Abstract
Interleukin (IL)-18 is a potent stimulator of immunity and augments the severity of type II collagen-induced arthritis (CIA) in rats and mice by enhancing T helper 1 (Th1) cell activation, which increases the production of proinflammatory cytokines and arthritogenic antibodies. In this study, we show that recombinant IL-18 (rIL-18) also has a direct effect on normal rat chondrocytes maintained in vitro inducing them to produce proinflammatory factors including IL-6, regulated upon activation normal T cell expressed and secreted (RANTES), prostaglandin E2 (PGE2) and prostaglandin F2α (PGF2α) in a dose- and time-dependent manner. The production of matrix metalloproteinase (MMP)-13, nitric oxide (NO), tumour necrosis factor (TNF)-α and IL-1β were also enhanced, although less intensely. Neutralizing polyclonal anti-rIL-18 antibodies effectively blocked the production of IL-6, PGE2 and RANTES, as well as mRNA expression for the same products in addition to IL-18 and TNF-α. In contrast, neutralizing antibodies to IL-1β, TNF-α and IL-6 were ineffective in suppressing any of these products. Together, these findings suggest that IL-18 may play an important, possibly direct, role in mediating cartilage injury, which might not be amenable to treatment with currently utilized anti-cytokine agents. These findings suggest further that IL-18 antagonists might prove beneficial as anti-inflammatory and chondroprotective agents in the treatment of arthritis, and that the development of such agents for human use is worth consideration.
Keywords: anti-IL-18 antibody, cartilage, chondrocyte, IL-18, proinflammatory cytokine
Introduction
The destruction of articular cartilage matrix by inflamed synovial tissue is a characteristic finding of many types of arthritis. The biomechanical integrity of the cartilage matrix is dependent upon a balance between the anabolic and catabolic activities of chondrocytes. Externally and internally produced proinflammatory mediators activate chondrocytes to degrade the matrix, making cartilage more susceptible to injury. Unfortunately, the potential for cartilage matrix repair is limited. Certain cytokines, namely interleukin (IL)-1β and tumour necrosis factor (TNF)-α, play major roles in cartilage degradation [1,2]; however, others might similarly be involved [3]. One cytokine of interest is IL-18, a member of the IL-1 cytokine family. IL-18 is released as a propeptide, cleaved by IL-1β-converting enzyme to a biologically active 18 kDa product. IL-18 is expressed by a variety of cells, including monocytes/macrophages, dendritocytes, synovial fibroblasts, osteoblasts and chondrocytes [4–7]. IL-18 possesses immunoregulatory activities, including the stimulation of interferon (IFN)-γ production by T helper 1 (Th1) and natural killer cells [8,9], as well as other activities that promote inflammation, e.g. the enhancement of TNF-α, IL-1α, IL-1β and IL-6 production [10], the up-regulation of intercellular adhesion molecule (ICAM)-1 [11], the enhancement of NO and cyclooxygenase 2 (COX-2) production [6], and the recruitment and activation of neutrophils [12,13]. Thus, not unexpectedly, IL-18 is linked firmly to the destruction of cartilage and bone [3].
With regard to joint injury, Gracie et al. [14] have reported higher levels of IL-18 mRNA and protein in the joints of patients with rheumatoid arthritis (RA) than patients with osteoarthritis (OA). In using the collagen-induced arthritis model, it has been shown that IL-18 enhances the incidence and severity of joint injury in rats and mice [12,15,16]. In contrast, IL-18 knock-out mice develop clinically and histologically milder arthritis at a reduced incidence [15]. In vitro studies using normal human cartilage show that IL-18 enhances NO, IL-6 and glycosaminoglycan production [6], suggesting that similar events might occur in vivo. Recently, IL-18-induced joint inflammation has been shown to occur independently of IL-1 although it may remain, in part, dependent on TNF-α[17].
Our previous report showed that IL-18 plays a strong proinflammatory role in rat collagen-induced arthritis (CIA) [16]. This effect was associated with enhanced Th1 responses, as demonstrated by increased cytokine and anti-CII antibody production. Neutralizing anti-IL-18 antibodies attenuated these changes in addition to arthritis severity and incidence. To ascertain whether IL-18 also has a direct effect on articular cartilage, we stimulated normal rat chondrocytes in vitro with IL-18 and measured the expression of mRNA and production of key proinflammatory mediators that cause tissue injury. Our studies described here show that chondrocytes release substantial amounts of proinflammatory substances. Moreover, antibodies specific for IL-18, but not other cytokines, suppressed the production of these products. These data indicate that IL-18 may have a direct effect on chondrocytes resulting in cartilage matrix injury.
Materials and methods
Preparation of rat recombinant IL-18
Total RNA was extracted from rat lymphoid node cells with TRIzol Reagent (Life Technologies, Grand Island, NY, USA). First-strand cDNA synthesis was performed with a cDNA cycle kit (Invitrogen, San Diego, CA, USA). Primer set pairs designed from a rat IL-18 sequence data were used to clone IL-18 (sense, ATGGCTGCAATACCAGAAGAAG; anti-sense, ACTTTGATGTAAGTTAGTAAG). The polymerase chain reaction (PCR) product was cloned into the pET-19b expression vector, expressed in Escherichia coli, and purified as a histidine-tagged fusion protein. Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE), Piecer gelcode blue staining and limulus assays were used to confirm rIL-18 purity, as reported earlier [16].
Chondrocyte isolation and culture
Articular cartilage was pooled from the shoulders, knees and hips of 4–5-week-old BB rats. Chondrocytes were isolated by enzymatic digestion using 0.25% trypsin/ethylenediamine tetraacetic acid (EDTA) solution for 30 min followed by 4 h digestion with 0.2% collagenase in Dulbecco's modified Eagle medium (DMEM)/F-12 medium containing 10% fetal calf serum (FCS) and penicillin/streptomycin [18]. The freshly isolated chondrocytes were washed, suspended in complete medium, cultured at 1 × 105 cells/ml in six-well plates for 7–10 days, and the medium changed every 3 days. On reaching confluence, chondrocytes were treated with trypsin/EDTA and reseeded into 24-well plates for cytokine measurement or 10-cm culture plates for mRNA studies. Complete medium was replaced with medium containing 0.2% FCS for 12–24 h before rIL-18 stimulation. To ensure the chondrocyte phenotype, only first-passage cells were studied. Fibroblast and dendritic cells were not observed in the cultures.
Measurement of cytokines
Chondrocyte monolayers were incubated in 24-well plates for 6, 24 and 48 h with rat rIL-18 or medium alone. The supernatants were divided into aliquots and cytokines measured using commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA).
RNA isolation and reverse transcription–polymerase chain reaction (RT–PCR). Chondrocytes grown in 10 ml culture dishes were stimulated in vitro with rIL-18 (100 ng/ml of medium) for 0–48 h. After cell lysis with TRIzol reagent (Invitrogen), total RNA was isolated following the manufacture's instructions. First-strand cDNA synthesis was performed with a cloned avian myeloblastosis virus (AMV) first-strand cDNA synthesis kit (Invitrogen). Conventional PCR was performed using primer pairs purchased from BioSource (Camarillo, CA, USA) in a 50-µl reaction mixture containing 5 µl of 10× PCR buffer, 4 µl of 2.5 mM deoxyribonucleoside (dNTP), 2 µl of primer pairs, 4 µl of 25 mM MgCl2, 10 µl of Q-Solution (Qiagen, Chatsworth, CA, USA), 0.3 µl of HotStar Taq DNA polymerase (Qiagen) and 1 µl of cDNA product. The reaction utilized a DNA thermal cycler (Perkin-Elmer, Irvine, CA, USA) for 35 cycles at 94°C for 45 s, 60°C for 45 s and 72°C for 2 min, with an initial activation step at 95°C for 15 min. PCR products were resolved by electrophoresis using a 1.5% agarose gel.
Measurement of mRNA expression by quantitative RT–PCR (qPCR)
For transcript quantification, 1 µg of total RNA was used for cDNA synthesis and diluted at a 1-µg RNA to 50 µl RT-mix ratio. qPCR reactions were performed in an ABI Prism 7900 HT Sequence Detection System (ABI Applied BioSystems, Foster City, CA, USA). Primers, probes and reagents for the reverse transcription and PCR were purchased from ABI Applied BioSystems. Results were normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and expressed as fold increase versus control using the 2-delta delta Ct method.
Immunoblotting
Matrix metalloproteinase (MMP)-13 production was detected by Western blotting. Aliquots of concentrated culture media from 10-ml dishes were resolved on 7.5% SDS–PAGE gel, then electrotransferred to nitrocellulose membrane at 250 mA for 1 h. Non-specific antibody binding was blocked with 5% non-fat dry milk in Tris-buffered saline (TBS)/0.1% Tween 20 at 25°C for 2 h. After washing, membrane was incubated overnight at 4°C with 1:300 diluted anti-MMP-13 monoclonal antibody (mAb) (Calbiochem, La Jolla, CA, USA). The membrane was washed and reacted with peroxidase-conjugated goat anti-mouse immunoglobulin (IgG) (Sigma, St. Louis, MO, USA) at 25°C for 2 h. Immunoreactive bands were visualized using SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL, USA), and exposure to CL-XPosure film (Pierce).
Inhibition of rIL-18-induced cytokine production with anti-cytokine antibodies
Antibodies to rIL-18 were produced in rabbits and IgG isolated from a protein-A column [16]. Chondrocytes were cultured for 24 h in 10-cm dishes with 100 ng/ml of rIL-18 and increasing amounts of anti-IL-18 or normal rabbit IgG. Cytokines in the supernatant were measured by ELISA. For inhibition of mRNA expression, cells were cultured with 100 ng/ml of rIL-18 and 5 µg/ml of anti-rIL-18 IgG or normal IgG. Efforts were made to inhibit cytokine mRNA expression with antibodies to IL-6 (2 µg/ml), IL-1β (10 µg/ml) and TNF-α (10 µg/ml) antibodies (R&D Systems). These products and concentrations are non-toxic and are twofold (or greater) than those reported by others to be effective in comparable systems [19,20], or described in information provided by the supplier. Total RNA was isolated from the aforementioned cultures after 6 h exposure to rIL-18 and antibody and subjected to qPCR study and the data were evaluated by 2-delta delta Ct analysis.
Results
Rat rIL-18 stimulates chondrocytes to express mRNA for of proinflammatory mediators
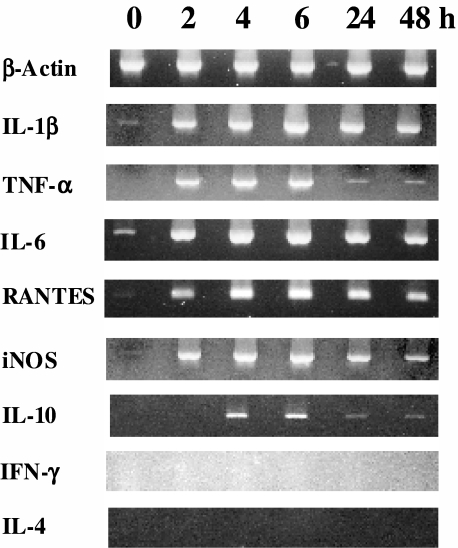
Conventional RT–PCR was used to ascertain the expression of mRNA for β-actin, IL-1β, TNF-α, IL-6, regulated upon activation normal T cell expressed and secreted (RANTES), inducible macrophage type nitric oxide synthase (iNOS), IL-10, IFN and rIL-4 by chondrocytes cultured 2–48 h with rIL-18. Data presented in Fig. 1 show that IL-18 stimulated mRNA expression of all these substances except IFN-γ and IL-4. Expression levels were highest at 4–6 h and decreased after 24–48 h culture. IL-10 mRNA was weakly expressed at 4–6 h. Only small amounts of IL-1β, IL-6, RANTES and iNOS mRNA were detected in unstimulated cultures.
Fig. 1.
Conventional reverse transcription–polymerase chain reaction (RT–PCR) analysis of recombinant interleukin (rIL)-18-induced mRNA expression of proinflammatory mediators by rat chondrocytes. Cells were cultured with 100 ng/ml of rat rIL-18 for 2, 4, 6, 24 and 48 h or medium alone (0 h). Total RNA was isolated and reverse transcribed. The PCR products were separated on 1.5% agarose gels and stained with ethidium bromide.
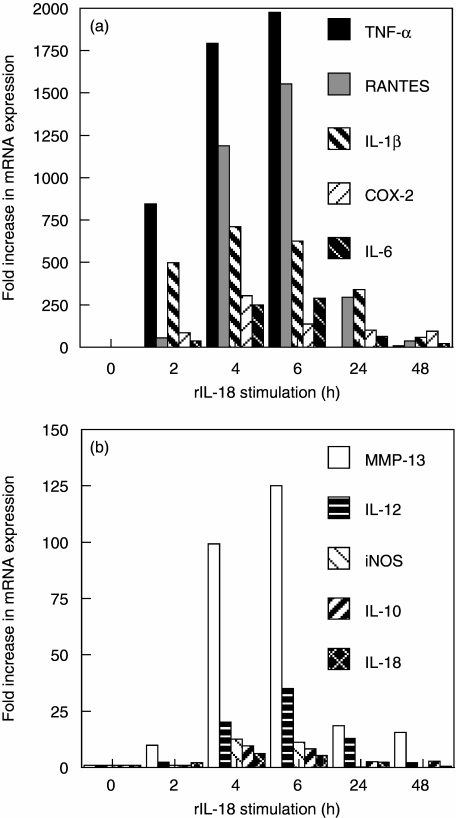
These results were verified by qPCR, as shown in Fig. 2. Message for TNF-α and IL-1β was 844 and 499-fold greater in cultures stimulated with rIL-18 for 2 h than control. Cultures maintained for 2, 4, 6, 24 and 48 h demonstrated that message peaked at 4 or 6 h for all the factors. Message for TNF-α, RANTES, IL-1β, IL-6 and COX-2 peaked at levels 1977, 1553, 711, 290 and 305-fold above control, respectively. Message for MMP-13, IL-12, iNOS, IL-10, IL-18 and MMP-8 were detected at lower peak levels of 125, 35, 13, 10, 6 and 2.1-fold increases, respectively.
Fig. 2.
Real-time polymerase chain reaction (PCR) analysis of recombinant interleukin (rIL)-18-induced mRNA expression of proinflammatory mediators by rat chondrocytes. Cells were cultured with 100 ng/ml of rat rIL-18 for 2, 4, 6, 24 and 48 h or medium alone (0 h). Total RNA was isolated, reverse transcribed, and subjected to quantitative PCR. The results were normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase and expressed as x-fold increase versus control using the 2 delta-delta Ct method.
Rat rIL-18 stimulates chondrocytes to produce proinflammatory mediators
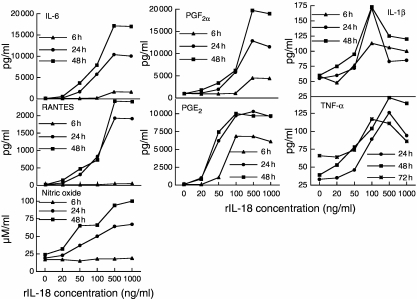
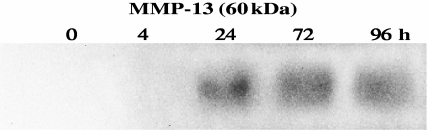
Exogenous rIL-18 stimulated chondrocytes to secrete proinflammatory mediators and MMP-13 in a time- and dose-dependent manner as measured by ELISA (Fig. 3) or Western blotting (Fig. 4). IL-6, prostaglandin F2α (PGF2α), RANTES, IL-1β, TNF-α and NO appeared in supernatants after 6 h stimulation and reached peak levels at 48 h (245, 20, 120, 2.9, 3.6 and fourfold peak increases, respectively). Prostaglandin E2 (PGE2) production was an exception, peaking at 24 h (143-fold increase). IL-6, PGF2α, RANTES, TNF-α and NO production rose with increasing amounts of rIL-18. Although substantial amounts of product were detected with 50 ng/ml of rIL-18, maximal levels were often reached with 500 ng/ml. IL-1β levels peaked with 100 ng/ml of rIL-18, but fell with 500 ng/ml. MMP-13 was detected as a 60-kDa proenzyme at 24 h and remained elevated at 96 h (Fig. 4). IL-10 and IFN-γ products were undetectable.
Fig. 3.
Rat recombinant interleukin (rIL)-18 stimulates rat articular chondrocytes to produce a variety of proinflammatory products. The chondrocytes were cultured with increasing amounts of rIL-18 or medium for 6, 24 and 48 h. Levels of IL-6, prostaglandin E2, prostaglandin F2α, regulated upon activation normal T cell expressed and secreted (RANTES), interleukin (IL)-1β, tumour necrosis factor (TNF)-α and nitric oxide (NO) secreted into the culture supernatant were measured as described in Materials and methods. These products were secreted in a dose- and time-dependent manner.
Fig. 4.
The production of matrix metalloproteinase-13 by recombinant interleukin (rIL)-18-stimulated chondrocytes demonstrated by Western blotting. Chondrocytes were stimulated with 100 ng/ml of rIL-18 for 4, 24, 72 and 96 h or cultured with medium; cells lysed at 0 h provided a background value.
Anti-rIL-18 antibody inhibits IL-18 induced chondrocyte mRNA expression and the production of proinflammatory mediators
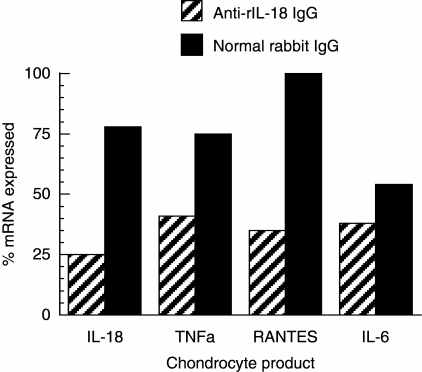
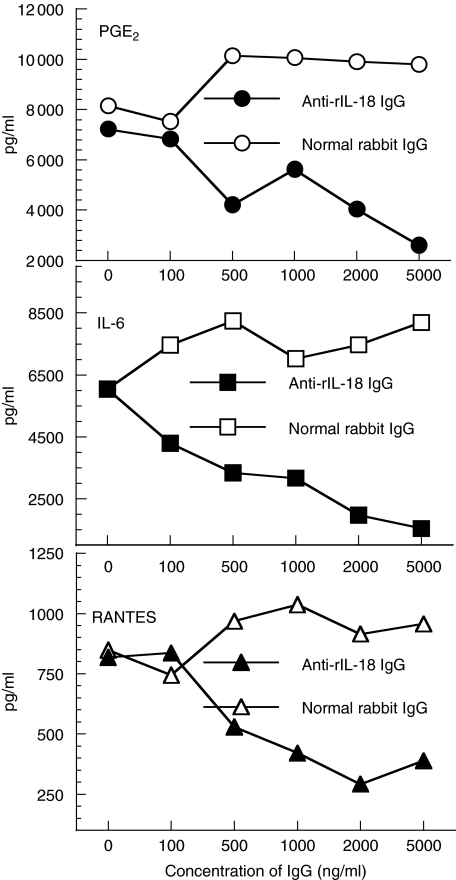
To determine if the effects of rIL-18 on chondrocytes could be attenuated, anti-rIL-18 IgG was added to cultures stimulated with rIL-18. Message for IL-18, TNF-α, RANTES and IL-6 fell to levels below 50% of control cultures treated with normal IgG (Fig. 5). Levels of PGE2, IL-6 and RANTES product also fell in a dose-related manner (Fig. 5); IL-1β, NO and PGF2α levels fell, although less notably (data not shown). Efforts to block the actions of rIL-18 on chondrocytes by simultaneously adding anti-IL-6, anti-IL-1β or anti-TNF-α antibodies were not successful and often produced small increases of the amount message expressed versus cultures where normal IgG was added (data not shown). These cultures were maintained for 6 h using the same conditions described in Fig. 6 and in Materials and methods. The cause and biological relevance of the apparent synergistic interaction between rIL-18 and these antibodies are unclear, and might be a topic of future interest.
Fig. 5.
Anti-recombinant interleukin (rIL)-18 antibodies inhibit the expression of mRNA for proinflammatory mediators by rIL-18-stimulated chondrocytes. Cells were cultured with 5 µg/ml of anti-rIL-18 IgG or normal rabbit IgG and 100 ng/ml of rIL-18 for 6 h. Total RNA was isolated, reverse transcribed and subjected to quantitative polymerase chain reaction analysis. The results are expressed as described in Fig. 3.
Fig. 6.
Anti-recombinant interleukin (rIL)-18 antibodies inhibit the production of proinflammatory mediators stimulated by rIL-18. Chondrocytes cultures were stimulated with 100 ng/ml of rIL-18 and increasing amounts of anti-rIL-18 IgG or normal rabbit IgG added. A commercial enzyme-linked immunosorbent assay system was used to quantify IL-6, prostaglandin E2 and regulated upon activation normal T cell expressed and secreted (RANTES) released into supernatants collected after 24 h culture. Anti-rIL-18 antibody suppressed the production of proinflammatory mediators in a dose-related manner.
Discussion
Unravelling the complexities of immune-mediated polyarthritis is a challenge, whether investigated in human or in experimental animal systems. Collagen-induced arthritis is initiated by autoreactive anti-CII antibodies, which bind to cartilage, fix complement and trigger synovitis by a variety of pathways. This process occurs whether antibodies are produced actively or administered passively [21,22]. Th1 cells are essential for anti-CII antibody production and infiltrate the synovium to sustain inflammation. All these events lead to the production of proinflammatory cytokines, chemokines and other substances, which promote cartilage injury.
IL-18 is produced by activated synovial macrophages and fibroblasts [4] and is believed to be instrumental in joint injury CIA and in RA. We have shown that rIL-18 enhances the incidence and severity of CIA in rats, in addition to the production of IFN-γ, IL-6, TNF-α and IL-2 by splenocytes [16]. Importantly, arthritis severity can be attenuated with neutralizing anti-rIL-18 antibodies or other IL-18 specific antagonists [23]. These findings, plus increased mRNA expression for IL-18 by inflamed rat synovium [16] and the presence of elevated levels of IL-18 in the synovial fluids and tissues of RA patients suggest that IL-18 might participate in cartilage injury [6,14]. This possibility is supported further by the work of Joosten et al. [17], who injected wild-type, TNF-α- and IL-1α,β-deficient C57BL/6 mice intra-articularly with an IL-18-expressing adenovirus vector. Histological examination of the joints of all three strains disclosed pronounced inflammation and cartilage proteoglycan loss; such changes were absent in the joints of control mice injected with non-expressing adenovirus.
The studies reported here show that rat chondrocytes stimulated in vitro with rIL-18 produce a variety of proinflammatory substances. IL-6 was the greatest, reaching values 245-fold above control. The importance of IL-6 in the pathogenesis of RA and experimental arthritis is well established. IL-6 is significantly higher in RA synovial fluid than in OA samples [24,25]. In the CIA model, IL-6 knock-out mice are protected against arthritis [26,27]. We have also found that the synovia of rats with CIA express mRNA for IL-6 at levels 37 times greater than messages for IL-1α/β, TNF-α/β, IFN-γ and IL-2; moreover, the synovial production of IL-6 protein was 200 times greater in arthritic rats than non-arthritic rats (our unpublished data). Our work described here, showing that IL-18 stimulates chondrocytes to produce IL-6, IL-1β and TNF-α, complements the work of others who investigated the effects of IL-18 on synovial cells and splenocytes [14,16]. The roles of these mediators in the pathogenesis of RA, and to a lesser degree osteoarthritis, are well established [28,29]. Although IL-1β and TNF-α stimulate IL-6 synthesis [30], we were unable to block IL-6 expression by adding neutralizing antibodies to either cytokine to IL-18-stimulated chondrocyte cultures, suggesting that IL-18 is a potent and possibly an independent activator of IL-6.
Although often thought of as T lymphocyte-related product, synovial fibroblasts and a host of other cells [31] produce RANTES (CCL5). Our studies show that rIL-18-stimulated chondrocytes produce large amounts of RANTES, complementing the work of Alaaeddine et al. [32], who found increased RANTES in chondrocytes obtained from patients with osteoarthritis. RANTES is a strong chemoattractant for eosinophils, monocytes and T lymphocytes, specifically CD4+/CD45RO+ memory T cells [33,34]. Ex vivo studies show that RANTES also stimulates chondrocytes to produce iNOS and IL-6 [32]. RANTES production, like IL-6, is stimulated by IL-1β and TNF-α, which were elevated in our system [32,35]. The importance of RANTES in experimental arthritis has been demonstrated by Plater-Zyberk et al. [36], who showed that metRANTES, a chemically modified antagonist of RANTES, attenuates CIA.
Chondrocytes stimulated with IL-18 also produced large quantities of PGE2 and PGF2α. PGE2 is an important mediator of inflammation in RA and OA and triggers osteolysis [37]. PGE2, which is biologically more potent than most other prostaglandins, rose more rapidly than the other products quantified. Message for COX-2 also rose quickly and remained elevated at 72 h. Levels of PGE2 exceeded those of non-stimulated controls by 79-fold at 6 h and peaked at 143-fold at 24 h. PGF2α also reached a high level, although more slowly than PGE2. PGF2α is linked closely to joint injury and is capable of down-regulating TGF-β-induced osteoblast activation [38].
iNOS is active in endothelial cells, synovial cells and chondrocytes, where it generates high levels of NO in response to lipopolysaccharide (LPS), IL-1β, TNF-α and, as shown here, IL-18. The overproduction of iNOS, and consequently NO, occurs in the synovia of rheumatoid patients [39,40] and rats with CIA [41]. Nitric oxide exerts numerous proinflammatory effects, including increased vascular permeability [42], PGE2 synthesis [43], MMP production, nuclear factor (NF)-κB gene activation and DNA fragmentation. NO also suppresses CII and proteoglycan synthesis and TGF-β-induced chondrocyte proliferation. The potential role of NO in cartilage destruction is thus substantial [44]. Nevertheless, NO possesses some anti-inflammatory properties, including the down-regulation of PGE2, IL-6 and IL-8 in human chondrocytes [45]. The dual effect of NO might be related to the rate and amount of product generated, in addition to its rate of clearance. Stadler et al. [46] have shown that low levels of NO stimulation increased chondrocyte PGE2 synthesis, whereas a high level was inhibitory. Our studies show that rIL-18 stimulated relatively low levels of iNOS expression, which was accompanied by increased production of PGE2, suggesting that iNOS was promoting inflammation.
MMPs are zinc-dependent enzymes that degrade extracellular matrix. MMP-13 (collagenase-3) is expressed by synovial cells and chondrocytes and cleaves CII ∼10 times faster than MMP-1 [47]. The over-expression of MMP-13 occurs in RA synovium [48] and OA cartilage [49]. Interestingly, mice expressing the MMP-13 transgene develop OA-like changes and transient synovitis, as do mice injected intra-articularly with an MMP-13 expressing adenovirus [50]. In our studies, rIL-18 significantly induced MMP-13 expression by chondrocytes at mRNA and protein levels, indicating that IL-18 mediates matrix degradation.
Lastly, we found that PGE2, IL-6 and RANTES produced by IL-18-stimulated chondrocytes, as well as message for IL-1β, NO and PGF2α, could be suppressed effectively by neutralizing antibodies to IL-18, but not to IL-1β, TNF-α and IL-6. This observation could have potential clinical relevance. That is, if current and future biological treatments designed to inhibit the actions of IL-1β, TNF-α and IL-6 do not inhibit IL-18 production, it might continue to activate chondrocytes resulting in continued cartilage injury. Together, we believe that the findings reported here support the further investigation of IL-18 as a mediator of cartilage injury and as a target for future therapeutic intervention.
Acknowledgments
These studies were funded by a Merit Review Grant provided by the Department of Veterans Affairs and with additional support from USPHS grants AR39166 and AR43589. We would like to acknowledge Jesse B Cremer for his sustained support.
References
- 1.Le J, Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987;56:234–48. [PubMed] [Google Scholar]
- 2.Lotz M, Blanco FJ, von Kempis J, et al. Cytokine regulation of chondrocyte functions. J Rheumatol Suppl. 1995;43:104–8. [PubMed] [Google Scholar]
- 3.Ghayur T, Banerjee S, Hugunin M, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386:619–23. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 4.Gillespie MT, Horwood NJ. Interleukin-18: perspectives on the newest interleukin. Cytokine Growth Factor Rev. 1998;9:109–16. doi: 10.1016/s1359-6101(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 5.Stoll S, Jonuleit H, Schmitt E, et al. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur J Immunol. 1998;28:3231–9. doi: 10.1002/(SICI)1521-4141(199810)28:10<3231::AID-IMMU3231>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 6.Olee T, Hashimoto S, Quach J, Lotz M. IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses. J Immunol. 1999;162:1096–100. [PubMed] [Google Scholar]
- 7.Udagawa N, Horwood NJ, Elliott J, et al. Interleukin-18 (interferon-gamma-inducing factor) is produced by osteoblasts and acts via granulocyte/macrophage colony-stimulating factor and not via interferon-gamma to inhibit osteoclast formation. J Exp Med. 1997;185:1005–12. doi: 10.1084/jem.185.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okamura H, Tsutsui H, Kashiwamura S, Yoshimoto T, Nakanishi K. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol. 1998;70:281–312. doi: 10.1016/s0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- 9.Hunter CA, Timans J, Pisacane P, et al. Comparison of the effects of interleukin-1 alpha, interleukin-1 beta and interferon-gamma-inducing factor on the production of interferon-gamma by natural killer. Eur J Immunol. 1997;27:2787–92. doi: 10.1002/eji.1830271107. [DOI] [PubMed] [Google Scholar]
- 10.Netea MG, Kullberg BJ, Verschueren I, Van Der Meer JW. Interleukin-18 induces production of proinflammatory cytokines in mice: no intermediate role for the cytokines of the tumor necrosis factor family and interleukin-1beta. Eur J Immunol. 2000;30:3057–60. doi: 10.1002/1521-4141(200010)30:10<3057::AID-IMMU3057>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 11.Kohka H, Yoshino T, Iwagaki H, et al. Interleukin-18/interferon-gamma-inducing factor, a novel cytokine, up-regulates ICAM-1 (CD54) expression in KG-1 cells. J Leukoc Biol. 1998;64:519–27. doi: 10.1002/jlb.64.4.519. [DOI] [PubMed] [Google Scholar]
- 12.Cannetti CA, Leung BP, Culshaw S, McInnes IB, Cunha FQ, Liew FY. IL-18 enhances collagen-induced arthritis by recruiting neutrophils via TNF-alpha and leukotriene B4. J Immunol. 2003;171:1009–15. doi: 10.4049/jimmunol.171.2.1009. [DOI] [PubMed] [Google Scholar]
- 13.Leung BP, Culshaw S, Gracie JA, et al. A role for IL-18 in neutrophil activation. J Immunol. 2001;167:2879–86. doi: 10.4049/jimmunol.167.5.2879. [DOI] [PubMed] [Google Scholar]
- 14.Gracie JA, Forsey RJ, Chan WL, et al. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999;104:1393–401. doi: 10.1172/JCI7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei XQ, Leung BP, Arthur HM, McInnes IB, Liew FY. Reduced incidence and severity of collagen-induced arthritis in mice lacking IL-18. J Immunol. 2001;166:517–21. doi: 10.4049/jimmunol.166.1.517. [DOI] [PubMed] [Google Scholar]
- 16.Ye XJ, Tang B, Ma Z, Kang AH, Myers LK, Cremer MA. The roles of interleukin-18 in collagen-induced arthritis in the BB rat. Clin Exp Immunol. 2004;136:440–7. doi: 10.1111/j.1365-2249.2004.02430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joosten LA, Smeets RL, Koenders MI, et al. Interleukin-18 promotes joint inflammation and induces interleukin-1-driven cartilage destruction. Am J Pathol. 2004;165:959–67. doi: 10.1016/S0002-9440(10)63357-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto T, Tsukazaki T, Enomoto H, Iwasaki K, Yamashita S. Effects of interleukin-1 beta on insulin-like growth factor-I autocrine/paracrine axis in cultured rat articular chondrocytes. Ann Rheum Dis. 1994;53:128–33. doi: 10.1136/ard.53.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horan J, Dean DD, Kieswetter K, Schwartz Z, Boyan BD. Evidence that interleukin-1, but not interleukin-6, affects costochondral chondrocyte proliferation, differentiation, and matrix synthesis through an autocrine pathway. J Bone Miner Res. 1996;11:1119–29. doi: 10.1002/jbmr.5650110811. [DOI] [PubMed] [Google Scholar]
- 20.Martensson K, Chrysis D, Savendahl L. Interleukin-1beta and TNF-alpha act in synergy to inhibit longitudinal growth in fetal rat metatarsal bones. J Bone Miner Res. 2004;19:1805–12. doi: 10.1359/JBMR.040805. [DOI] [PubMed] [Google Scholar]
- 21.Stuart JM, Cremer MA, Townes AS, Kang AH. Type II collagen-induced arthritis in rats. Passive transfer with serum and evidence that IgG anticollagen antibodies can cause arthritis. J Exp Med. 1982;155:1–16. doi: 10.1084/jem.155.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terato K, Hasty KA, Reife RA, Cremer MA, Kang AH, Stuart JM. Induction of arthritis with monoclonal antibodies to collagen. J Immunol. 1992;148:2103–8. [PubMed] [Google Scholar]
- 23.Banda NK, Vondracek A, Kraus D, et al. Mechanisms of inhibition of collagen-induced arthritis by murine IL-18 binding protein. J Immunol. 2003;170:2100–5. doi: 10.4049/jimmunol.170.4.2100. [DOI] [PubMed] [Google Scholar]
- 24.Guerne PA, Zuraw BL, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989;83:585–92. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houssiau FA, Devogelaer JP, Van Damme J, de Deuxchaisnes CN, Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988;31:784–8. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- 26.Alonzi T, Fattori E, Lazzaro D, et al. Interleukin 6 is required for the development of collagen-induced arthritis. J Exp Med. 1998;187:461–8. doi: 10.1084/jem.187.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasai M, Saeki Y, Ohshima S, et al. Delayed onset and reduced severity of collagen-induced arthritis in interleukin-6-deficient mice. Arthritis Rheum. 1999;42:1635–43. doi: 10.1002/1529-0131(199908)42:8<1635::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 28.Maini RN, Elliott M, Brennan FM, Williams RO, Feldmann M. TNF blockade in rheumatoid arthritis: implications for therapy and pathogenesis. APMIS. 1997;105:257–63. doi: 10.1111/j.1699-0463.1997.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 29.Towle CA, Hung HH, Bonassar LJ, Treadwell BV, Mangham DC. Detection of interleukin-1 in the cartilage of patients with osteoarthritis: a possible autocrine/paracrine role in pathogenesis. Osteoarthritis Cartilage. 1997;5:293–300. doi: 10.1016/s1063-4584(97)80008-8. [DOI] [PubMed] [Google Scholar]
- 30.Bender S, Haubeck HD, Van de Leur E, et al. Interleukin-1 beta induces synthesis and secretion of interleukin-6 in human chondrocytes. FEBS Lett. 1990;263:321–4. doi: 10.1016/0014-5793(90)81404-c. [DOI] [PubMed] [Google Scholar]
- 31.Rathanaswami P, Hachicha M, Sadick M, Schall TJ, McColl SR. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J Biol Chem. 1993;268:5834–9. [PubMed] [Google Scholar]
- 32.Alaaeddine N, Olee T, Hashimoto S, Creighton-Achermann L, Lotz M. Production of the chemokine RANTES by articular chondrocytes and role in cartilage degradation. Arthritis Rheum. 2001;44:1633–43. doi: 10.1002/1529-0131(200107)44:7<1633::AID-ART286>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 33.Alam R, Stafford S, Forsythe P, et al. RANTES is a chemotactic and activating factor for human eosinophils. J Immunol. 1993;150:3442–8. [PubMed] [Google Scholar]
- 34.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–71. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 35.Hirano F, Komura K, Fukawa E, Makino I. Tumor necrosis factor alpha (TNF-alpha)-induced RANTES chemokine expression via activation of NF-kappaB and p38 MAP kinase: roles of TNF-alpha in alcoholic liver diseases. J Hepatol. 2003;38:483–9. doi: 10.1016/s0168-8278(02)00456-7. [DOI] [PubMed] [Google Scholar]
- 36.Plater-Zyberk C, Hoogewerf AJ, Proudfoot AE, Power CA, Wells TN. Effect of a CC chemokine receptor antagonist on collagen induced arthritis in DBA/1 mice. Immunol Lett. 1997;57:117–20. doi: 10.1016/s0165-2478(97)00075-8. [DOI] [PubMed] [Google Scholar]
- 37.Robinson DR, Tashjian AH, Jr, Levine L. Prostaglandin-induced bone resorption by rheumatoid synovia. Trans Assoc Am Physicians. 1975;88:146–60. [PubMed] [Google Scholar]
- 38.Klein-Nulend J, Semeins CM, Burger EH. Prostaglandin mediated modulation of transforming growth factor-beta metabolism in primary mouse osteoblastic cells in vitro. J Cell Physiol. 1996;168:1–7. doi: 10.1002/(SICI)1097-4652(199607)168:1<1::AID-JCP1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 39.Sakurai H, Kohsaka H, Liu MF, et al. Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J Clin Invest. 1995;96:2357–63. doi: 10.1172/JCI118292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farrell AJ, Blake DR, Palmer RM, Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis. 1992;51:1219–22. doi: 10.1136/ard.51.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cannon GW, Openshaw SJ, Hibbs JB, Jr, Hoidal JR, Huecksteadt TP, Griffiths MM. Nitric oxide production during adjuvant-induced and collagen-induced arthritis. Arthritis Rheum. 1996;39:1677–84. doi: 10.1002/art.1780391010. [DOI] [PubMed] [Google Scholar]
- 42.Mayhan WG. Role of nitric oxide in modulating permeability of hamster cheek pouch in response to adenosine 5′-diphosphate and bradykinin. Inflammation. 1992;16:295–305. doi: 10.1007/BF00917622. [DOI] [PubMed] [Google Scholar]
- 43.Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA. 1993;90:7240–4. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van de Loo FA, Arntz OJ, van Enckevort FH, van Lent PL, van den Berg WB. Reduced cartilage proteoglycan loss during zymosan-induced gonarthritis in NOS2-deficient mice and in anti-interleukin-1-treated wild-type mice with unabated joint inflammation. Arthritis Rheum. 1998;41:634–46. doi: 10.1002/1529-0131(199804)41:4<634::AID-ART10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 45.Henrotin YE, Zheng SX, Deby GP, Labasse AH, Crielaard JM, Reginster JY. Nitric oxide downregulates interleukin 1beta (IL-1beta) stimulated IL-6, IL-8, and prostaglandin E2 production by human chondrocytes. J Rheumatol. 1998;25:1595–601. [PubMed] [Google Scholar]
- 46.Stadler J, Stefanovic-Racic M, Billiar TR, et al. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J Immunol. 1991;147:3915–20. [PubMed] [Google Scholar]
- 47.Mitchell PG, Magna HA, Reeves LM, et al. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–8. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Konttinen YT, Ainola M, Valleala H, et al. Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: different profiles in trauma and rheumatoid arthritis. Ann Rheum Dis. 1999;58:691–7. doi: 10.1136/ard.58.11.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–94. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 50.Joronen K, Ala-aho R, Majuri ML, Alenius H, Kahari VM, Vuorio E. Adenovirus mediated intra-articular expression of collagenase-3 (MMP-13) induces inflammatory arthritis in mice. Ann Rheum Dis. 2004;63:656–64. doi: 10.1136/ard.2003.009720. [DOI] [PMC free article] [PubMed] [Google Scholar]