls before and after consumption of probiotic yogurt (data not shown).
Fig. 5.
Suppressive effect of cell-free extracts (CFE) of Lactobacillus reuteri RC-14 and L. rhamnosus GR-1 on the in vitro proliferative responses of peripheral blood mononuclear cells (PBMC). PBMC obtained from five healthy controls were cultured with (+) or without (–) PMA, ionomycin and CFE. Results are expressed as mean optical density (OD) at 575 nm +s.d., with higher OD corresponding to higher proliferation rate.
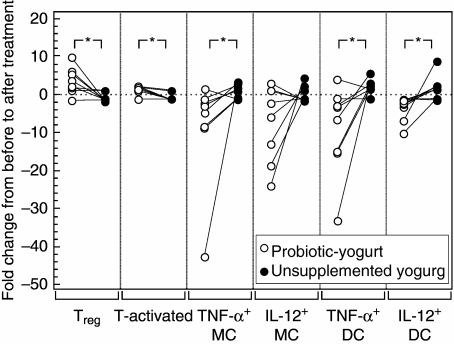
Immunomodulatory properties of unsupplemented yogurt
In the follow-up of eight IBD patients no significant changes were observed in the percentage of Treg cells, activated T cells or TNF-α/IL-12-producing monocytes or DC following the 30-day intake of unsupplemented yogurt. The lack of changes were contrary to the significant changes that followed the intake of probiotic yogurt, as indicated in Fig. 6.
Fig. 6.
Comparison of fold changes in the numbers of regulatory T cells (Treg, CD4+ CD25high), activated T cells (CD4+ CD25+) and tumour necrosis factor (TNF)-α- and interleukin (IL)-12-producing monocytes (MC) and dendritic cells (DC) in inflammatory bowel disease (IBD) patients following treatment with probiotic yogurt or unsupplemented yogurt. Individuals are indicated by connective lines. *Change following treatment with probiotic yogurt significantly different from change following treatment with unsupplemented yogurt (P < 0.05).
Discussion
The results of this study demonstrate that the consumption of probiotic yogurt can result in an increased proportion of putative CD4+ CD25+ Treg cells (CD4+ CD25high) in the peripheral blood of IBD patients. This effect has not been reported previously in humans, although a recent study in a mouse colitis model showed that a dried probiotic cocktail protected against chemically induced intestinal inflammation by the induction of CD4+ TGF-β-bearing Treg cells [5]. Expansion of the peripheral pool of CD4+ CD25high cells is particularly interesting in light of recent data, suggesting that in active Crohn's disease and ulcerative colitis these cells have adequate suppressive function, but the peripheral pool is numerically insufficient in supplying enough of them to the intestinal inflammatory lesions [27]. The expansion of peripheral CD4+ CD25+ Treg cells in IBD patients may therefore have fundamental importance for promoting and maintaining remission. It is noteworthy that the percentages of CD4+ CD25high cells in the patients before and after treatment in the present study are consistent with the values reported by Maul and co-workers [27] for patients with active and inactive IBD, respectively.
The probiotic yogurt intake was associated with a number of potentially anti-inflammatory changes that are in harmony with the putative immunosuppressive role of the expanded CD4+ CD25high cell population. The treatment was followed by a decrease in serum IL-12 concentration and a decreased percentage of TNF-α- and IL-12-producing monocytes and myeloid DC. Monocyte-produced TNF-α is one of the central final mediators in the inflammatory cascade of IBD. Its aetiological significance is well demonstrated by the success of TNF-α antibody treatments in inducing and maintaining remission [28]. IL-12 is the primary cytokine in directing T cell differentiation towards Th1 effector cells and thus is considered to be among the major cytokines in the pathogenesis of Crohn's disease [9]. An indirect correlation was observed between production of TNF-α and IL-12 by monocytes and DC and the numbers of CD4+ CD25high cells in IBD patients. The causal relationship in such a correlation may be bidirectional. On one hand, low numbers of IL-12-producing monocytes and DC may indicate the expansion of an immature population of these potential antigen-presenting cells, DC in particular, which may then direct the T cell differentiation towards Treg cells [29]. There is some indication that this would be a characteristic immunosuppressive response to Gram-positive commensal bacteria − an unlikely threat to the host [30]. On the other hand, established expansion of the Treg cell population is likely to suppress the proinflammatory responses by monocytes and DC [31,32]. The increase in CD4+ CD25high cells was also paralleled by reduced ex vivo production of IL-2 by T cells in response to polyclonal stimulus, a characteristic in vitro effect of CD4+ CD25+ Treg cells [33]. Down-regulated T cell responsiveness was indicated further by reduced expression of the early T cell activation marker CD69 in response to ex vivo stimulus. However, the lack of influence on other T cell cytokines than IL-2 implies that the treatment did not have any considerable influence on the peripheral Th1/Th2 balance.
The data with unsupplemented yogurt indicate that the anti-inflammatory effects seen in the current study were dependent upon the presence of the Lactobacillus probiotic strains GR-1 and RC-14. The immunosuppressive capacity of these strains is supported by the finding that CFE of GR-1 and RC-14 inhibited the proliferative response of PBMC to polyclonal stimulus in vitro. Notably, the anti-inflammatory effects seen here are also consistent with previous in vitro data from our group, indicating that spent media from GR-1 culture inhibited the production of proinflammatory cytokines, including TNF and IL-12, with no effect on IL-10 production by murine macrophages exposed to E. coli or LPS [34].
The ability to expand the peripheral pool of CD4+ CD25+ Treg cells could be beneficial in a wide variety of applications: in addition to the presumable ability to prevent and treat IBD, as suggested by animal studies [5,35], these cells can prevent and limit reactions to allergens, inhibit organ transplant rejections [36,37] and prevent autoimmune diseases, including arthritis [38] and insulitis as well as autoimmune thyroiditis and gastritis [39,40]. Further studies will determine whether or not significant expansion of the CD4+ CD25high cell population, associated with the intake of probiotic yogurt, is evident only in IBD patients. In the current study, only a minor expansion was seen in healthy controls. Such a difference to IBD patients is in agreement with previous studies indicating that probiotic therapies have commonly distinct effects on subjects with healthy versus inflamed mucosa [41]. Overall, fewer and generally more moderate changes in immunological parameters were observed in healthy subjects in this study. The effects were, however, in line with the anti-inflammatory effects seen in IBD patients, including a decrease in basal concentrations of IL-12- and TNF-α-producing monocytes and myeloid DC as well as a decrease in serum TNF-α concentrations. In contrast, faecal TNF-α concentration increased during the treatment, a reminder that peripheral effects and local effects in the intestine can be dramatically different.
Conclusion
Short-term consumption of yogurt supplemented with Lactobacillus strains GR-1 and RC-14 promoted the formation of a desirable anti-inflammatory environment in the peripheral blood of IBD patients, and showed no harmful effects in these patients or control subjects. This effect was associated with an increase in the presence of CD4+ CD25high cells, a putative population of CD4+ CD25+ Treg cells. Further clinical studies are now warranted to confirm the immunosuppressive functions and assess whether these peripheral effects are reflective of beneficial anti-inflammatory action locally in the intestine, resulting in clinical benefits such as prolonged remission of IBD.
In terms of the potential application of this nutritional supplement to patients with underlying complaints, such as HIV/AIDS patients with chronic diarrhoea or undernourished adults and children in developing countries, the findings supported at least acceptability of the taste and texture of the yogurt [42,43], and its safe use for 1 month. A preliminary study of its use by HIC/AIDS patients with chronic diarrhoea in Nigeria has shown that cessation of the diarrhoea occurs within 2 days of its use [44].
In short, probiotic yogurt can have nutritional as well as beneficial immune modulatory effects in patients with serious underlying disease.
Acknowledgments
We wish to thank Dr M. R. Belsheim and Dr R. Reynolds for their clinical assistance and Ms. L. Moyer for her invaluable help in recruiting and following the participants. This project was supported by a grant from NSERC Canada. P. V. K. was supported by the Academy of Finland.
References
- 1.Isolauri E, Kirjavainen PV, Salminen S. Probiotics: a role in the treatment of intestinal infection and inflammation? Gut. 2002;50:54–9. doi: 10.1136/gut.50.suppl_3.iii54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Sullivan GC, Kelly P, O'Halloran S, et al. Probiotics: an emerging therapy. Curr Pharm Des. 2005;11:3–10. doi: 10.2174/1381612053382368. [DOI] [PubMed] [Google Scholar]
- 3.Kirjavainen PV. Probiotics and the management of food allergy. In: Mattila-Sandholm T, Saarela M, editors. Functional dairy products. Cambridge, UK: Woodhead Publishing; 2003. pp. 108–31. [Google Scholar]
- 4.Fedorak RN, Madsen KL. Probiotics and the management of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:286–99. doi: 10.1097/00054725-200405000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol. 2005;174:3237–46. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
- 6.Allez M, Mayer L. Regulatory T cells: peace keepers in the gut. Inflamm Bowel Dis. 2004;10:666–76. doi: 10.1097/00054725-200409000-00027. [DOI] [PubMed] [Google Scholar]
- 7.Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunol Rev. 2005;204:184–94. doi: 10.1111/j.0105-2896.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 8.Robinson DS, Larche M, Durham SR. Tregs and allergic disease. J Clin Invest. 2004;114:1389–97. doi: 10.1172/JCI23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–33. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 10.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci USA. 2002;99:351–8. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stagg AJ, Hart AL, Knight SC, Kamm MA. The dendritic cell: its role in intestinal inflammation and relationship with gut bacteria. Gut. 2003;52:1522–9. doi: 10.1136/gut.52.10.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao Y, Li W, Kaplan MH, Chang C-H. Interleukin (IL)-4 inhibits IL-10 to promote IL-12 production by dendritic cells. J Exp Med. 2005;201:1899–903. doi: 10.1084/jem.20050324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pessi T, Sutas Y, Hurme M, Isolauri E. Interleukin-10 generation in atopic children following oral Lactobacillus rhamnosus GG. Clin Exp Allergy. 2000;30:1804–8. doi: 10.1046/j.1365-2222.2000.00948.x. [DOI] [PubMed] [Google Scholar]
- 14.Di Caro S, Tao H, Grillo A, et al. Effects of Lactobacillus GG on genes expression pattern in small bowel mucosa. Dig Liver Dis. 2005;37:320–9. doi: 10.1016/j.dld.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Rautava S, Kalliomaki M, Isolauri E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol. 2002;109:119–21. doi: 10.1067/mai.2002.120273. [DOI] [PubMed] [Google Scholar]
- 16.Park H-B, Paik D-J, Jang E, Hong S, Youn J. Acquisition of anergic and suppressive activities in transforming growth factor-{beta}-costimulated CD4+CD25- T cells. Int Immunol. 2004;16:1203–13. doi: 10.1093/intimm/dxh123. [DOI] [PubMed] [Google Scholar]
- 17.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-{beta}-producing regulatory T cells from CD4+CD25- precursors. J Immunol. 2002;169:4183–9. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 18.Goudy KS, Burkhardt BR, Wasserfall C, et al. Systemic overexpression of IL-10 induces CD4+CD25+ cell populations in vivo and ameliorates type 1 diabetes in nonobese diabetic mice in a dose-dependent fashion. J Immunol. 2003;171:2270–8. doi: 10.4049/jimmunol.171.5.2270. [DOI] [PubMed] [Google Scholar]
- 19.Reid G, Bruce AW, Fraser N, Heinemann C, Owen J, Henning B. Oral probiotics can resolve urogenital infections. FEMS Immunol Med Microbiol. 2001;30:49–52. doi: 10.1111/j.1574-695X.2001.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 20.Bueno C, Almeida J, Alguero MC, et al. Flow cytometric analysis of cytokine production by normal human peripheral blood dendritic cells and monocytes: comparative analysis of different stimuli, secretion-blocking agents and incubation periods. Cytometry. 2001;46:33–40. [PubMed] [Google Scholar]
- 21.Almeida J, Bueno C, Alguero MC, et al. Extensive characterization of the immunophenotype and pattern of cytokine production by distinct subpopulations of normal human peripheral blood MHC II+/lineage- cells. Clin Exp Immunol. 1999;118:392–401. doi: 10.1046/j.1365-2249.1999.01078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin Immunol. 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Azim T, Halder RC, Sarker MS, et al. Cytokines in the stools of children with complicated shigellosis. Clin Diagn Lab Immunol. 1995;2:492–5. doi: 10.1128/cdli.2.4.492-495.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burton JP, Cadieux PA, Reid G. Improved understanding of the bacterial vaginal microbiota of women before and after probiotic instillation. Appl Environ Microbiol. 2003;69:97–101. doi: 10.1128/AEM.69.1.97-101.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cadieux P, Burton J, Gardiner G, et al. Lactobacillus strains and vaginal ecology. JAMA. 2002;287:1940–1. doi: 10.1001/jama.287.15.1940. [DOI] [PubMed] [Google Scholar]
- 26.Berger RL. More powerful tests from confidence interval p values. Am Stat. 1996;50:314–18. [Google Scholar]
- 27.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–78. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 28.Travassos WJ, Cheifetz AS. Infliximab: use inflammatory bowel disease. Curr Treat Options Gastroenterol. 2005;8:187–96. doi: 10.1007/s11938-005-0011-2. [DOI] [PubMed] [Google Scholar]
- 29.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4+ T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–22. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlsson H, Larsson P, Wold AE, Rudin A. Pattern of cytokine responses to gram-positive and Gram-negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells. Infect Immun. 2004;72:2671–8. doi: 10.1128/IAI.72.5.2671-2678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taams LS, van Amelsfort JM, Tiemessen MM, et al. Modulation of monocyte/macrophage function by human CD4+CD25+ regulatory T cells. Hum Immunol. 2005;66:222–30. doi: 10.1016/j.humimm.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge. human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–80. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 33.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SO, Sheik HI, Ha S-D, Martins A, Reid G. G-CSF mediated inhibition of JNK is a key mechanism for Lactobacillus rhamnosus-induced anti-inflammatory effects in macrophages. Cell Microbiol. 2006;8:1958–71. doi: 10.1111/j.1462-5822.2006.00763.x. [DOI] [PubMed] [Google Scholar]
- 35.Maloy KJ, Antonelli LR, Lefevre M, Powrie F. Cure of innate intestinal immune pathology by CD4+CD25+ regulatory T cells. Immunol Lett. 2005;97:189–92. doi: 10.1016/j.imlet.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Meloni F, Vitulo P, Bianco AM, et al. Regulatory CD4+CD25+ T cells in the peripheral blood of lung transplant recipients: correlation with transplant outcome. Transplantation. 2004;77:762–6. doi: 10.1097/01.tp.0000116565.86752.6b. [DOI] [PubMed] [Google Scholar]
- 37.Waldmann H, Graca L, Cobbold S, Adams E, Tone M, Tone Y. Regulatory T cells and organ transplantation. Semin Immunol. 2004;16:119–26. doi: 10.1016/j.smim.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 38.de Kleer IM, Wedderburn LR, Taams LS, et al. CD4+CD25bright regulatory T cells actively regulate inflammation in the joints of patients with the remitting form of juvenile idiopathic arthritis. J Immunol. 2004;172:6435–43. doi: 10.4049/jimmunol.172.10.6435. [DOI] [PubMed] [Google Scholar]
- 39.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–18. [PubMed] [Google Scholar]
- 40.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 41.Isolauri E, Rautava S, Kalliomaki M, Kirjavainen P, Salminen S. Role of probiotics in food hypersensitivity. Curr Opin Allergy Clin Immunol. 2002;2:263–71. doi: 10.1097/00130832-200206000-00018. [DOI] [PubMed] [Google Scholar]
- 42.Hekmat S, Reid G. Survival of Lactobacillus reuteri RC-14 and Lactobacillus rhamnosus GR-1 in milk. Int J Food Sci and Technol. 2007;42:615–619. [Google Scholar]
- 43.Hekmat S, Reid G. Sensory properties of probiotic yogurt is comparable to standard yogurt. Nutr Res. 2006;26:163–166. [Google Scholar]
- 44.Anukam KC, Osazuwa EO, Osadolor BE, Bruce AW, Reid G. Yogurt containing probiotic Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 helps resolve moderate diarrhea and increases CD4 count in HIV/AIDS patients. J Clin Gastroenterol. in press. [DOI] [PubMed]