Abstract
Chronic obstructive pulmonary disease (COPD) is an inflammatory disorder characterized by the presence of non-fully reversible airflow limitation. The study was undertaken to investigate the involvement of alpha-1-antitrypsin (α1AT) and T lymphocyte subsets in the pathogenesis of COPD. Blood samples of 50 subjects, including 25 healthy volunteers and 25 patients with COPD, were analysed. Serum trypsin inhibitory capacity (STIC) was determined by enzymatic assay. CD4+ and CD8+ T lymphocytes were enumerated in heparinized blood using a fluorescence activated cell sorter counter. The STIC in COPD patients was found to be decreased significantly than in controls (P < 0.01). In COPD patients with lower expression levels of α1AT, a highly significant decrease in the number of CD4+ T lymphocytes (P < 0.0009) and CD4/CD8 ratio was observed compared with control subjects (P < 0.008). The mean ± standard error of CD8+ lymphocytes was found to be little different (only marginally decreased) in COPD patients compared to healthy controls; however, an alteration in the individual count of CD8+ lymphocytes cells was observed in COPD patients. Using linear regression analysis, a negative correlation was observed between STIC and CD4+ lymphocytes and CD8+ lymphocytes (r = −0.40, P < 0.04; r = −0.42, P < 0.03, respectively) in COPD patients. An alteration in α1AT and T lymphocyte subsets in COPD patients suggested that interplay of these factors may be responsible for the progression of COPD.
Keywords: alpha-1-antitrypsin, CD4+ T lymphocytes, CD8+ T lymphocytes, chronic obstructive pulmonary disease, inhibitory capacity, serum trypsin
Introduction
Chronic obstructive pulmonary disease (COPD) is a slowly progressive disorder of the lungs characterized by the presence of non-fully reversible airflow limitation [1,2]. This airflow limitation has been attributed to an inflammatory process in the peripheral airways due to diverse factors [3].
Cigarette smoking is known to produce alterations in inflammatory cell populations in peripheral blood, bronchoalveolar lavage fluid (BALF) and in the airway mucosa that are independent of airflow obstruction. An increase in total leucocyte numbers, an increased CD8+ cell subpopulation and a reduced CD4/CD8 cell ratio in the peripheral blood of smokers compared with non-smokers have been reported [4]. Studies have also revealed increased CD8+ T lymphocytes and smooth muscle area in the peripheral airways of the COPD subjects [5].
Although cigarette smoking is the major cause of inflammation and COPD, only 20% of smokers develop clinically recognizable disease, suggesting the involvement of host factors in the pathogenesis of COPD [2,6]. Impaired expression of alpha-1-antitrypsin (α1AT) in the body is considered to be the second major cause of COPD. α1AT is a protease inhibitor, inhibiting the activity of various proteases which are liberated in human plasma. These proteases, if left unchecked by the protease inhibitor, can cause destruction of normal tissues. α1AT is an important protein that neutralizes the action of various proteases and thus prevents the proteolytic damage of tissues in the body [7]. Thus, a deficiency of α1AT in the body may lead to early onset of COPD and finally to emphysema.
The role of T lymphocytes in pulmonary diseases has also been the subject of considerable interest. T lymphocyte abnormalities have been reported to lead to chronic infections and inflammation, resulting in local release of proteolytic enzymes which might be implicated indirectly with the biological activity of α1AT [5,8–10]. Lymphocytes are known to modulate inflammatory processes in the airways of asthmatics [11–13]. However, little has been published on their role in the pathogenesis of COPD [14–16]. A significant increase in the percentage and total number of CD8+ T lymphocytes in COPD smokers has been reported compared to non-COPD smokers or to healthy non-smokers [17]. An increase in the CD4/CD8 ratio has been reported to be associated with a higher forced expiratory volume in 1 s (FEV1) as a percentage of that predicted in non-smoking COPD subjects compared with non-smoking healthy subjects [18]. However, to date, an association of T cell functions and α1AT with the progression of COPD has not been studied. Therefore, the present study was designed to investigate the possible role of α1AT and T lymphocyte subsets in the pathogenesis of COPD.
Materials and methods
Subjects
The study population consisted of 25 patients diagnosed with COPD at Guru Teg Bahadur Hospital, Shahdara (18 males and seven females; age 58.3 ± 10.6; 15 smokers, 10 non-smokers) and 25 control volunteers (18 males and seven females; age 56.9 ± 7.8; 15 smokers, 10 non-smokers) without respiratory symptoms. Diagnosis of COPD was made as per the guidelines of the American Thoracic Society [19]. In COPD patients, the mean ± standard deviation (s.d.) of percentage predicted value of FEV1 was found to be 65.1 ± 19.8. None of the subjects was receiving oral or inhaled corticosteroids or any other immunosuppressive treatment. The patients included in the present study were neither alcoholic nor had consumed drugs of abuse at any stage of their life. They also had no clinically diagnosable infection and had had no acute exacerbation for the last 6 months. All patients were negative for HIV serology.
Heparinized and non-heparinized blood samples were collected from patients and controls after informed consent. Sera prepared from the non-heparinized blood were stored at −20°C until analysis. The heparinized blood was used to enumerate the CD4+ and CD8+ T lymphocytes and also to determine the CD4+/CD8+ ratio.
Serum trypsin inhibitory capacity (STIC)
STIC was determined by enzymatic assay using alpha-N-benzoyl-L-arginine-p-nitroanilide (BAPNA; Sigma, St. Louis, MO, USA) as substrate [20]. The assay was carried out at 25°C using a water bath. Serum sample (15.0 µl) was added to 3.0 ml of Tris-HCl (0.1 M) containing 200.0 µl of trypsin (Sigma) (0.03% w/v in 0.0025 M HCl) and incubated at 25°C for 15 min. Then 3.0 ml of BAPNA solution (63.5 mg percentage) was added and incubated at 25°C. After 10 min, 750.0 µl of acetic acid (30%) was added and mixed thoroughly. Absorbance was measured at 410 nm and the STIC was expressed as mg of trypsin inhibited/ml of sample.
Analysis of mononuclear cell subsets
The CD4+ and CD8+ T lymphocytes were enumerated in uncoagulated blood of control subjects and COPD patients using the fluorescence activated cell sorter (FACSCount) system (version 1.3) from M/S Becton Dickinson Immunocytometry Systems (SanJose, CA, USA) [21,22]. The specific fluorescein isothiocyanate (FITC)-conjugated mouse monoclonal antibodies (Becton Dickinson) derived from the hybridization of mouse NS-1 myeloma cells with spleen cells from BALB/c mice immunized with human peripheral blood T lymphocytes were used. Two reagent tubes having antibodies against CD4+ and CD8+ were vortexed separately upside-down and upright for 5–10 s. The tubes were opened using a coring station and 50.0 µl of whole blood was transferred to each tube. The tubes were capped, vortexed upright very gently for 5 s and incubated at room temperature for 1 h. After incubation 50.0 µl fixative (5% formaldehyde) was added to the tubes. The control beads supplied with the kit (Becton Dickinson) were used at different concentrations in the assay. The tubes containing samples were then run on the FACSCount system to determine the absolute number of CD4+ and CD8+ T lymphocytes.
Statistical analysis
Group data were expressed as mean ± standard error (s.e.). Student's t-test and spss version 10.0 were used to carry out statistical analysis. Probability values of P < 0.05 were considered significant. Linear regression analysis was used to identify variables or group of variables that were associated strongly with STIC.
Results
Twenty-five COPD patients and 25 control subjects fulfilled the inclusion criteria. All the individuals included in the study were from the Northern plains of India.
STIC
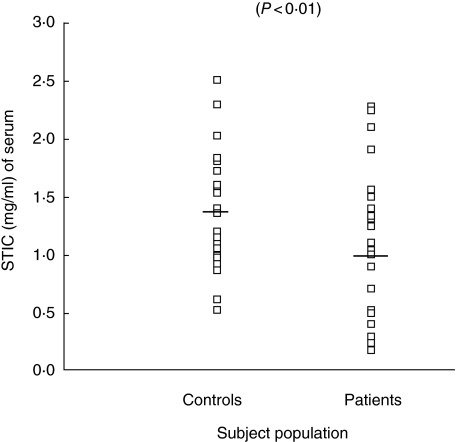
Sera were obtained from the blood of 25 COPD patients and 25 controls. STIC of all the sera was determined using enzymatic assay. The mean ± s.e. of STIC was found to be 1.08 ± 0.12 mg of trypsin inhibited/ml of serum in COPD patients, while the mean ± s.e. in controls was recorded to be 1.40 ± 0.10 mg of trypsin inhibited/ml of serum (Fig. 1). The decrease in STIC of patients was found to be statistically significant (P < 0.01) compared to controls. No significant difference in STIC was observed when COPD patients were subgrouped into smokers and non-smokers (P < 0.9).
Fig. 1.
Individual values of serum trypsin inhibitory capacity (STIC) in serum samples of chronic obstructive pulmonary disease (COPD) patients and control subjects.
CD4+ and CD8+ cells
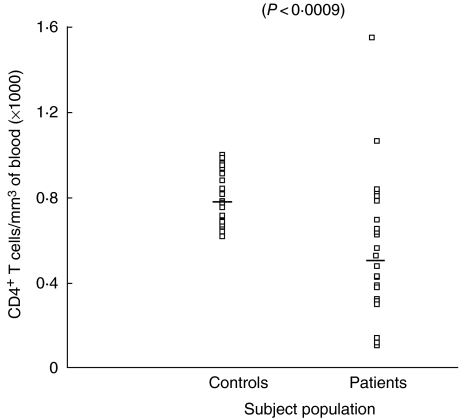
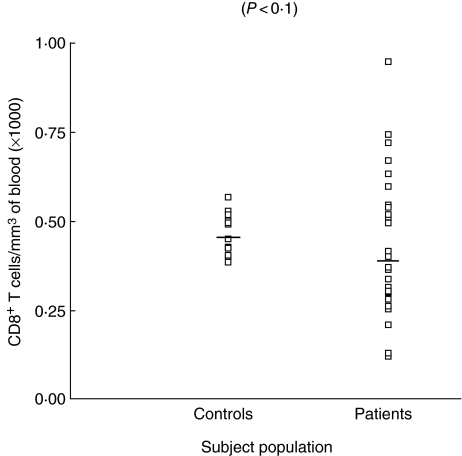
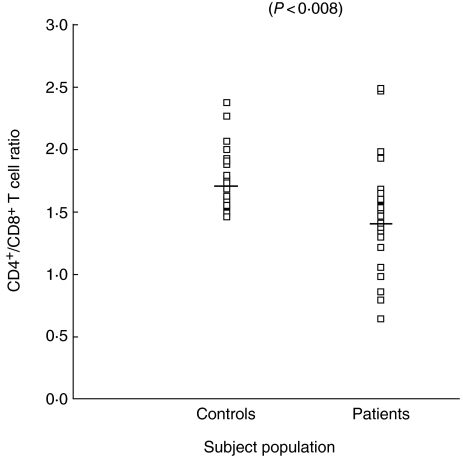
CD4+ and CD8+ cells were enumerated in the whole blood of all subjects included in the study. The number of CD4+ cells in patients was found to be 543 ± 65 cell/mm3 (range 101–1579), while in controls it was 792 ± 25 cells/mm3 of blood with a range of 612–996 cells/mm3. The decrease in number of CD4+ cell count was statistically significant in patients with COPD compared to controls (P < 0.0009). The mean ± s.e. of CD8+ lymphocytes was found to be only marginally decreased in COPD patients than controls, the difference being of no significance (P < 0.1). However, when individual counts of CD8+ lymphocytes in patients were compared with controls, the range of CD8+ lymphocytes in patients was found to be highly variable compared to controls. The number of CD8+ was found to be 383 ± 41 cell/mm3 of blood (range 72–944 cells/mm3) in patients, while in controls it was 446 ± 10.2 cells/mm3 of blood (range 384–564 cell/mm3). The number of CD4+ and CD8+ cells are shown in Figs 2 and 3, respectively. However, when the CD4+/CD8+ ratio was computed, a highly significant decrease was observed in patients (P < 0.008). The CD4+/CD8+ ratio in patients was found to be 1.4 ± 0.09, while in controls it was 1.7 ± 0.05. Individual values for the CD4/CD8 ratio are shown in Fig. 4.
Fig. 2.
Number of CD4+ T lymphocytes in blood of chronic obstructive pulmonary disease (COPD) patients and control subjects.
Fig. 3.
Number of CD8+ T lymphocytes in blood of chronic obstructive pulmonary disease (COPD) patients and control subjects.
Fig. 4.
Ratio of CD4+/CD8+ T lymphocytes in blood of chronic obstructive pulmonary disease (COPD) patients and control subjects.
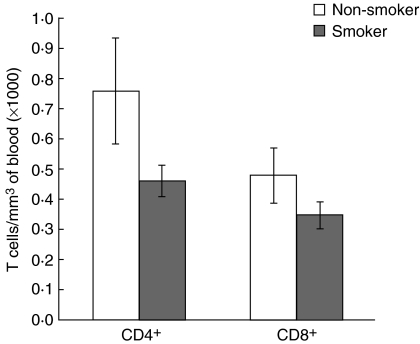
Also, a significant decrease in the number of CD4+ T lymphocytes was observed in smoking COPD subjects compared to non-smoking COPD subjects (P < 0.03). However, no significant alteration in the number of CD8+ T cells was observed in the two subgroups (smoking and non-smoking) COPD patients (Fig. 5).
Fig. 5.
Mean values of number of CD4+ and CD8+ T lymphocytes in blood of smoking and non-smoking chronic obstructive pulmonary disease (COPD) subjects.
Relationship of STIC with T cell subsets
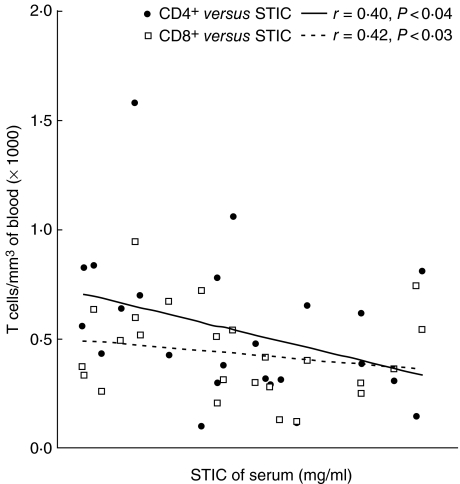
A linear regression analysis was used to identify the association of STIC with number of CD4+ and CD8+ lymphocytes. The analysis revealed a significant inverse relationship of CD4+ and CD8+ lymphocytes with STIC. The values ofcorrelation coefficients (r) in two cases were 0.40 (P < 0.04) and 0.42 (P < 0.03), respectively, in COPD patients, suggesting that the number of T lymphocytes was associated with STIC in COPD patients (Fig. 6).
Fig. 6.
Relationship between the number of T lymphocytes and serum trypsin inhibitory capacity (STIC) of chronic obstructive pulmonary disease (COPD) patients.
Discussion
The results of the present study revealed a significant decrease in serum α1AT activity in COPD patients compared to controls. Mean ± s.e. of the STIC value in controls was 1.40 ± 0.10 mg of trypsin inhibited/ml of serum. The normal value of STIC in the control population has been reported in the range from 1.1 to 2.1 mg of trypsin inhibited/ml of serum [23,24]. Ihrig et al. [25] found the mean value of STIC in controls to be 1.32 ± 0.28 mg of trypsin inhibited/ml of serum. Mean STIC values in COPD patients as observed in the present study were 1.08 ± 0.12 mg of trypsin inhibited/ml of serum. These values were found to be decreased significantly in COPD patients in comparison to controls (P < 0.01).
Deficiency of α1AT in the body leads to early onset of COPD and finally to emphysema, and both these conditions are preceded by inflammation. To the best of our knowledge, an association of T cell functions and α1AT with the progression of COPD has not yet been investigated. We studied the T lymphocyte count in COPD patients with decreased levels of α1AT. The results revealed that the COPD patients with α1AT deficiency had significantly decreased numbers of CD4+ T lymphocytes (P < 0.0009) and a decreased CD4/CD8 ratio in peripheral blood compared with the control subjects (P < 0.008). The decrease in number of CD4+ T lymphocytes and CD4/CD8 ratio was found to be highly significant. A marginal decrease in number of CD8+ cells was also observed; however, it was not significant. Although the difference in the mean ± s.e. of CD8+ cells in COPD patients was marginal, the variation in the range of CD8+ count was found to be greater in patients compared to controls. The number of CD8+ cells was found to range from 72 to 944 cell/mm3 of blood in patients, while in controls it was 384–564 cell/mm3, also indicating an alteration in the development of CD8+ cells. Almost similar observations were made by Ginn et al. [26] and Miller et al. [4], who demonstrated a relative decrease in CD4+ T lymphocytes and an increase in CD8+ T lymphocytes with a low CD4+/CD8+ ratio in the peripheral blood of heavy smokers [4,26]. The results of the current study were not in agreement with Kim et al. [8], who reported no significant difference in CD4+ and CD8+ T lymphocytes and the CD4/CD8 ratio in COPD patients and controls. An increase in CD4+ (T helper) and CD8+ (suppressor/cytotoxic) T cells in the airways and lung parenchyma of COPD patients was observed by Saetta et al. [10] and O'Shaughnessy et al. [9]. The difference in patient selection and their geographic location may have contributed to the discrepancy in findings. Several other studies have reported variable observations with respect to T lymphocytes count. Di Stefano et al. [27] observed a significant decrease in number of CD8+ cells in patients with severe COPD compared to those having mild/moderate COPD and control smokers. They did not observe any significant difference in the number of CD4+ cells and the CD4/CD8 ratio. Further, it has also been reported that the percentage of CD8+ T lymphocytes in peripheral blood is significantly higher in non-smoking patients with COPD compared with non-smoking control subjects and a lower CD4+/CD8+ ratio is associated with lower FEV1 in patients with COPD [18].
The CD4+ T cells act as a regulatory element in the activation as well as the deletion of CD8+ T cells. The ability of anergic CD4+ T cells has been reported to suppress CD8+ T cell activation through the induction of apoptosis, and on the need for CD8+ T cells for antigen recognition in inducing cell death in CD4+ T cells. Moreover, the central role of CD4+ T cells in the maintenance of peripheral regulation and tolerance has been described widely [28]. Several reports have observed that an imbalance between cytotoxic CD8+ T lymphocytes and helper CD4+ T lymphocytes may contribute to the abnormal inflammatory process in the airways of patients with COPD to make the disorder a lymphocyte-driven inflammatory condition [5,12,29]. A low CD4+/CD8+ ratio has been a characteristic feature of the pulmonary inflammatory response in COPD [5,10,30,31]. A decreased ratio of CD4+/CD8+ T lymphocytes in conjunction with the decreased synthesis of functional α1AT in the Indian population may therefore be contributory to induce inflammation preceding to COPD. However, the reflection of such abnormalities in the form of circulatory markers in the patients remains unclear.
In order to study the role of smoking in COPD, De Jong et al. [18] investigated lymphocyte subsets in the peripheral blood of smoking and non-smoking COPD patients and healthy control subjects. They did not find significant differences in lymphocyte subsets when either total groups or smoking subjects of both groups were compared. However, the percentage of CD8+ lymphocytes was significantly higher in non-smoking COPD subjects than non-smoking healthy controls. They showed further that within the group of non-smoking COPD subjects, a higher CD4+/CD8+ ratio in peripheral blood was associated with better lung function. Whether these findings represent a consequence of the disease, i.e. spillover of the pulmonary inflammatory process or a potential pathogenic mechanism, which may be related to susceptibility to COPD in some smokers is not known. Miller et al. [4] did not find any significant difference in the total number of T lymphocytes and T cell subsets in light or moderate smokers compared with non-smokers, but reported that numbers of circulating CD8+ T cells were increased and CD4+ cells decreased in heavy smokers. Similarly, Costabel et al. [32] did not find significant differences in the proportion of CD4+ and CD8+ lymphocytes in the systemic blood of young healthy smokers compared to healthy non-smokers, despite obvious differences in bronchoalveolar lavage fluid. Overall, the results of these studies seem to indicate that cigarette smoking can cause alterations in the number of circulating immunoregulatory T cells, probably reversible after quitting smoking [4]. We have attempted to analyse the results to determine the relationship between smoking and subsets of T lymphocytes. A significant decrease in the number of CD4+ T lymphocytes was observed in smoking COPD subjects compared to non-smoking COPD subjects. However, no significant alteration in the number of CD8+ T lymphocytes and CD4+/CD8+ ratio was observed in smoking COPD subjects compared to non-smoking COPD subjects. The results revealed that lower α1AT and smoking may together alter the level of T lymphocytes and thus contribute to the progression of COPD.
Linear regression data analysis showed a negative correlation between the number of CD4+ cells and level of STIC, as well as the number of CD8+ cells and the level of STIC. Such a correlation may suggest that alteration in CD4+ T lymphocytes and the CD8+ T lymphocyte subset may be associated with the biological activity of α1AT. It has been reported that the proteolytic enzymes play an important role in the early events of lymphocyte activation and support the hypothesis that serum inhibitors of proteases, such as α1AT, contribute to the modulation of immune response [33]. The results have revealed that α1AT deficiency with T lymphocyte abnormalities might be involved in the pathogenesis of airflow limitation in COPD patients.
It has been observed that an imbalance between cytotoxic CD8+ T lymphocytes and helper CD4+ T lymphocytes may contribute to the abnormal inflammatory process in the airways of patients with COPD [5,9,29]. These cells release inflammatory cytokines and proteases that cause an imbalance of the proinflammatory and protective mediators found in healthy lungs. The pathology of COPD is that of a chronic inflammatory process with tissue damage and repair processes; many cytokines play a role in this condition. We investigated type 1 [interleukin (IL)-2 and interferon (IFN)-γ] and type 2 (IL-4 and IL-10) serum cytokines in COPD patients to support our findings (data not shown). We observed an increase in the level of IL-4 and IL-10 in COPD patients compared to controls (P < 0.0001). An increase in the level of IL-4 and IL-10 revealed that the response was predominantly of the Th-2 type. A significant decrease in the levels of IFN-γ and IL-2 was observed in COPD patients compared to controls (P < 0.0001). The decrease in the number of IL-2 and IFN-γ demonstrated that the Th-1 type response in patients was suppressed.
The present study describes alterations in peripheral blood immunological parameters and anti-protease synthesis. Impairment of the T lymphocyte function might invite chronic infection leading to inflammation, which may result in local release of proteolytic enzymes in the body. When the host is not able to neutralize these proteases due to deficient synthesis of a protease inhibitor, i.e. α1AT, might lead to serious complications and progression of respiratory diseases, including COPD. Thus, a complex interaction of α1AT and changes in the balance of T lymphocyte subsets may be responsible for the development of COPD.
Acknowledgments
J. Gupta would like to thank Director IGIB and Council of Scientific and Industrial Research for providing the necessary facilities and funding, respectively.
References
- 1.Snider GL. Changes in COPD occurrence. Chronic obstructive pulmonary disease: a definition and implications of structural determinants of airflow obstruction for epidemiology. Am Rev Respir Dis. 1989;140:S3–8. doi: 10.1164/ajrccm/140.3_Pt_2.S3. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1:1645–8. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive pulmonary disease. N Eng J Med. 1968;278:1355–60. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- 4.Miller LG, Goldstein G, Murphy M, Ginns LC. Reversible alterations in immunoregulatory T cells in smoking. Chest. 1982;5:526–9. doi: 10.1378/chest.82.5.526. [DOI] [PubMed] [Google Scholar]
- 5.Saetta M, Stefano AD, Turato G, et al. CD8+ T lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:822–6. doi: 10.1164/ajrccm.157.3.9709027. [DOI] [PubMed] [Google Scholar]
- 6.Diener CF, Burrows B. Further observations on the course and prognosis of chronic obstructive pulmonary disease. Am Rev Respir Dis. 1975;111:719–24. doi: 10.1164/arrd.1975.111.6.719. [DOI] [PubMed] [Google Scholar]
- 7.Gadek JS, Fells GA, Zimmerman RL, Rennard SI, Crystal RG. Antielastases of the human alveolar structures. Implications for the protease–antiprotease theory of emphysema. J Clin Invest. 1981;68:889–98. doi: 10.1172/JCI110344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim W-D, Kim W-S, Koh Y, et al. Abnormal peripheral blood T lymphocyte subsets in a subgroup of patients with COPD. Chest. 2002;122:437–44. doi: 10.1378/chest.122.2.437. [DOI] [PubMed] [Google Scholar]
- 9.O'Shaughnessy TC, Ansari TW, Barnes NC, Jeffery PK. Inflammation in bronchial biopsies of subjects with chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med. 1997;155:852–7. doi: 10.1164/ajrccm.155.3.9117016. [DOI] [PubMed] [Google Scholar]
- 10.Saetta M, Baraldo S, Corbin L, et al. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:711–17. doi: 10.1164/ajrccm.160.2.9812020. [DOI] [PubMed] [Google Scholar]
- 11.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 12.Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC. Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–15. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez MC, Diaz P, Galleguillos FR, Ancic P, Cromwell O, Kay AB. Allergen-induced recruitment of bronchoalveolar helper (OKT4) and suppressor (OKT8) T-cells in asthma. Relative increases in OKT8 cells in single early responders compared with those in late-phase responders. Am Rev Respir Dis. 1987;136:600–4. doi: 10.1164/ajrccm/136.3.600. [DOI] [PubMed] [Google Scholar]
- 14.Fournier M, Lebargy F, Le Roy Ladurie F, Lenormand E, Pariente R. Intraepithelial T lymphocyte subsets in the airways of normal subjects and of patients with chronic bronchitis. Am Rev Respir Dis. 1989;140:737–42. doi: 10.1164/ajrccm/140.3.737. [DOI] [PubMed] [Google Scholar]
- 15.Bosken CH, Hards J, Gatter K, Hogg JC. Characterization of the inflammatory reaction in the peripheral airways of cigarette smokers using immunocytochemistry. Am Rev Respir Dis. 1992;145:911–7. doi: 10.1164/ajrccm/145.4_Pt_1.911. [DOI] [PubMed] [Google Scholar]
- 16.Saetta M, Stefano AD, Maestrelli P, et al. Activated T lymphocytes and macrophages in bronchial mucosa of subjects with chronic bronchitis. Am Rev Respir Dis. 1993;147:301–6. doi: 10.1164/ajrccm/147.2.301. [DOI] [PubMed] [Google Scholar]
- 17.Chrysofakis G, Tzanakis N, Kyriakoy D, et al. Perforin expression and cytotoxic activity of sputum CD8+ lymphocytes in patients with COPD. Chest. 2004;125:71–6. doi: 10.1378/chest.125.1.71. [DOI] [PubMed] [Google Scholar]
- 18.De Jong JW, Van der Belt-Gritter B, Koeter GH, Postma DS. Peripheral blood lymphocyte cell subsets in subjects with chronic obstructive pulmonary disease: association with smoking, IgE and lung function. Respir Med. 1997;91:67–76. doi: 10.1016/s0954-6111(97)90070-6. [DOI] [PubMed] [Google Scholar]
- 19.American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1987;136:225–44. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- 20.Dietz AA, Rubinstein HM, Hodges LK. Use of alpha-N-benzoyl-L-arginine-p-nitroanilide as trypsin substrate in estimation of alpha 1-antitrypsin. Clin Chem. 1976;22:1754–5. [PMC free article] [PubMed] [Google Scholar]
- 21.Landey A, Ho JL, Hom D, et al. The rapid manual method for CD4+ T cell quantitation for use in developing countries. AIDS. 1993;7:1565–8. doi: 10.1097/00002030-199312000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Koepke J, Landey A. Precision and accuracy of absolute lymphocyte counts. Clin Immunol Immunopathol. 1989;52:19–27. doi: 10.1016/0090-1229(89)90189-x. [DOI] [PubMed] [Google Scholar]
- 23.Lieberman J, Gaidulis L, Garoutte B, Mittman C. Identification and characteristics of the common alpha 1-antitrypsin phenotypes. Chest. 1972;62:557–64. doi: 10.1378/chest.62.5.557. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad A, Siddiqui MA, Abdullah AK, Qureshi MU, Rizvi SS, Tajuddin M. Serum alpha-1 antitrypsin in chronic obstructive lung disease. Indian J Med Sci. 1979;33:91–6. [PubMed] [Google Scholar]
- 25.Ihrig J, Schwartz HJ, Rynbrandt DJ, Kleinerman J. Serum trypsin inhibitory capacity and pi phenotypes. II. Prevalence of alpha1-antitrypsin deficiency in an allergy population. Am J Clin Pathol. 1975;64:297–303. doi: 10.1093/ajcp/64.3.297. [DOI] [PubMed] [Google Scholar]
- 26.Ginns LC, Goldenheim PD, Miller LG, et al. T lymphocyte subsets in smoking and lung cancer: analysis of monoclonal antibodies and flow cytometry. Am Rev Respir Dis. 1982;126:265–9. doi: 10.1164/arrd.1982.126.2.265. [DOI] [PubMed] [Google Scholar]
- 27.Di Stefano A, Capelli A, Lusuardi M, et al. Decreased T lymphocyte infiltration in bronchial biopsies of subjects with severe chronic obstructive pulmonary disease. Clin Exp Allergy. 2001;31:893–902. doi: 10.1046/j.1365-2222.2001.01098.x. [DOI] [PubMed] [Google Scholar]
- 28.Frasca L, Piazza C, Piccolella E. CD4+ T cells orchestrate both amplification and deletion of CD8+ T cells. Crit Rev Immunol. 1998;18:569–94. doi: 10.1615/critrevimmunol.v18.i6.50. [DOI] [PubMed] [Google Scholar]
- 29.Leckie MJ, Jenkins GR, Khan J, et al. Sputum T lymphocytes in asthma, COPD and healthy subjects have the phenotype of activated intraepithelial T cells (CD69+ CD103+) Thorax. 2003;58:23–9. doi: 10.1136/thorax.58.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeffery PK. Structural and inflammatory changes in COPD: a comparison with asthma. Thorax. 1998;53:129–36. doi: 10.1136/thx.53.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cosio MG, Guerassimov A. Chronic obstructive pulmonary disease. Inflammation of small airways and lung parenchyma. Am J Respir Crit Care Med. 1999;160:S21–5. doi: 10.1164/ajrccm.160.supplement_1.7. [DOI] [PubMed] [Google Scholar]
- 32.Costabel U, Bross KJ, Reuter C, Rühle K, Matthys H. Alterations in immunoregulatory T-cell subsets in cigarette smokers. A phenotypic analysis of bronchoalveolar and blood lymphocytes. Chest. 1986;89:39–44. doi: 10.1378/chest.90.1.39. [DOI] [PubMed] [Google Scholar]
- 33.Bata J, Deviller P, Vallier P, Revillard JP. Modification of lymphocyte DNA synthesis by alpha 1-antitrypsin. Ann Immunol. 1981;132C:275–86. doi: 10.1016/0769-2625(81)90077-5. [DOI] [PubMed] [Google Scholar]